Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors
Abstract
:1. Introduction
2. Configurations of PMRs
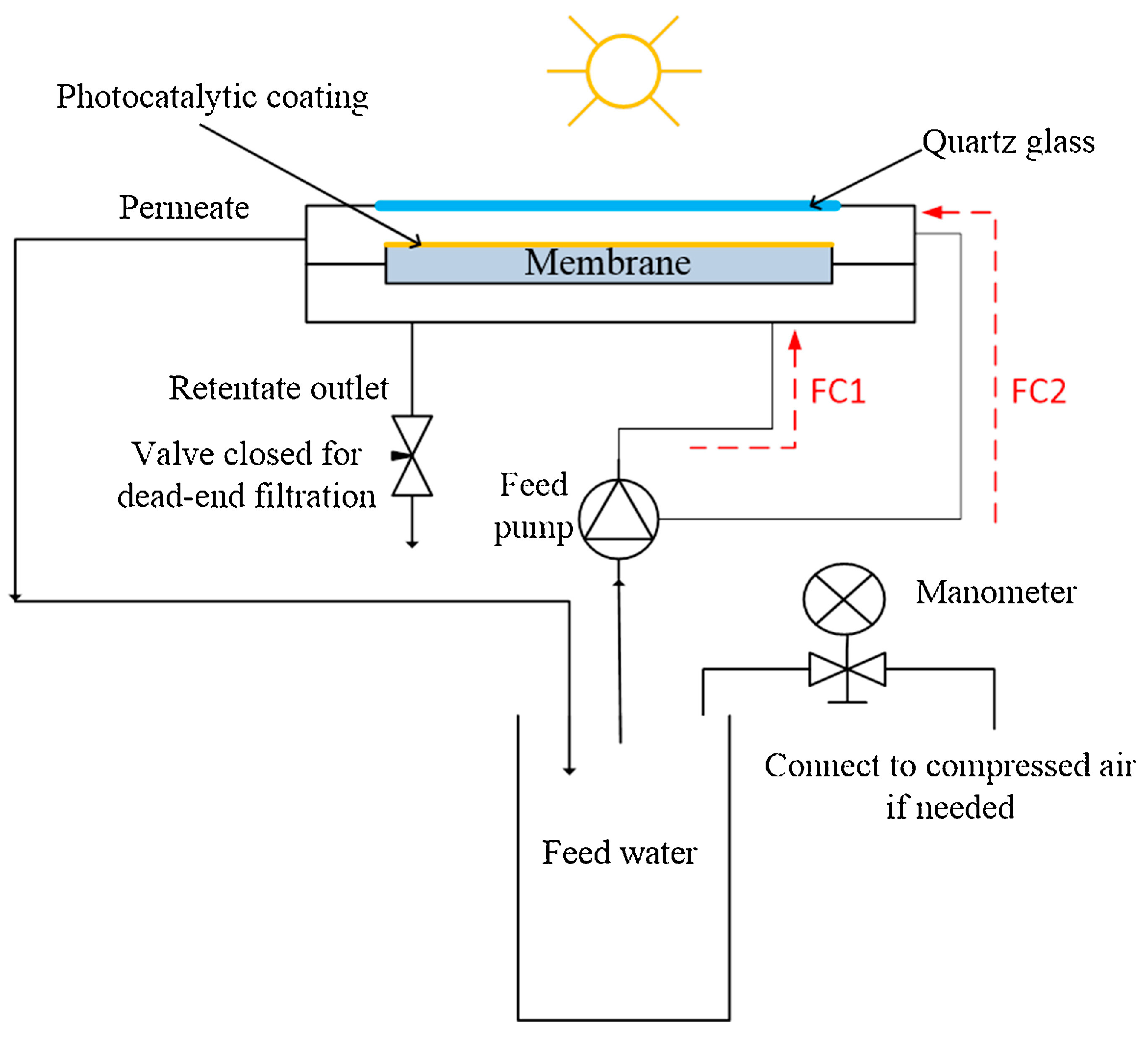
2.1. PMRs with Immobilized Photocatalyst
2.2. PMRs with Suspended Photocatalyst
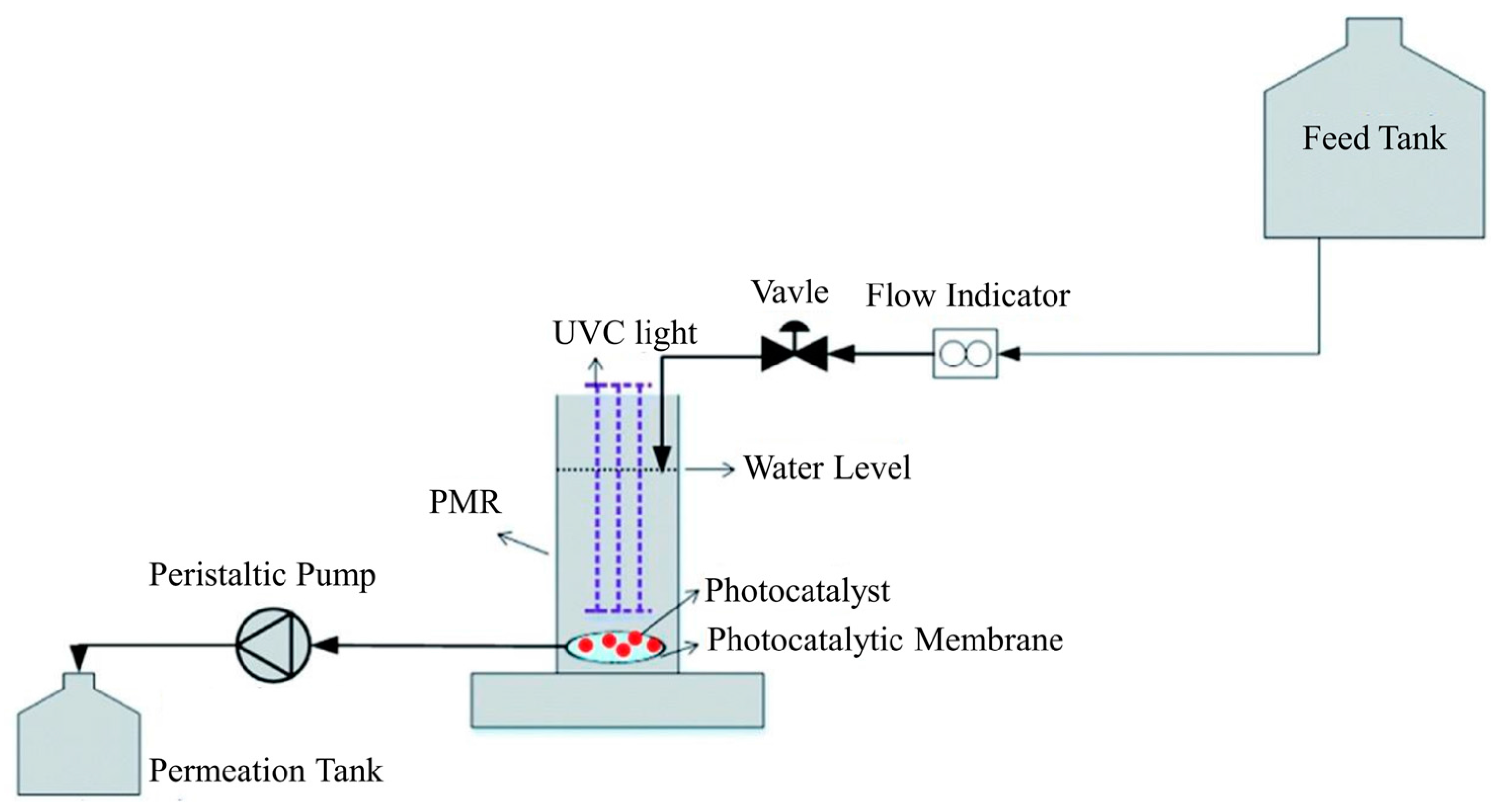
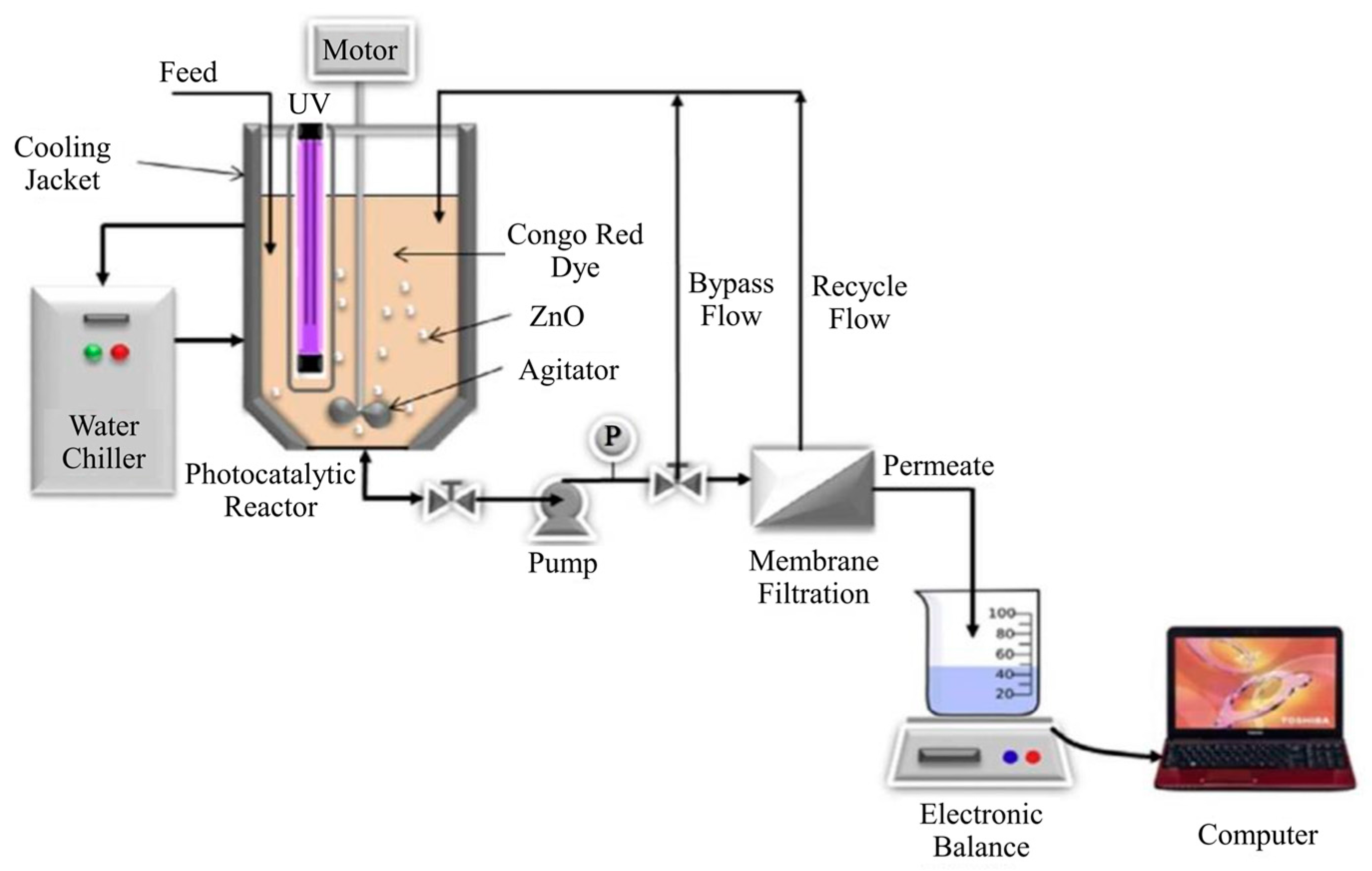
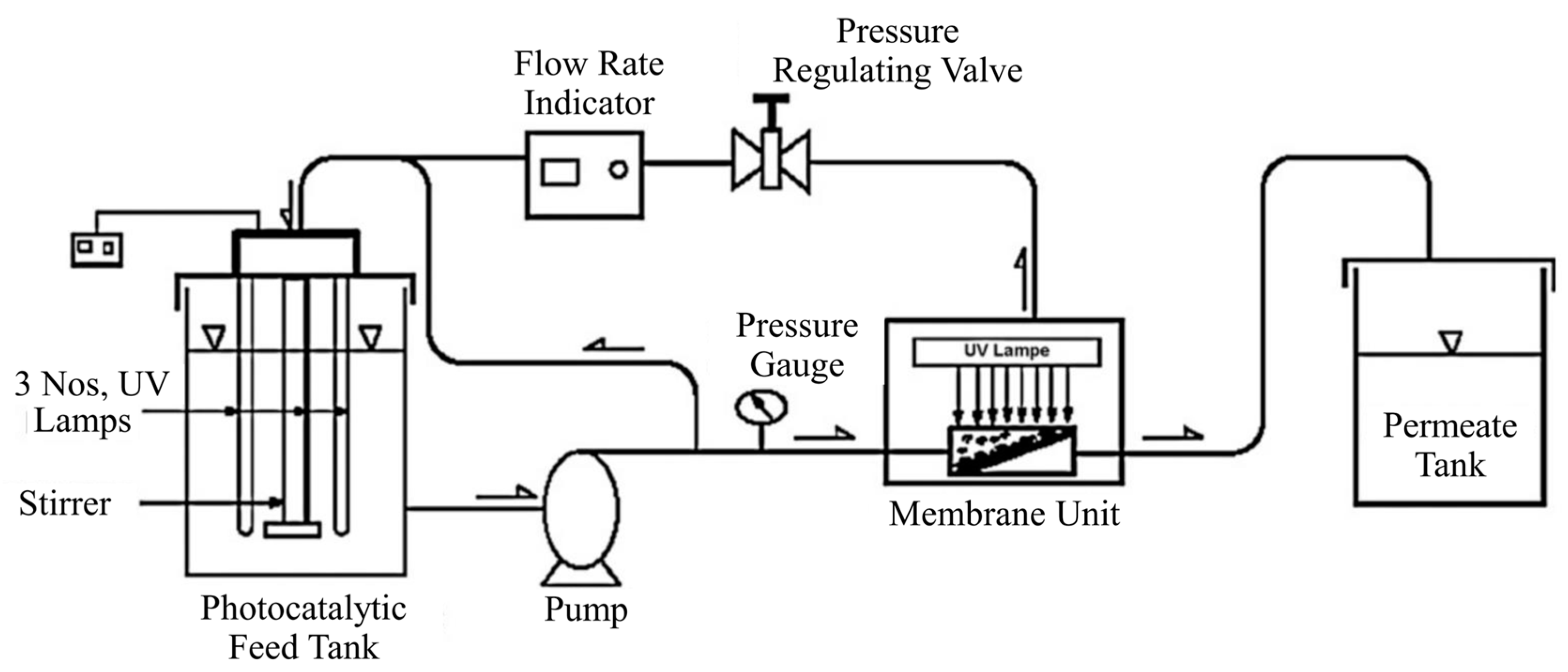
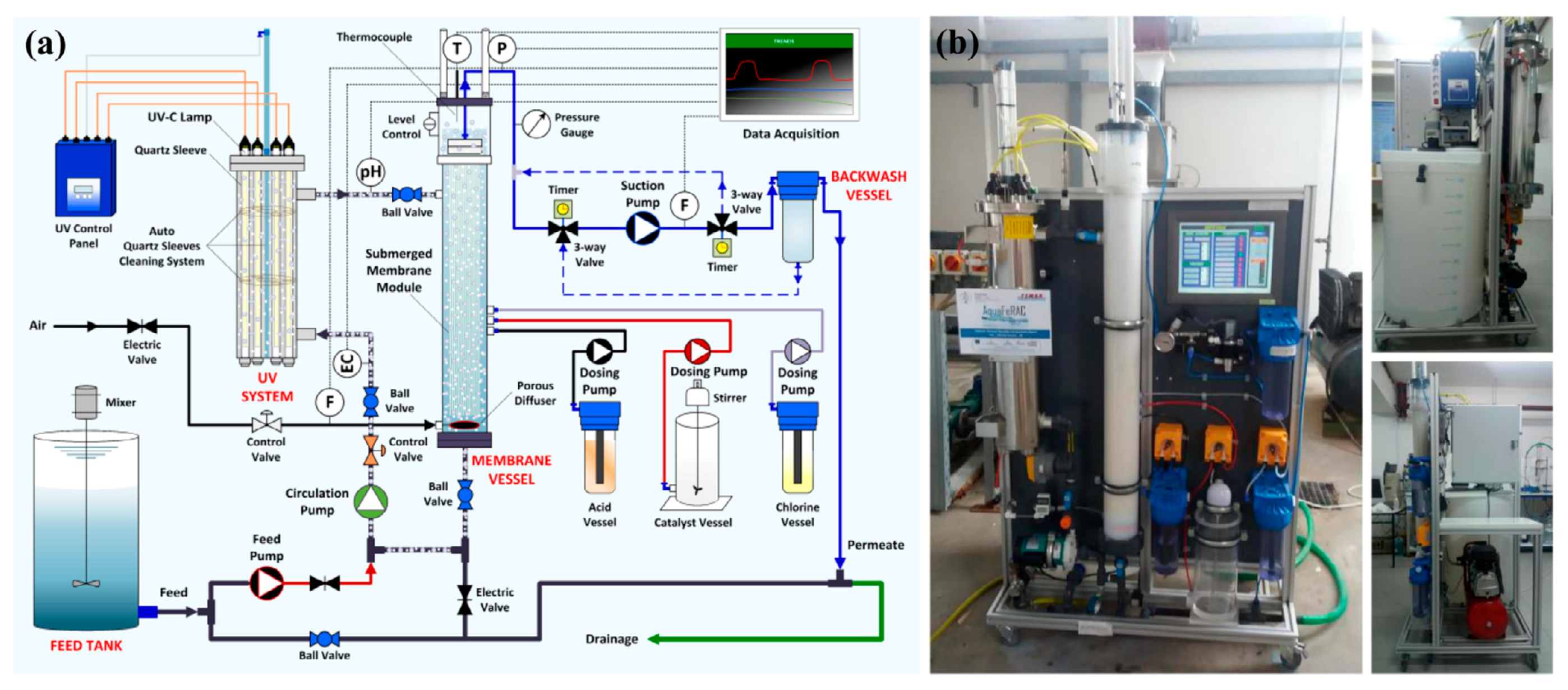
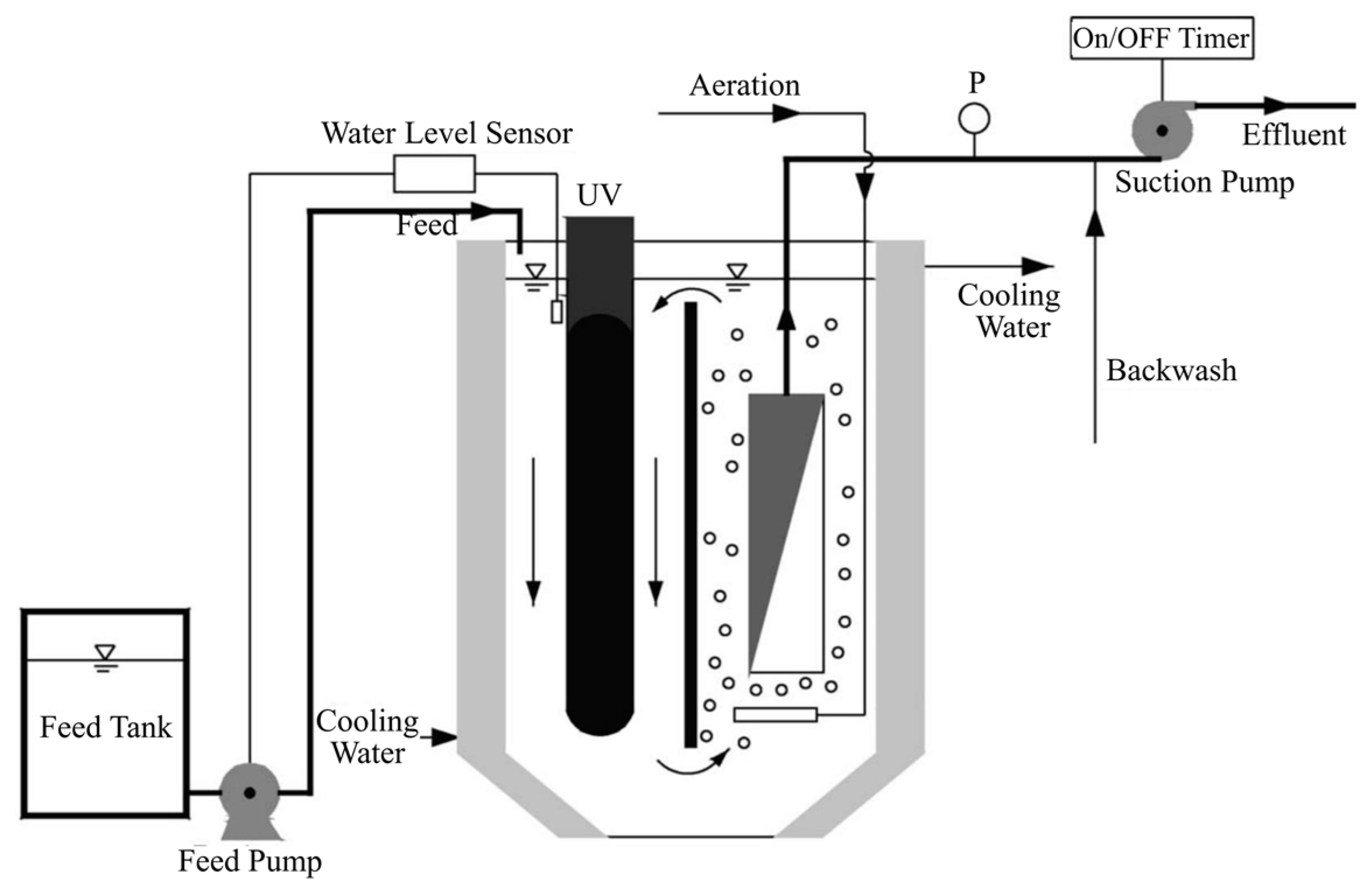
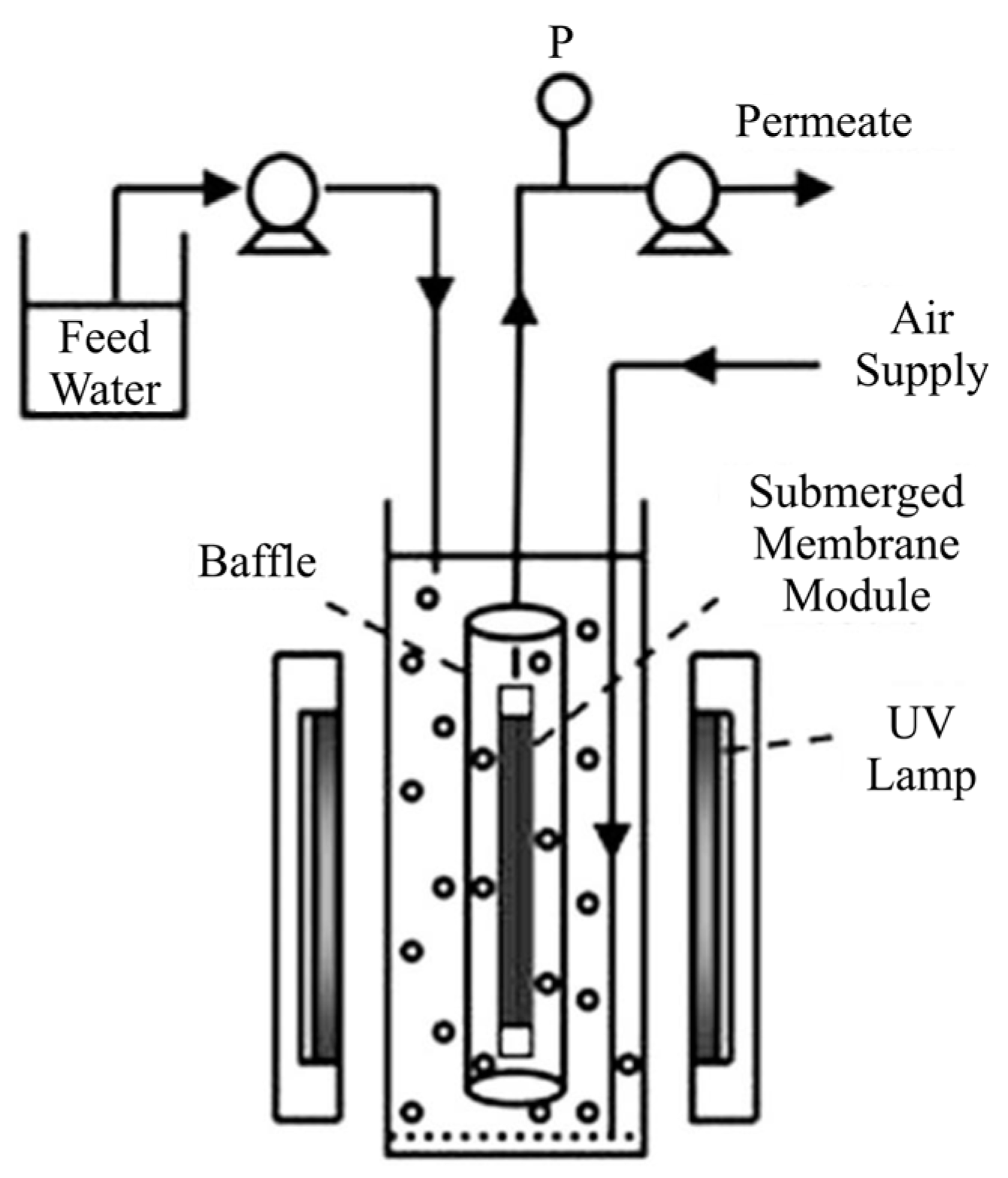
2.2.1. Split-Type PMRs with Suspended Photocatalyst
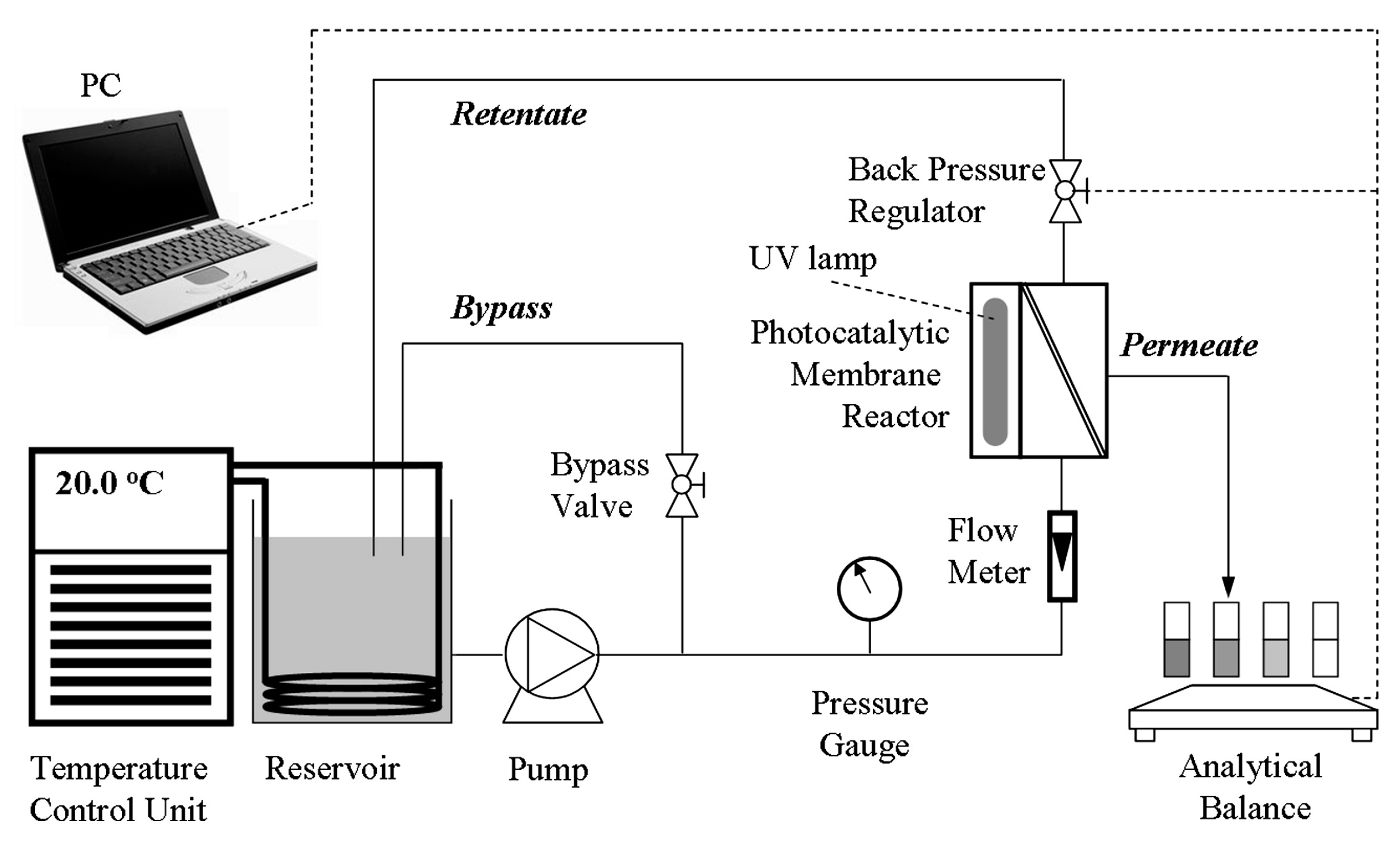
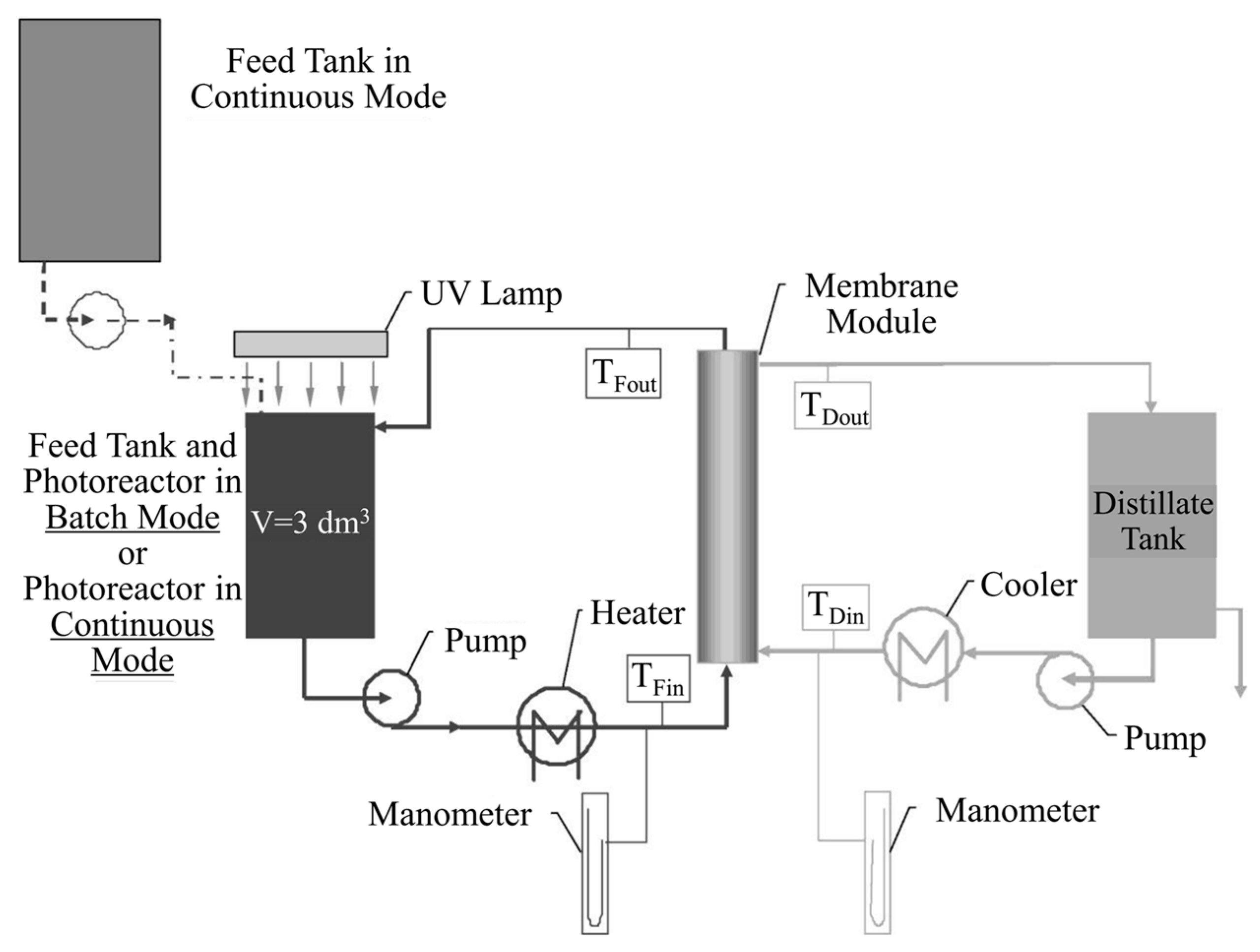
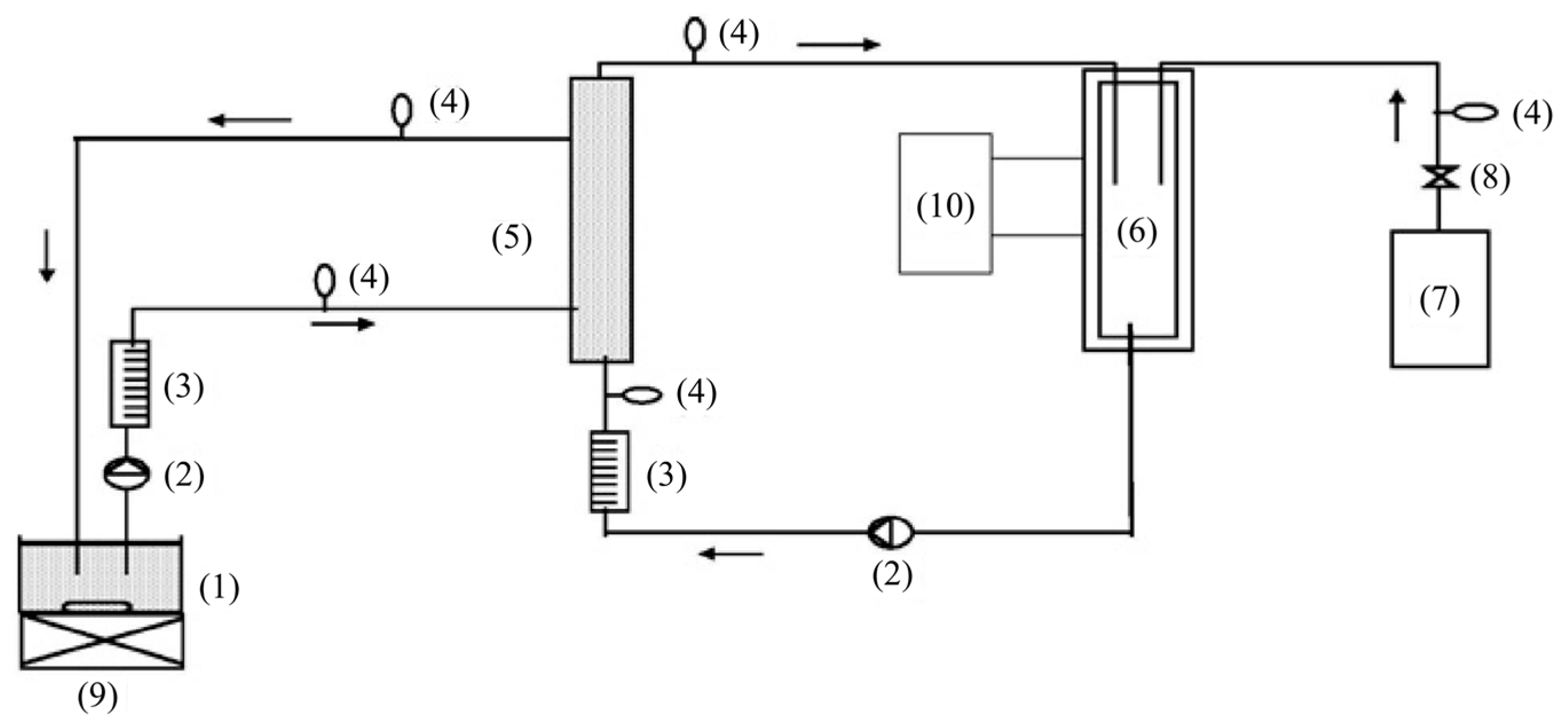
2.2.2. Integrative-Type PMRs with Suspended Photocatalyst
2.3. Novel PMR Configurations
2.3.1. Coupling Photocatalysis with Membrane Distillation
2.3.2. Coupling Photocatalysis with Dialysis
2.3.3. Coupling Photocatalysis with Pervaporation
2.4. Evaluation of Different PMR Configurations
3. Influencing Factors of PMR
3.1. Photocatalyst
3.1.1. Structures and Properties of Photocatalyst
3.1.2. Photocatalyst Loading
3.2. Light Source
3.2.1. Light Wavelength
3.2.2. Light Intensity
- (1)
- At low light intensity, reactions involving electron–hole formation are predominant while electron—hole recombination can be ignored, thus the reaction rate increases linearly with the increase of light intensity;
- (2)
- At middle light intensity, the electron—hole pair separation and recombination process compete with each other, resulting in relative lower reaction rate, which ultimately lies on the square root of the light intensity;
- (3)
- At high light intensity, the reaction rate is not affected by the light intensity.
3.3. Water Quality
3.3.1. Initial Pollutant Concentration
3.3.2. pH
3.3.3. Temperature
3.3.4. Inorganic Ions
3.4. Aeration
3.5. Membrane
3.5.1. Membrane Material
3.5.2. Membrane Pore Size
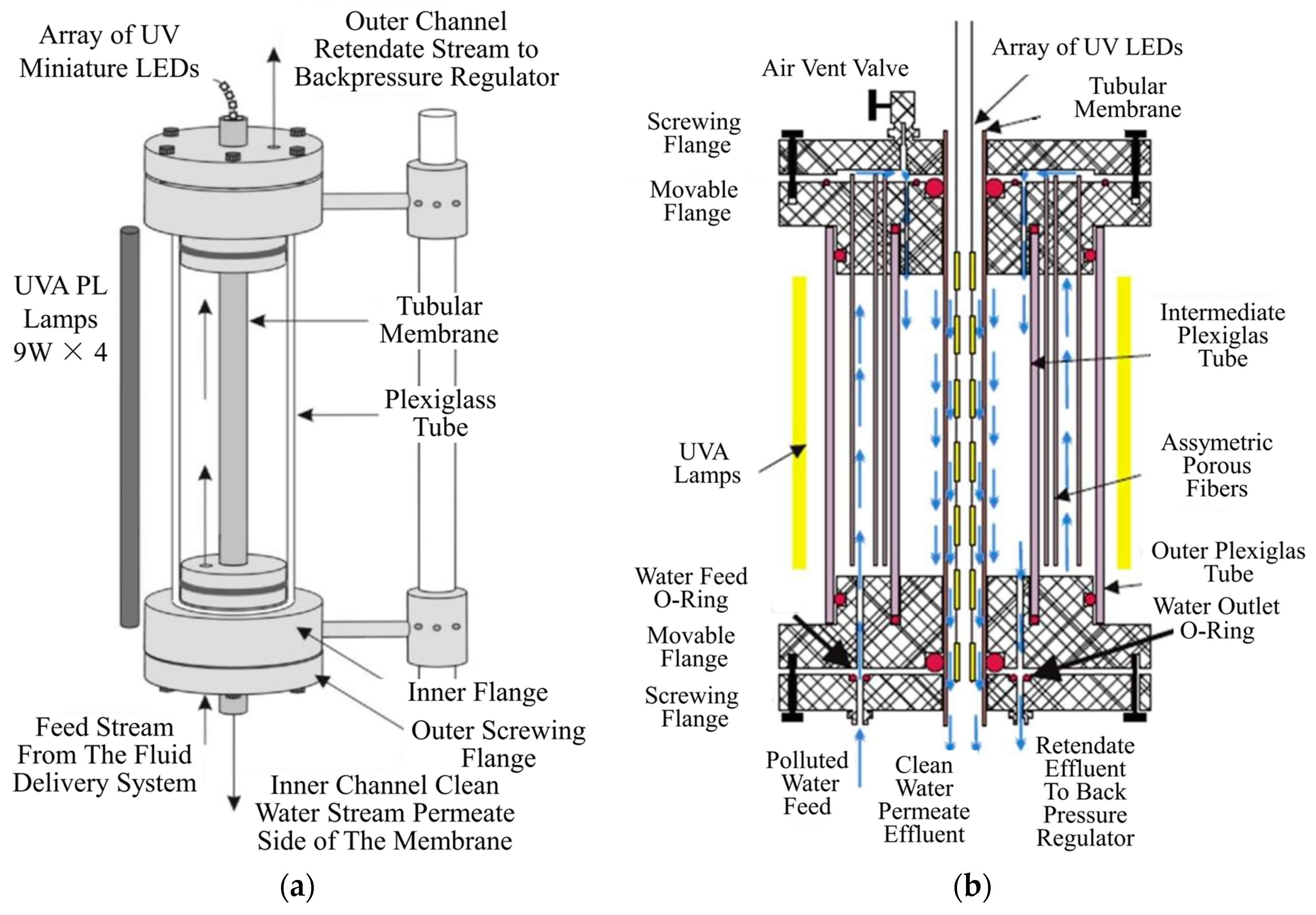
3.5.3. Membrane Configuration
3.5.4. Other Parameters
4. Future Challenges and Prospects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Khraisheh, M.; Wu, L.; Al-Muhtaseb, A.H.; Al-Ghouti, M.A. Photocatalytic disinfection of Escherichia coli using TiO2 P25 and Cu-doped TiO2. J. Ind. Eng. Chem. 2015, 28, 369–376. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’Shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, D.; Zhang, W.; Wang, Q.; Wang, Y.; Wang, P. Ag@helical chiral TiO2 nanofibers for visible light photocatalytic degradation of 17α-ethinylestradiol. Environ. Sci. Pollut. Res. 2015, 22, 10444–10451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N-doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Murakami, N.; Ono, A.; Nakamura, M.; Tsubota, T.; Ohno, T. Development of a visible-light-responsive rutile rod by site-selective modification of iron (III) ion on {111} exposed crystal faces. Appl. Catal. B Environ. 2010, 97, 115–119. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S.; Rao, N.N. Visible light assisted photodegradation of halocarbons on the dye modified TiO2 surface using visible light. Sol. Energy Mater. Sol. Cells 2006, 90, 1013–1020. [Google Scholar] [CrossRef]
