Conversion of Cellulose to Lactic Acid by Using ZrO2–Al2O3 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
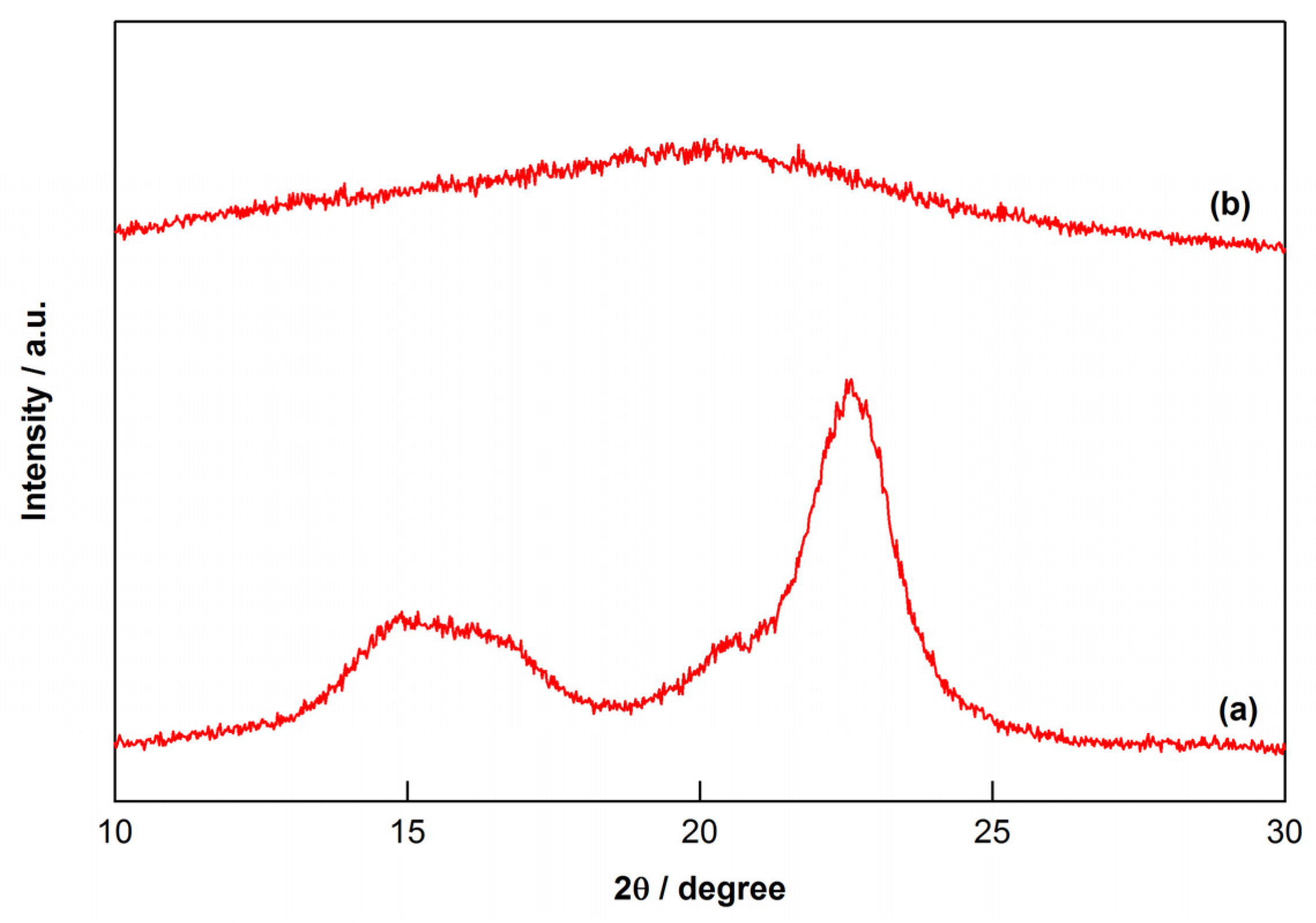
2.1. Effect of Ball-Milling Treatment on Cellulose Conversion
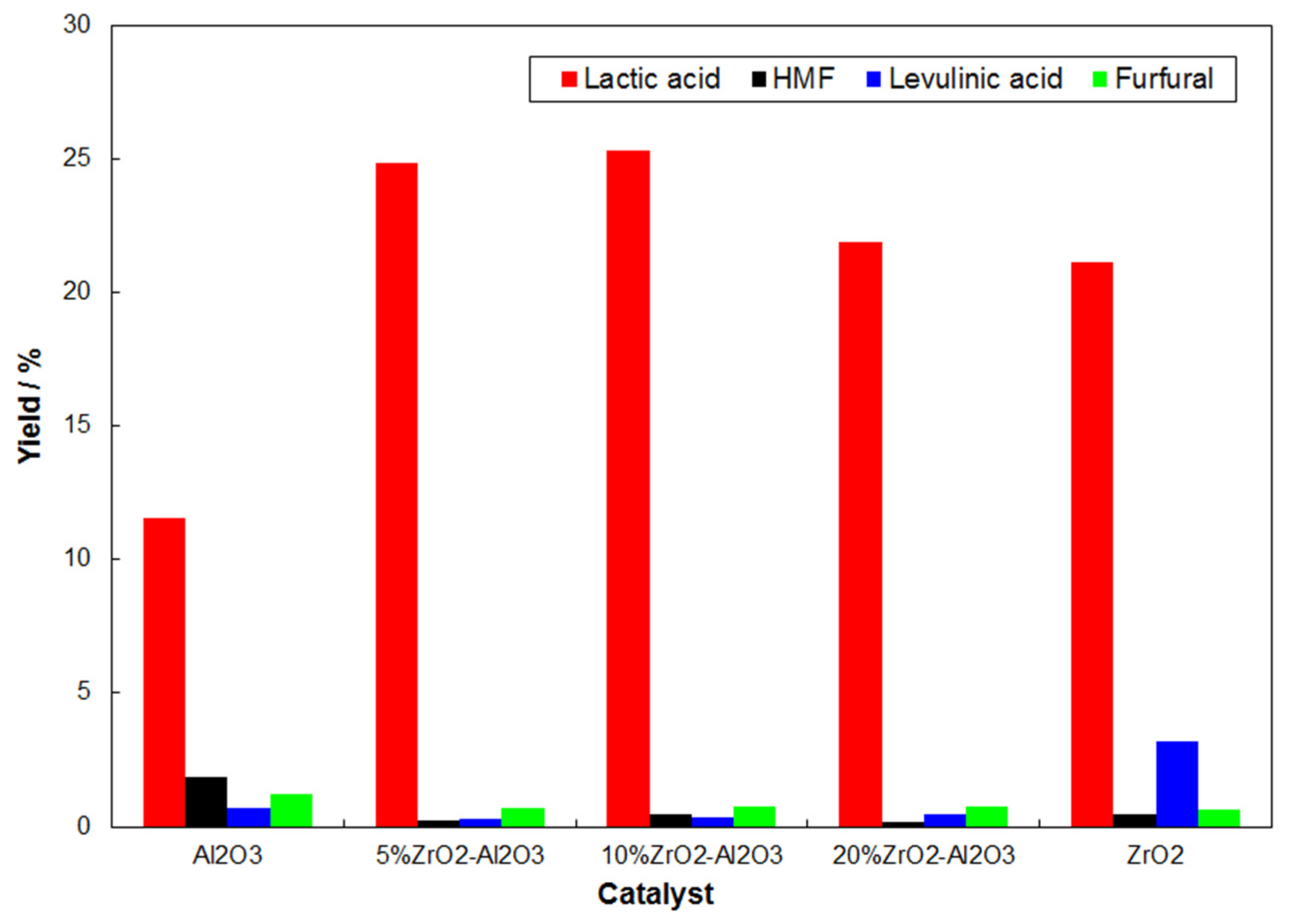
2.2. Catalytic Conversion of Cellulose to Lactic Acid by Using ZrO2–Al2O3
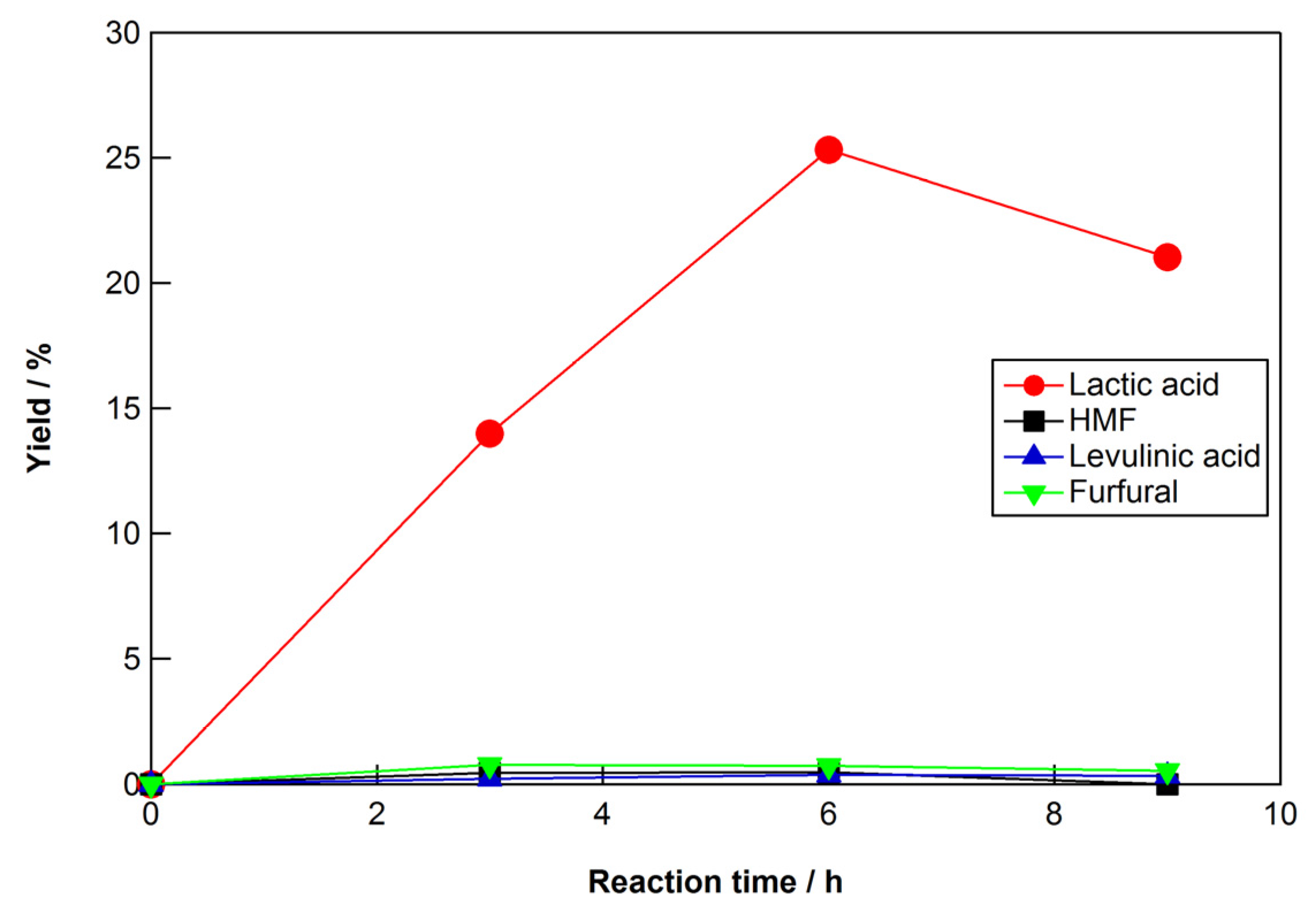
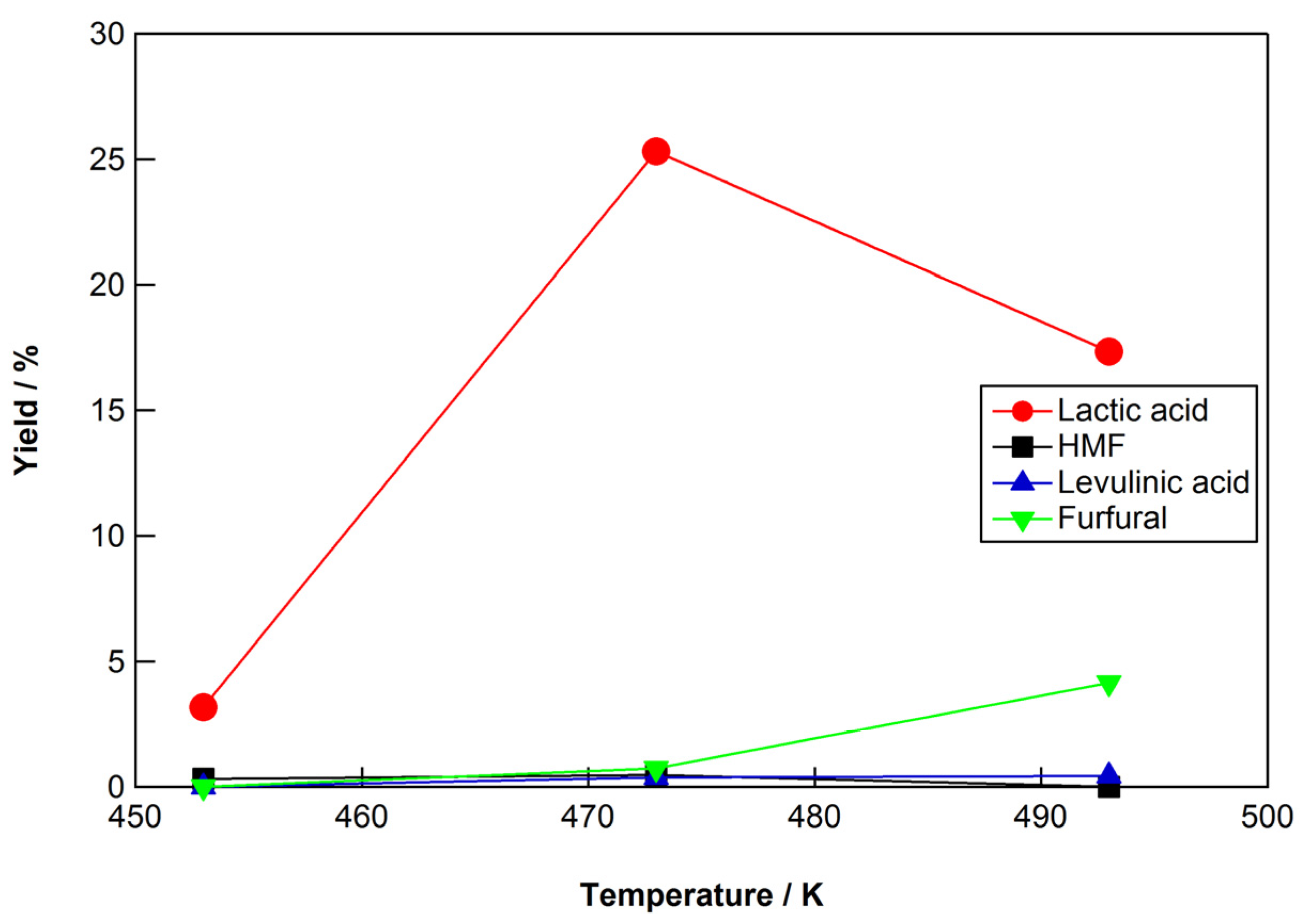
2.3. Reaction Conditions
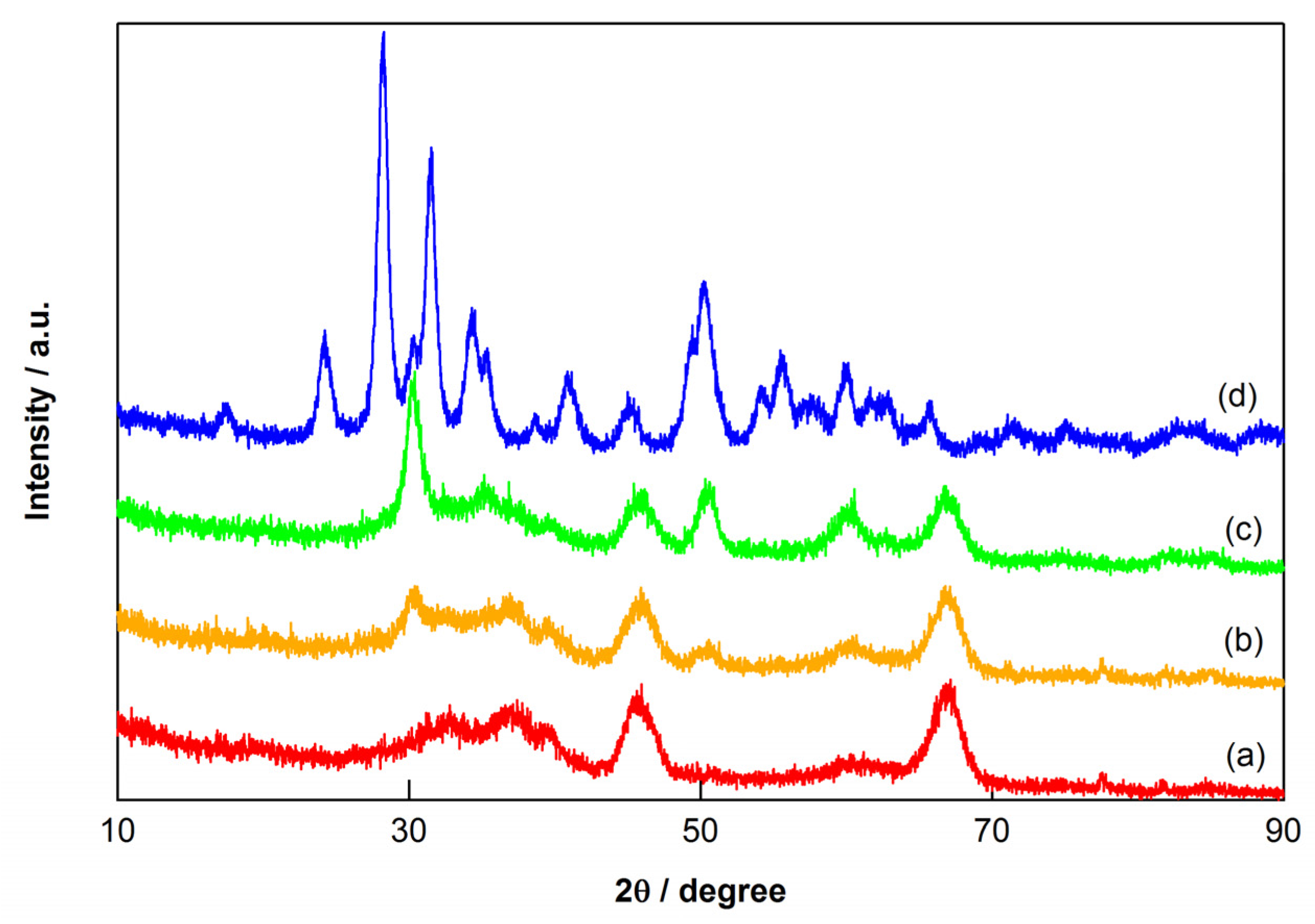
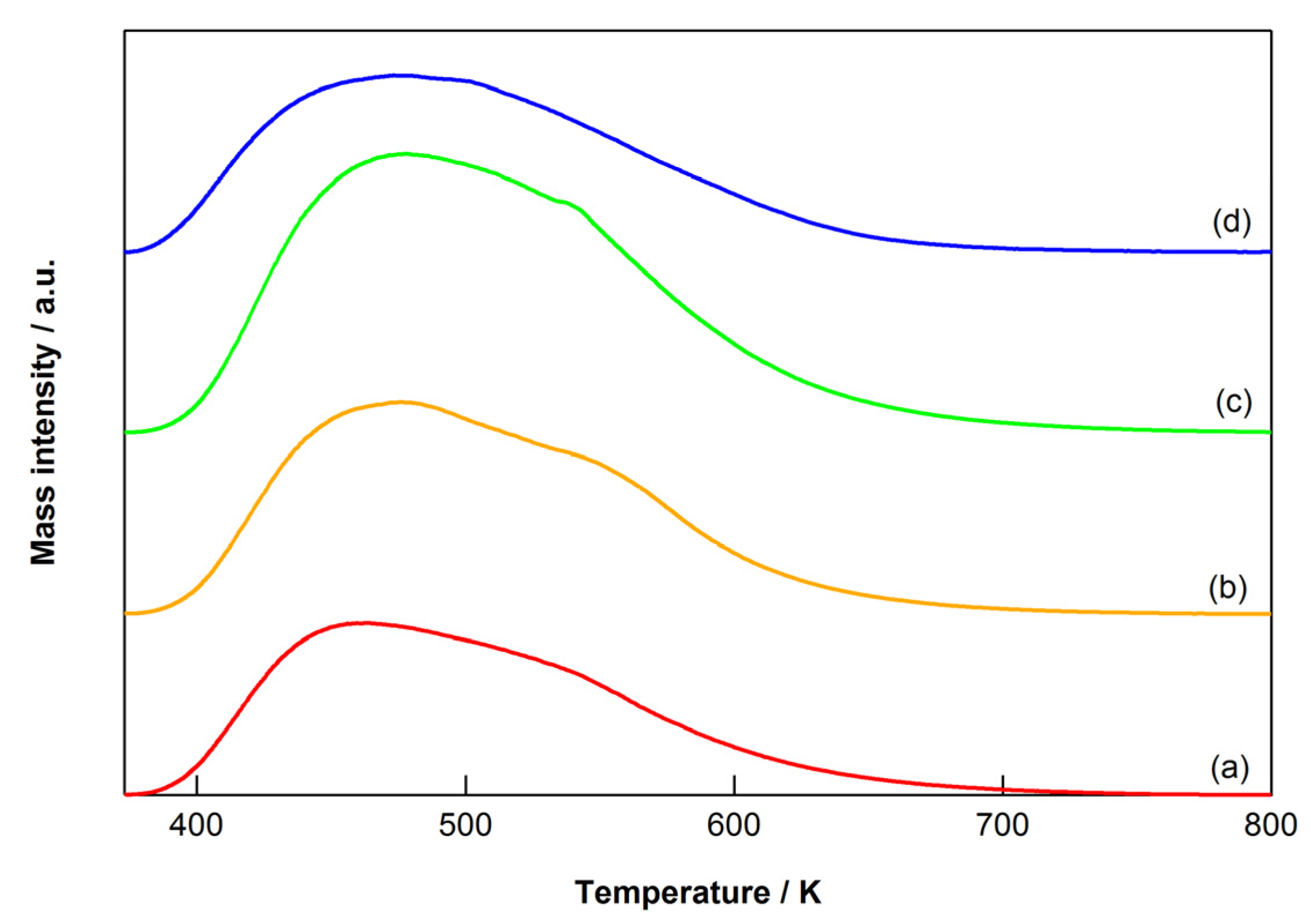
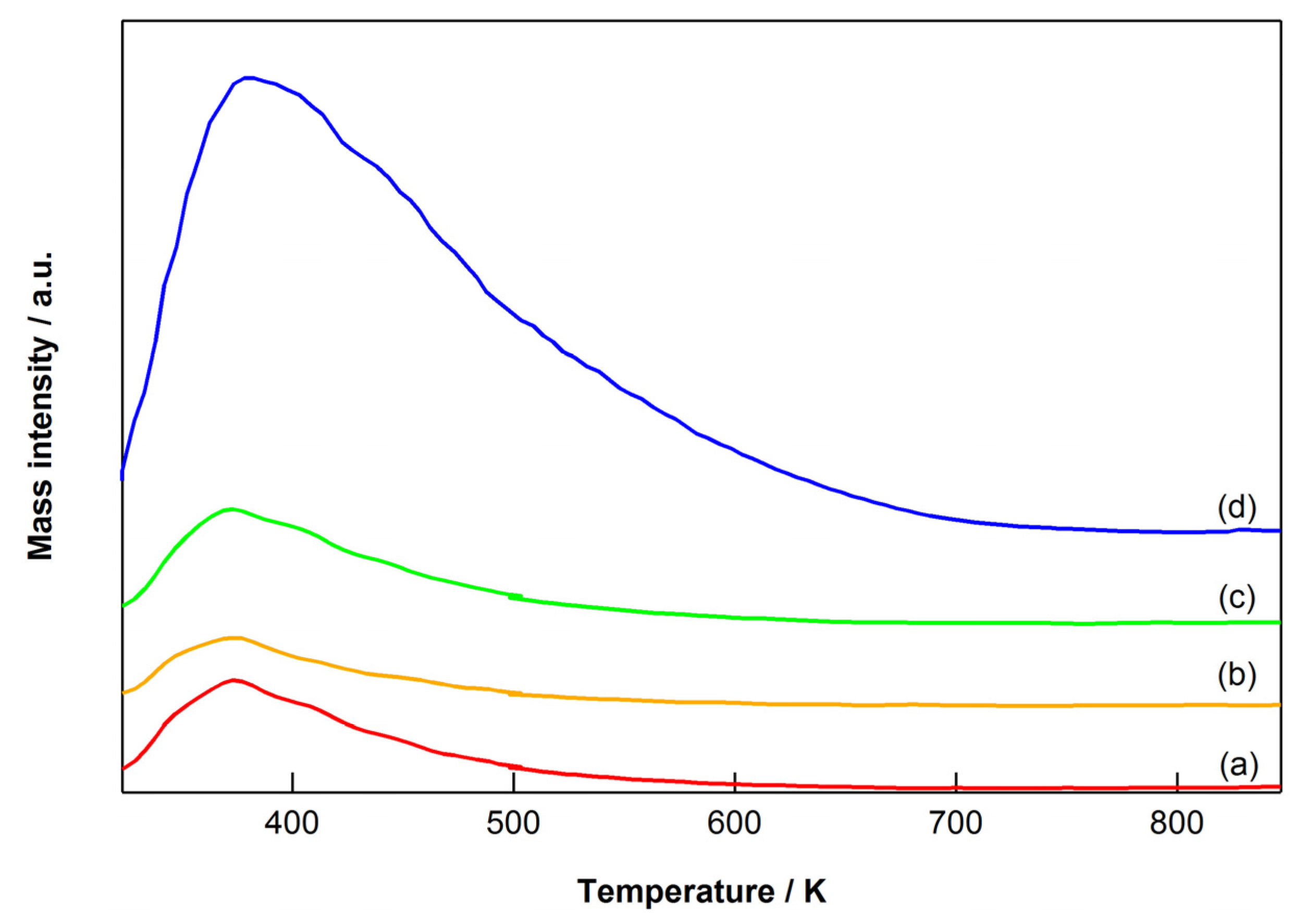
2.4. Characterization
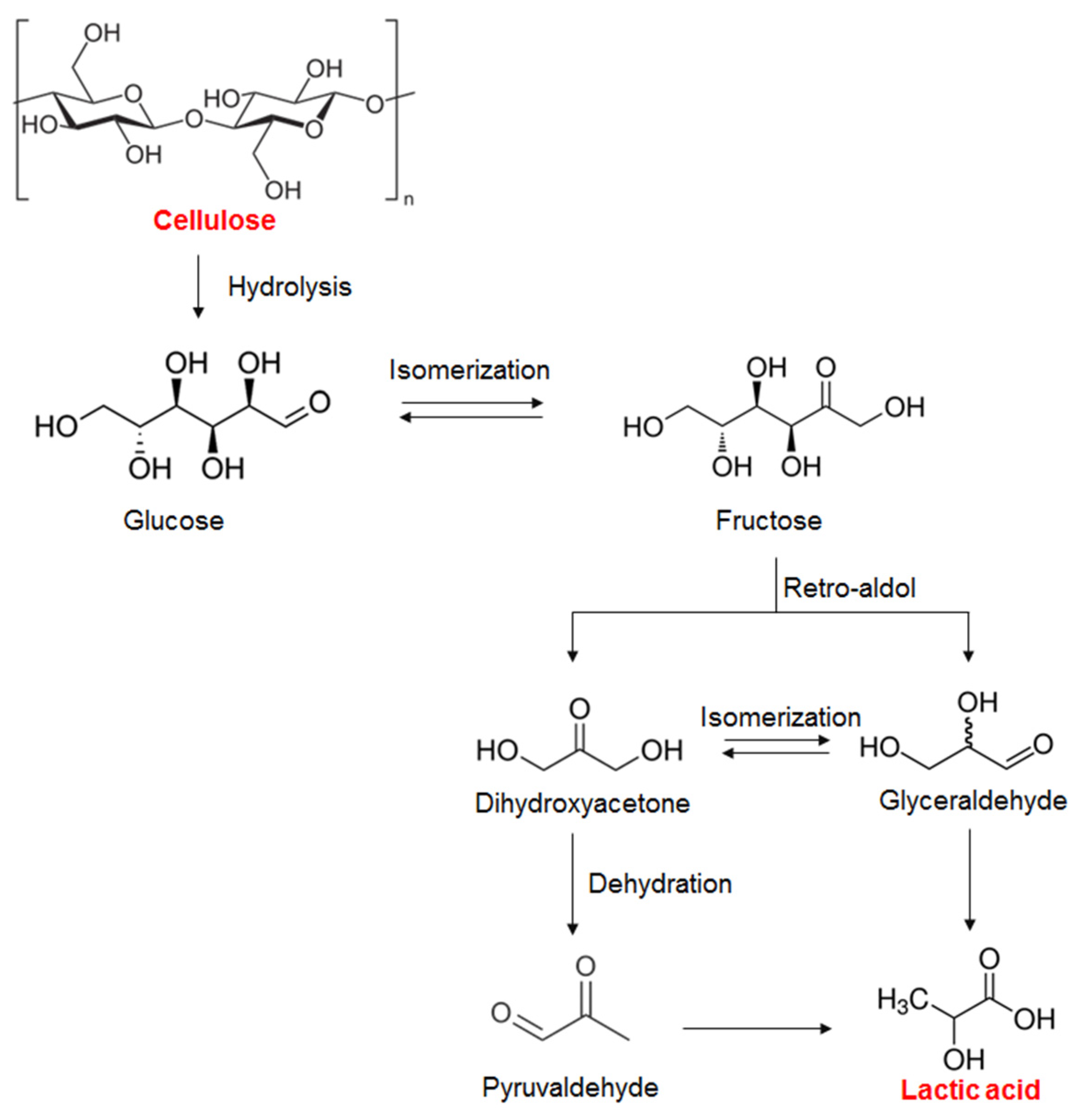
2.5. Reaction Pathway
3. Materials and Methods
3.1. Materials
3.2. Catalytic Reaction
3.3. Catalyst Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Komanoya, T.; Guha, S.K.; Hara, K.; Fukuoka, A. Conversion of cellulose into renewable chemicals by supported metal catalysis. Appl. Catal. A 2011, 409–410, 13–20. