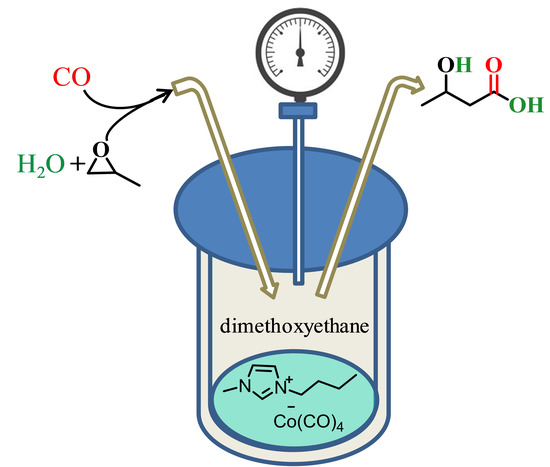
Direct Conversion of Propylene Oxide to 3-Hydroxy Butyric Acid Using a Cobalt Carbonyl Ionic Liquid Catalyst
Abstract
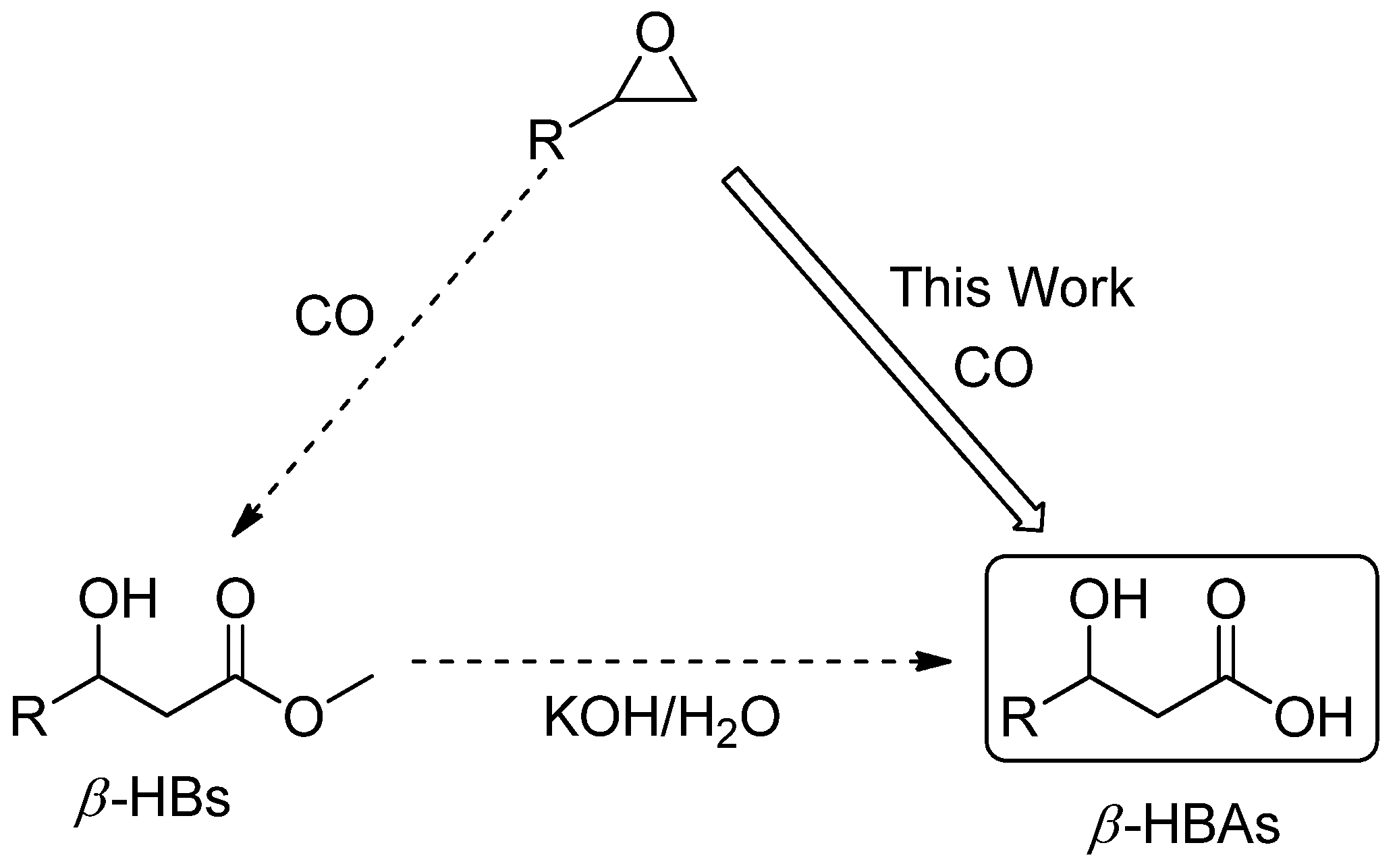
:1. Introduction
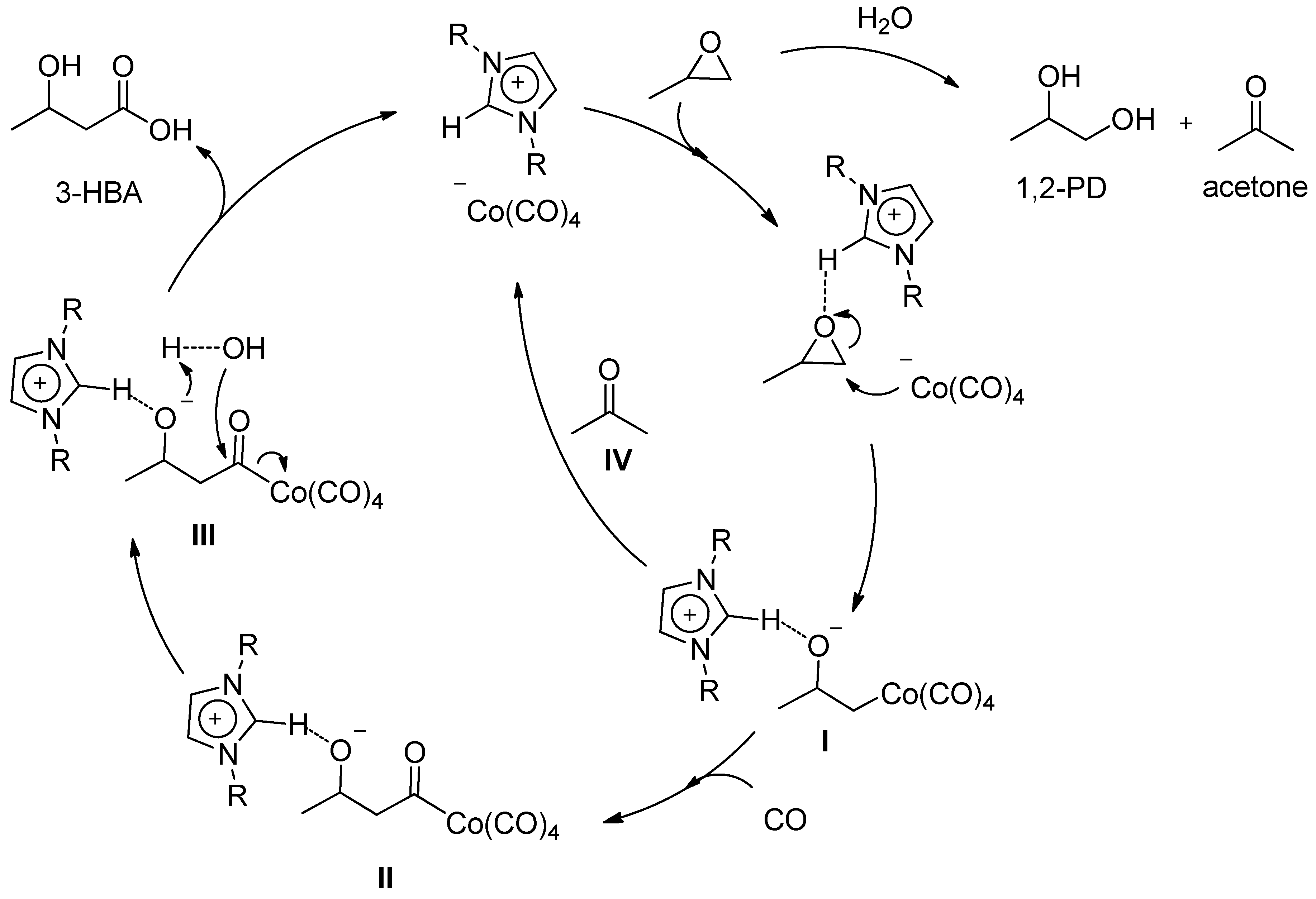
2. Results and Discussion
3. Experimental Section
3.1. General Considerations and Physical Measurements
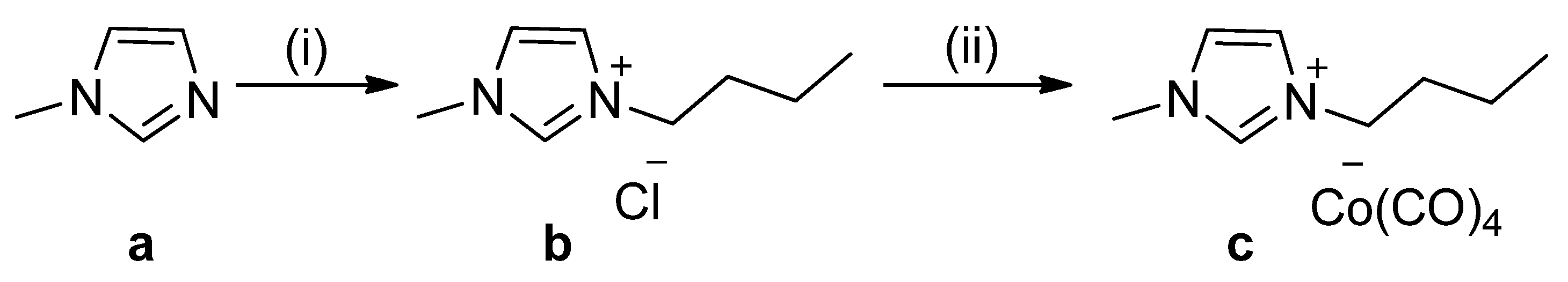
3.2. Synthesis of 1-Butyl-3-methylimidazolium Cobalt TetraCarbonyl [Bmim][Co(CO)4] [14] (C)
3.3. Epoxide Ring-Opening Carbonylation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Della Pina, C.; Falletta, E.; Rossi, M. A green approach to chemical building blocks. The case of 3-hydroxypropanoic acid. Green Chem. 2011, 13, 1624–1632. [Google Scholar] [CrossRef]
- Chiba, T.; Nakai, T. A Synthetic Approach to (+)−Thienamycin from Methyl (R)-3-Hydroxybutanoate—A New Entry to (3r,4r)-3-[(R)-1-Hydroxyethyl]-4-Acetoxy-2-Azetidinone. Chem. Lett. 1985, 14, 651–654. [Google Scholar] [CrossRef]
- Seebach, D.; Albert, M.; Arvidsson, P.I.; Rueping, M.; Schreiber, J.V. From the biopolymer PHB to biological investigations of unnatural beta- and gamma-peptides. Chimia 2001, 55, 345–353. [Google Scholar]