- Ghows, N.; Entezari, M.H. Fast and easy synthesis of core—Shell nanocrystal (CdS/TiO2) at low temperature by micro-emulsion under ultrasound. Ultrason. Sonochem. 2011, 18, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, C.; Peng, T.; Zhou, X.; Qu, J. Plasmon-induced inactivation of enteric pathogenic microorganisms with Ag–AgI/Al2O3 under visible-light irradiation. Environ. Sci. Technol. 2010, 44, 7058–7062. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Ong, W.; Tan, L.; Ng, Y.H.; Yong, S.; Chai, S. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. 2016, 23, 11549–11567. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Ali, I. (Eds.) Water treatment by membrane filtration techniques. In Environmental Water, 1st ed.; Elsevier B.V.: Oxford, UK, 2013; Volume 5, pp. 135–154. [Google Scholar]
- Jean Christophe, S.; Bengu, B.S. Current and emerging membrane processes for water treatment. In Membrane Technology: Membranes for Water Treatment, 1st ed.; Peinemann, K.V., Nunes, S.P., Eds.; Wiley: Weinheim, Germany, 2010; Volume 4, pp. 53–91. [Google Scholar]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Sabate, J.; Anderson, M.A.; Aguado, M.A.; Giménez, J.; Cervera-March, S.; Hill, C.G. Comparison of TiO2 powder suspensions and TiO2 ceramic membranes supported on glass as photocatalytic systems in the reduction of chromium (VI). J. Mol. Catal. 1992, 71, 57–68. [Google Scholar] [CrossRef]
- Chester, G.; Anderson, M.; Read, H.; Esplugas, S. A jacketed annular membrane photocatalytic reactor for wastewater treatment: Degradation of formic acid and atrazine. J. Photochem. Photobio. A Chem. 1993, 71, 291–297. [Google Scholar] [CrossRef]
- Bellobono, I.R.; Bonardi, M.; Castellano, L.; Selli, E.; Righetto, L. Degradation of some chloro-aliphatic water contaminants by photocatalytic membranes immobilizing titanium dioxide. J. Photochem. Photobiol. A Chem. 1992, 67, 109–115. [Google Scholar] [CrossRef]
- Molinari, R.; Marino, T.; Argurio, P. Photocatalytic membrane reactors for hydrogen production from water. Int. J. Hydrog. Energy 2014, 39, 7247–7261. [Google Scholar] [CrossRef]
- Mascolo, G.; Comparelli, R.; Curri, M.L.; Lovecchio, G.; Lopez, A.; Agostiano, A. Photocatalytic degradation of methyl red by TiO2: Comparison of the efficiency of immobilized nanoparticles versus conventional suspended catalyst. J. Hazard. Mater. 2007, 142, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Palmisano, L.; Drioli, E.; Schiavello, M. Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification. J. Membr. Sci. 2002, 206, 399–415. [Google Scholar] [CrossRef]
- Dijkstra, M.; Buwalda, H.; De Jong, A.; Michorius, A.; Winkelman, J.; Beenackers, A. Experimental comparison of three reactor designs for photocatalytic water purification. Chem. Eng. Sci. 2001, 56, 547–555. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W.; Molinari, R.; Palmisano, L.; Loddo, V. Photocatalytic membrane reactors: Fundamentals, membrane materials and operational issues A2–Basile, Angelo. Handb. Membr. React. 2013, 2, 236–295. [Google Scholar]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Molinari, R.; Palmisano, L.; Loddo, V.; Mozia, S.; Morawski, A.W. Photocatalytic membrane reactors: Configurations, performance and applications in water treatment and chemical production A2–Basile, Angelo. Handb. Membr. React. 2013, 2, 808–845. [Google Scholar]