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A. Biomass Valorization in High-temperature Liquid Water. J. Jpn. Petrol. Inst. 2014, 57, 155–163. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Sato, O.; Mimura, N.; Shirai, M. One-pot conversion of cellulose to isosorbide using supported metal catalysts and ion-exchange resin. Catal. Commun. 2015, 67, 59–63. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of Lactic Acid/Lactates from Biomass and Their Catalytic Transformations to Commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; Oliveira, R.P.D.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Wang, B.; Zhang, Q.; Wan, X.; Tang, Z.; Wang, Y.; Zhu, C.; Cao, Z.; Wang, G.; et al. Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nat. Commun. 2013, 4, 2141. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Wang, F.-F.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. One-pot catalytic conversion of carbohydrate biomass to lactic acid using an ErCl3 catalyst. Appl. Catal. A 2014, 482, 78–83. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Fan, W.; Lin, H. Effect of redox properties of LaCoO3 perovskite catalyst on production of lactic acid from cellulosic biomass. Catal. Today 2016, 269, 56–64. [Google Scholar] [CrossRef]
- Coman, S.M.; Verziu, M.; Tirsoaga, A.; Jurca, B.; Teodorescu, C.; Kuncser, V.; Parvulescu, V.I.; Scholz, G.; Kemnitz, E. NbF5–AlF3 Catalysts: Design, Synthesis, and Application in Lactic Acid Synthesis from Cellulose. ACS Catal. 2015, 5, 3013–3026. [Google Scholar] [CrossRef]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid. Appl. Catal. B 2011, 105, 171–181. [Google Scholar] [CrossRef]