- Ren, Q.; Ruth, K.; Thony-Meyer, L.; Zinn, M. Enatiomerically pure hydroxycarboxylic acids: Current approaches and future perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, M.; Endo, T.; Saito, T. Fermentative production of (R)-(−)-3-hydroxybutyrate using 3-hydroxybutyrate dehydrogenase null mutant of Ralstonia eutropha and recombinant Escherichia coli. J. Biosci. Bioeng. 2006, 102, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Ugwu, C.U. Biotechnological production of (R)-3-hydroxybutyric acid monomer. J. Biotechnol. 2007, 132, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.C.; Ramachandran, P.V. The Boron Approach to Asymmetric-Synthesis. Pure Appl. Chem. 1991, 63, 307–316. [Google Scholar] [CrossRef]
- Noyori, R.; Kitamura, M.; Ohkuma, T. Toward efficient asymmetric hydrogenation: Architectural and functional engineering of chiral molecular catalysts. Proc. Natl. Acad. Sci. USA 2004, 101, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- De Roo, G.; Kellerhals, M.B.; Ren, Q.; Witholt, B.; Kessler, B. Production of chiral R-3-hydroxyalkanoic acids and R-3-hydroxyalkanoic acid methylesters via hydrolytic degradation of polyhydroxyalkanoate synthesized by pseudomonads. Biotechnol. Bioeng. 