- Méricq, J.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Moslehyani, A.; Ismail, A.F.; Othman, M.; Matsuura, T. Hydrocarbon degradation and separation of bilge water via a novel TiO2-HNTs/PVDF-based photocatalytic membrane reactor (PMR). RSC Adv. 2015, 5, 14147–14155. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405, 48–56. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Jin, P.; Tang, H.; Wang, H.; Chen, Y. Highly hydrophilic poly (vinylidene fluoride)/meso-titania hybrid mesoporous membrane for photocatalytic membrane reactor in water. Sci. Rep. UK 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Improved hydrodynamic permeability and antifouling properties of poly (vinylidene fluoride) membranes using polydopamine nanoparticles as additives. J. Membr. Sci. 2014, 457, 73–81. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Morphological study of co-extruded dual-layer hollow fiber membranes incorporated with different TiO2 loadings. J. Membr. Sci. 2015, 479, 123–131. [Google Scholar] [CrossRef]
- Li, J.; Yan, B.; Shao, X.; Wang, S.; Tian, H.; Zhang, Q. Influence of Ag/TiO2 nanoparticle on the surface hydrophilicity and visible-light response activity of polyvinylidene fluoride membrane. Appl. Surf. Sci. 2015, 324, 82–89. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Yu, Z.; Zhan, Y.; Ma, L.; Zhang, L. Preparation and characterization of a novel PVDF ultrafiltration membrane by blending with TiO2-HNTs nanocomposites. Appl. Surf. Sci. 2016, 371, 624–632. [Google Scholar] [CrossRef]
- Alberti, A.; Bongiorno, C.; Pellegrino, G.; Sanzaro, S.; Smecca, E.; Condorelli, G.G.; Giuffrida, A.E.; Cicala, G.; Latteri, A.; Ognibene, G. Low temperature sputtered TiO2 nano sheaths on electrospun PES fibers as high porosity photoactive material. RSC Adv. 2015, 5, 73444–73450. [Google Scholar] [CrossRef]
- Fischer, K.; Kühnert, M.; Gläser, R.; Schulze, A. Photocatalytic degradation and toxicity evaluation of diclofenac by nanotubular titanium dioxide–PES membrane in a static and continuous setup. RSC Adv. 2015, 5, 16340–16348. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Nackaerts, R.; Thwala, J.M.; Mamba, B.B.; Verliefde, A. Hydrophilic fouling-resistant GO-ZnO/PES membranes for wastewater reclamation. J. Membr. Sci. 2017, 524, 43–55. [Google Scholar] [CrossRef]
- Kleine, J.; Peinemann, K.; Schuster, C.; Warnecke, H. Multifunctional system for treatment of wastewaters from adhesive-producing industries: Separation of solids and oxidation of dissolved pollutants using doted microfiltration membranes. Chem. Eng. Sci. 2002, 57, 1661–1664. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Liu, H.; Wang, K.; Ling, L.; Zhang, K. Robust electrospinning cellulose acetate@TiO2 ultrafine fibers for dyeing water treatment by photocatalytic reactions. RSC Adv. 2015, 5, 40521–40530. [Google Scholar] [CrossRef]
- Shet, A.; Vidya, S.K. Solar light mediated photocatalytic degradation of phenol using Ag core–TiO2 shell (Ag@TiO2) nanoparticles in batch and fluidized bed reactor. Sol. Energy 2016, 127, 67–78. [Google Scholar] [CrossRef]