- Wattanapaphawong, P.; Reubroycharoen, P.; Yamaguchi, A. Conversion of cellulose into lactic acid using zirconium oxide catalysts. RSC Adv. 2017, 7, 18561–18568. [Google Scholar] [CrossRef]
- Teramoto, Y.; Tanaka, N.; Lee, S.-H.; Endo, T. Pretreatment of eucalyptus wood chips for enzymatic saccharification using combined sulfuric acid-free ethanol cooking and ball milling. Biotechnol. Bioeng. 2008, 99, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Bando, K.K.; Shirai, M. Gaseous Fuel Production from Nonrecyclable Paper Wastes Using Supported Metal Catalysts in High-Temperature Liquid Water. ChemSusChem 2010, 3, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Sato, O.; Mimura, N.; Hirosaki, Y.; Kobayashi, H.; Fukuoka, A.; Shirai, M. Direct Production of Sugar Alcohols from Wood Chips using Supported Platinum Catalysts in Water. Catal. Commun. 2014, 54, 22–26. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.H.; Wang, Y.; Franz, J.A.; White, J.M.; Holladay, J.E. Effects of Crystallinity on Dilute Acid Hydrolysis of Cellulose by Cellulose Ball-Milling Study. Energy Fuels 2006, 20, 807–811. [Google Scholar] [CrossRef]
- Said, A.E.-A.A.; El-Wahab, M.M.M.A.; El-Aal, M.A. Effect of ZrO2 on the catalytic performance of nano γ-Al2O3 in dehydration of methanol to dimethyl ether at relatively low temperature. Res. Chem. Intermed. 2016, 42, 1537–1556. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, A.; Zhao, Z.; Wan, G.; Gao, Z.; Jiang, G.; Chi, K.; Chuang, K.H. Preparation, characterization and hydrotreating performances of ZrO2–Al2O3-supported NiMo catalysts. Catal. Today 2010, 149, 62–68. [Google Scholar] [CrossRef]
- Manrı́quez, M.E.; López, T.; Gómez, R.; Navarrete, J. Preparation of TiO2–ZrO2 mixed oxides with controlled acid–basic properties. J. Mol. Catal. A 2004, 220, 229–237. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Yang, C.; Wei, W.; Li, W.-H.; Sun, Y.-H. Surface properties and CO adsorption on zirconia polymorphs. J. Mol. Catal. A 2005, 227, 119–124. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schuth, F. Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes. ChemSusChem 2009, 2, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xi, J.; Wang, Y. Recent advances in the catalytic production of glucose from lignocellulosic biomass. Green Chem. 2015, 17, 737–751. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, X.; Tian, E.; Lin, H. Direct Conversion of Cellulose into Ethyl Lactate in Supercritical Ethanol–Water Solutions. ChemSusChem 2016, 9, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, X.; Tian, E.; Vattipalli, V.; Fan, W.; Lin, H. Mechanistic insights into the production of methyl lactate by catalytic conversion of carbohydrates on mesoporous Zr-SBA-15. J. Catal. 2016, 333, 207–216. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Janssen, K.P.F.; Delaet, C.; Stroobants, C.; Houthoofd, K.; Philippaerts, A.; De Jonghe, C.; Paul, J.S.; Jacobs, P.A.; Sels, B.F. Zeolite-catalysed conversion of C3 sugars to alkyl lactates. Green Chem. 2010, 12, 1083–1089. [Google Scholar] [CrossRef]
- Nakajima, K.; Noma, R.; Kitano, M.; Hara, M. Titania as an Early Transition Metal Oxide with a High Density of Lewis Acid Sites Workable in Water. J. Phys. Chem. C 2013, 117, 16028–16033. [Google Scholar] [CrossRef]
| Catalyst | Surface Area 1/m2 g−1 | Crystal Phase of ZrO2 2 | Amount of Acid Sites 3/mmol g−1 | Amount of Base Sites 4/mmol g−1 | Yield of Lactic Acid 5/% |
|---|---|---|---|---|---|
| 5%ZrO2–Al2O3 | 135 | - | 0.109 | 0.093 | 25.0 |
| 10%ZrO2–Al2O3 | 126 | Tetragonal | 0.131 | 0.057 | 25.3 |
| 20%ZrO2–Al2O3 | 130 | Tetragonal | 0.180 | 0.098 | 21.9 |
| ZrO2 6 | 101 | Monoclinic | 0.118 | 0.485 | 21.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanapaphawong, P.; Sato, O.; Sato, K.; Mimura, N.; Reubroycharoen, P.; Yamaguchi, A. Conversion of Cellulose to Lactic Acid by Using ZrO2–Al2O3 Catalysts. Catalysts 2017, 7, 221. https://doi.org/10.3390/catal7070221
Wattanapaphawong P, Sato O, Sato K, Mimura N, Reubroycharoen P, Yamaguchi A. Conversion of Cellulose to Lactic Acid by Using ZrO2–Al2O3 Catalysts. Catalysts. 2017; 7(7):221. https://doi.org/10.3390/catal7070221
Chicago/Turabian StyleWattanapaphawong, Panya, Osamu Sato, Koichi Sato, Naoki Mimura, Prasert Reubroycharoen, and Aritomo Yamaguchi. 2017. "Conversion of Cellulose to Lactic Acid by Using ZrO2–Al2O3 Catalysts" Catalysts 7, no. 7: 221. https://doi.org/10.3390/catal7070221