- Benhabiles, O.; Mahmoudi, H.; Lounici, H.; Goosen, M.F. Effectiveness of a photocatalytic organic membrane for solar degradation of methylene blue pollutant. Desalin. Water Treat. 2016, 57, 14067–14076. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Zulhairun, A.K.; Subramaniam, M.N.; Lau, W.J.; Ismail, A.F. Preparation of polysulfone-based PANI–TiO2 nanocomposite hollow fiber membranes for industrial dye rejection applications. RSC Adv. 2016, 6, 99764–99773. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Bhat, U.K.; Ismail, A.F.; Obaid, A.; Fun, H. Preparation and performance studies of polysulfone-sulfated nano-titania (S-TiO2) nanofiltration membranes for dye removal. RSC Adv. 2015, 5, 53874–53885. [Google Scholar] [CrossRef]
- Wang, X.; Shi, F.; Huang, W.; Fan, C. Synthesis of high quality TiO2 membranes on alumina supports and their photocatalytic activity. Thin Solid Films 2012, 520, 2488–2492. [Google Scholar] [CrossRef]
- Daels, N.; Radoicic, M.; Radetic, M.; Van Hulle, S.W.; De Clerck, K. Functionalisation of electrospun polymer nanofibre membranes with TiO2 nanoparticles in view of dissolved organic matter photodegradation. Sep. Purif. Technol. 2014, 133, 282–290. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B Environ. 2014, 160, 456–464. [Google Scholar] [CrossRef]
- Della Foglia, F.; Chiarello, G.L.; Dozzi, M.V.; Piseri, P.; Bettini, L.G.; Vinati, S.; Ducati, C.; Milani, P.; Selli, E. Hydrogen production by photocatalytic membranes fabricated by supersonic cluster beam deposition on glass fiber filters. Int. J. Hydrog. Energy 2014, 39, 13098–13104. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Liu, L.; Liu, Z.; Sun, D.D. Hierarchical 3D dendritic TiO2 nanospheres building with ultralong 1D nanoribbon/wires for high performance concurrent photocatalytic membrane water purification. Water Res. 2013, 47, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shao, J.; Wang, J.; Zhong, X. The removal of natural organic matter with LiCl-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, N.G.; Katsaros, F.K.; Kontos, A.G.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid ultrafiltration/photocatalytic membranes for efficient water treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Mohammad, A.W.; Kadhum, A.A.H. Effect of various zinc oxide nanoparticles in membrane photocatalytic reactor for Congo red dye treatment. Sep. Purif. Technol. 2014, 137, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Mozia, S.; Darowna, D.; Wróbel, R.; Morawski, A.W. A study on the stability of polyethersulfone ultrafiltration membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2015, 495, 176–186. [Google Scholar] [CrossRef]
- Choo, K.; Chang, D.; Park, K.; Kim, M. Use of an integrated photocatalysis/hollow fiber microfiltration system for the removal of trichloroethylene in water. J. Hazard. Mater. 2008, 152, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Phuntsho, S.; Vigneswaran, S. Effect of photocatalysis on the membrane hybrid system for wastewater treatment. Desalination 2008, 225, 235–248. [Google Scholar] [CrossRef]
- Sarasidis, V.C.; Plakas, K.V.; Patsios, S.I.; Karabelas, A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014, 239, 299–311. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Benotti, M.J.; Stanford, B.D.; Wert, E.C.; Snyder, S.A. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res. 2009, 43, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Garcia-Lopez, E.; Loddo, V.; Malato-Rodriguez, S.; Maldonado, I.; Marcì, G.; Molinari, R.; Palmisano, L. Degradation of lincomycin in aqueous medium: Coupling of solar photocatalysis and membrane separation. Sol. Energy 2005, 79, 402–408. [Google Scholar] [CrossRef]
- Fu, J.; Ji, M.; Wang, Z.; Jin, L.; An, D. A new submerged membrane photocatalysis reactor (SMPR) for fulvic acid removal using a nano-structured photocatalyst. J. Hazard. Mater. 2006, 131, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Q.; Chen, L.; Wang, J.; Cheng, R. Photocatalytic membrane reactor (PMR) for virus removal in water: Performance and mechanisms. Chem. Eng. J. 2015, 277, 124–129. [Google Scholar] [CrossRef]
- Huang, X.; Meng, Y.; Liang, P.; Qian, Y. Operational conditions of a membrane filtration reactor coupled with photocatalytic oxidation. Sep. Purif. Technol. 2007, 55, 165–172. [Google Scholar] [CrossRef]
- Jiang, L.; Choo, K. Photocatalytic mineralization of secondary effluent organic matter with mitigating fouling propensity in a submerged membrane photoreactor. Chem. Eng. J. 2016, 288, 798–805. [Google Scholar] [CrossRef]
- Fernández, R.L.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (MPR). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar] [CrossRef]
- Wang, P.; Fane, A.G.; Lim, T. Evaluation of a submerged membrane vis-LED photoreactor (sMPR) for carbamazepine degradation and TiO2 separation. Chem. Eng. J. 2013, 215, 240–251. [Google Scholar] [CrossRef]
- Chin, S.S.; Lim, T.M.; Chiang, K.; Fane, A.G. Hybrid low-pressure submerged membrane photoreactor for the removal of bisphenol A. Desalination 2007, 202, 253–261. [Google Scholar] [CrossRef]
- Deveci, E.Ü.; Dizge, N.; Yatmaz, H.C.; Aytepe, Y. Integrated process of fungal membrane bioreactor and photocatalytic membrane reactor for the treatment of industrial textile wastewater. Biochem. Eng. J. 2016, 105, 420–427. [Google Scholar] [CrossRef]
- Doruk, N.; Yatmaz, H.C.; Dizge, N. Degradation efficiency of textile and wood processing industry wastewater by photocatalytic process using in situ ultrafiltration membrane. CLEAN Soil Air Water 2016, 44, 224–231. [Google Scholar] [CrossRef]
- Darowna, D.; Grondzewska, S.; Morawski, A.W.; Mozia, S. Removal of non-steroidal anti-inflammatory drugs from primary and secondary effluents in a photocatalytic membrane reactor. J. Chem. Technol. Biot. 2014, 89, 1265–1273. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Xie, Z.; Wang, X.; Li, H.; Hoang, M.; Caruso, R.A. Methyl orange removal by combined visible-light photocatalysis and membrane distillation. Dyes Pigment. 2013, 98, 106–112. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W. The performance of a hybrid photocatalysis–MD system for the treatment of tap water contaminated with ibuprofen. Catal. Today 2012, 193, 213–220. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. Photocatalytic membrane reactor (PMR) coupling photocatalysis and membrane distillation—Effectiveness of removal of three azo dyes from water. Catal. Today 2007, 129, 3–8. [Google Scholar] [CrossRef]
- Mozia, S.; Toyoda, M.; Tsumura, T.; Inagaki, M.; Morawski, A.W. Comparison of effectiveness of methylene blue decomposition using pristine and carbon-coated TiO2 in a photocatalytic membrane reactor. Desalination 2007, 212, 141–151. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. A new photocatalytic membrane reactor (PMR) for removal of azo-dye Acid Red 18 from water. Appl. Catal. B Environ. 2005, 59, 131–137. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W.; Toyoda, M.; Tsumura, T. Effect of process parameters on photodegradation of Acid Yellow 36 in a hybrid photocatalysis—Membrane distillation system. Chem. Eng. J. 2009, 150, 152–159. [Google Scholar] [CrossRef]
- Azrague, K.; Aimar, P.; Benoit-Marquie, F.; Maurette, M.T. A new combination of a membrane and a photocatalytic reactor for the depollution of turbid water. Appl. Catal. B Environ. 2007, 72, 197–204. [Google Scholar] [CrossRef]
- Camera Roda, G.; Santarelli, F. Design of a pervaporation photocatalytic reactor for process intensification. Chem. Eng. Technol. 2012, 35, 1221–1228. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Santarelli, F. Intensification of water detoxification by integrating photocatalysis and pervaporation. J. Sol. Energy Eng. 2007, 129, 68–73. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F.; Choo, C.M. The impacts of various operating conditions on submerged membrane photocatalytic reactors (SMPR) for organic pollutant separation and degradation: A review. RSC Adv. 2015, 5, 97335–97348. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2-graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Shafaei, N.; Jahanshahi, M.; Peyravi, M.; Najafpour, Q. Self-cleaning behavior of nanocomposite membrane induced by photocatalytic WO3 nanoparticles for landfill leachate treatment. Korean J. Chem. Eng. 2016, 33, 2968–2981. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, H.; Sun, D.D. Hierarchical CuO/ZnO membranes for environmental applications under the irradiation of visible light. Int. J. Photoenergy 2011, 2012. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Li, Z.; An, X.; Zhou, L.; Zhang, Y.; Shiang, F.Q. Progress of applied research on TiO2 photocatalysis-membrane separation coupling technology in water and wastewater treatments. Chin. Sci. Bull. 2010, 55, 1345–1353. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; Mesones, S.; Casado, C.; van Grieken, R. Bacterial inactivation and degradation of organic molecules by titanium dioxide supported on porous stainless steel photocatalytic membranes. Chem. Eng. J. 2017, 318, 29–38. [Google Scholar] [CrossRef]
- Wang, W.; Irawan, A.; Ku, Y. Photocatalytic degradation of Acid Red 4 using a titanium dioxide membrane supported on a porous ceramic tube. Water Res. 2008, 42, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
- Szymański, K.; Morawski, A.W.; Mozia, S. Humic acids removal in a photocatalytic membrane reactor with a ceramic UF membrane. Chem. Eng. J. 2016, 305, 19–27. [Google Scholar] [CrossRef]
- Kertèsz, S.; Cakl, J.; Jiránková, H. Submerged hollow fiber microfiltration as a part of hybrid photocatalytic process for dye wastewater treatment. Desalination 2014, 343, 106–112. [Google Scholar] [CrossRef]
- Halim, R.; Utama, R.; Cox, S.; Le-Clech, P. Performances of submerged membrane photocatalysis reactor during treatment of humic substances. Membr. Water Treat. 2010, 1, 283–296. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Paz, Y. Preferential photodegradation—Why and how? C. R. Chim. 2006, 9, 774–787. [Google Scholar] [CrossRef]
- Damodar, R.; You, S.; Chiou, G. Investigation on the conditions mitigating membrane fouling caused by TiO2 deposition in a membrane photocatalytic reactor (MPR) used for dye wastewater treatment. J. Hazard. Mater. 2012, 203, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Influence of solution pH on the performance of photocatalytic membranes during dead-end filtration. Sep. Purif. Technol. 2013, 118, 406–414. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2-ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Hu, C.; Jimmy, C.Y.; Hao, Z.; Wong, P.K. Effects of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl. Catal. B Environ. 2003, 46, 35–47. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Su, Y.; Mao, K.; Wang, Q. Effect of water composition on TiO2 photocatalytic removal of endocrine disrupting compounds (EDCs) and estrogenic activity from secondary effluent. J. Hazard. Mater. 2012, 215, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Malato, S.; Weber, J. Solar photocatalytic degradation of humic acids as a model of organic compounds of landfill leachate in pilot-plant experiments: Influence of inorganic salts. Appl. Catal. B Environ. 2004, 53, 127–137. [Google Scholar] [CrossRef]
- Lan, Y.; Hu, C.; Hu, X.; Qu, J. Efficient destruction of pathogenic bacteria with AgBr/TiO2 under visible light irradiation. Appl. Catal. B Environ. 2007, 73, 354–360. [Google Scholar] [CrossRef]
- Ng, T.W.; Zhang, L.; Liu, J.; Huang, G.; Wang, W.; Wong, P.K. Visible-light-driven photocatalytic inactivation of Escherichia coli by magnetic Fe2O3-AgBr. Water Res. 2016, 90, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Darowna, D.; Wróbel, R.; Morawski, A.W.; Mozia, S. The influence of feed composition on fouling and stability of a polyethersulfone ultrafiltration membrane in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 310, 360–367. [Google Scholar] [CrossRef]
- Chin, S.S.; Lim, T.M.; Chiang, K.; Fane, A.G. Factors affecting the performance of a low-pressure submerged membrane photocatalytic reactor. Chem. Eng. J. 2007, 130, 53–63. [Google Scholar] [CrossRef]
- Du, X.; Qu, F.; Liang, H.; Li, K.; Bai, L.; Li, G. Control of submerged hollow fiber membrane fouling caused by fine particles in photocatalytic membrane reactors using bubbly flow: Shear stress and particle forces analysis. Sep. Purif. Technol. 2017, 172, 130–139. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T. Design and fabrication of hollow fiber membrane modules. J. Membr. Sci. 2017, 538, 96–107. [Google Scholar] [CrossRef]
- Xu, J.; Ruan, G.; Gao, X.; Pan, X.; Su, B.; Gao, C. Pilot study of inside-out and outside-in hollow fiber UF modules as direct pretreatment of seawater at low temperature for reverse osmosis. Desalination 2008, 219, 179–189. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.; Ou, S. Coupling of membrane separation with photocatalytic slurry reactor for advanced dye wastewater treatment. Sep. Purif. Technol. 2010, 76, 64–71. [Google Scholar] [CrossRef]


| Coating Method | Photocatalyst | Membrane | Characteristics | Ref. |
|---|---|---|---|---|
| Dip-coating | TiO2 nanoparticles | α-Al2O3 | TiO2-Al2O3 membrane was synthesized by dipping the α-Al2O3 disk into TiO2 sol. | [53] |
| Electrospraying TiO2 particles | TiO2 nanoparticles | Polyamide-6 nanofiber membrane | A colloid of TiO2 nanoparticles was added into the polyamide-6 solution before the electrospinning process. | [54] |
| Magnetron sputtering | TiO2 nanotubes | Polyethersulfone membrane | A titanium film was magnetron sputtered onto polyethersulfone membrane, and then anodized into TiO2 nanotubes. Subsequent crystallization of TiO2 to anatase structures was conducted at low temperatures. Enhanced photocatalytic performance was achieved by combining nanotubes with porous membrane. | [55] |
| Deposition of gas phase photocatalyst nanoparticles | TiO2 and Pt/TiO2 nano thin films | Glass fiber filters | TiO2 and Pt/TiO2 nanoparticles were prepared through flame spray pyrolysis, followed by expansion in a supersonic beam for the deposition on the glassfiber filters. | [56] |
| PMR with Immobilized Photocatalyst | PMR with Suspended Photocatalyst | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Configuration | Photocatalyst (Membrane) | Contaminant | Light Source | Tested Loading | Optimum Loading | Ref. |
|---|---|---|---|---|---|---|
| Immobilized | TiO2-porous stainless steel membrane | Methanol | UVA N/A | 0–34.0 g/m2 | 8.5 g/m2 | [98] |
| Immobilized | TiO2-ceramic membrane | Acid Red 4 | UVA 4 mW/cm2 | 0.03, 0.13, 0.29 and 0.44 g | 0.29 g | [99] |
| Immobilized | TiO2-PVDF membrane | Oily wastewater | UVA 0.333 mW/cm2 | 0–4 wt % | 2 wt % | [39] |
| Suspended | TiO2-P25 | Diclofenac | UVA 14.4 mW/cm2 | 0.3, 0.5 and 0.75 g/L | 0.5 g/L | [69] |
| Suspended | TiO2-P25 | Humic acids | UVC 0.154 mW/cm2 | 0.5, 1.0, 1.5 and 2.0 g/L | 1.5 g/L | [100] |
| Suspended | TiO2 | Acid Red 1 | UVC 62.91 mW/cm2 | 0–2 g/L | 0.5 g/L | [101] |
| Configuration | Photocatalyst (Membrane) | Contaminant | Tested pH Value | Optimum pH Value | Ref. |
|---|---|---|---|---|---|
| Suspended | TiO2-P25 | Diclofenac | 4.1, 6.2 and 7.5 | 6.2 | [69] |
| Suspended | TiO2-P25 | Humic acid | 3.0, 6.5 and 9.0 | 3.0 | [100] |
| Suspended | TiO2 | Fulvic acid | 3.4, 6.5, 8.2 and 10.3 | 3.4 | [73] |
| Immobilized | TiO2/Al2O3 membranes | Acid Orange 7 | 4.0, 6.0 and 8.0 | 4.0 | [106] |
| Suspended | TiO2-ZrO2 | Humic acid | 4.0, 7.0 and 10.0 | 4.0 | [107] |
| Material | Configuration | Stability | Cost | Fouling | Surface Area |
|---|---|---|---|---|---|
| polymer | inside-out hollow fiber | normal | low | severe | large |
| polymer | outside-in hollow fiber | normal | low | normal | large |
| polymer | flat sheet | normal | low | normal | normal |
| ceramic | tubular | excellent | high | normal | normal |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. https://doi.org/10.3390/catal7080224
Zheng X, Shen Z-P, Shi L, Cheng R, Yuan D-H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts. 2017; 7(8):224. https://doi.org/10.3390/catal7080224
Chicago/Turabian StyleZheng, Xiang, Zhi-Peng Shen, Lei Shi, Rong Cheng, and Dong-Hai Yuan. 2017. "Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors" Catalysts 7, no. 8: 224. https://doi.org/10.3390/catal7080224