2002, 77, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.M.; Wang, H.S.; Lv, Z.G.; Wang, Z.H.; Nie, T.; Zhang, W.W. Catalytic performance of [Bmim][Co(CO)(4)] functional ionic liquids for preparation of 1,3-propanediol by coupling of hydroesterification-hydrogenation from ethylene oxide. J. Organomet. Chem. 2011, 696, 3668–3672. [Google Scholar] [CrossRef]
- Rajendiran, S.; Park, K.; Lee, K.; Yoon, S. Ionic-Liquid-Based Heterogeneous Covalent Triazine Framework Cobalt Catalyst for the Direct Synthesis of Methyl 3-Hydroxybutyrate from Propylene Oxide. Inorg. Chem. 2017, 56, 7270–7277. [Google Scholar] [CrossRef] [PubMed]

| Entry | Solvent | Pressure (MPa) | Temperature (°C) | Time (h) | Conversion (%) b | Selectivity (%) b | |||
|---|---|---|---|---|---|---|---|---|---|
| 3-HBA | 2-HPHB g | Acetone | 1,2-PD h | ||||||
| 1 | H2O | 4.0 | 75 | 24 | 0.7 f | 29 | 42 | 14 | 15 |
| 2 | DME:H2O | 4.0 | 75 | 24 | 57 f | 42 | 31 | 14 | 13 |
| 3 c | DME:H2O | - | 75 | 24 | 63 f | - | - | 43 | 57 |
| 4 c | DME:H2O | 4.0 | 75 | 24 | 63 f | - | - | 44 | 56 |
| 5 c | DME:H2O | - | 40 | 24 | - | - | - | - | - |
| 6 | DME:H2O | 4.0 | 40 | 24 | 2 f | 2 | - | 96 | 2 |
| 7 d | DME:H2O | 4.0 | 75 | 24 | 39 f | 41 | 30 | 15 | 14 |
| 8 e | DME:H2O | 4.0 | 75 | 24 | 14 f | 42 | 29 | 16 | 13 |
| 9 | DME:H2O | 6.0 | 75 | 24 | 62 f | 44 | 31 | 12 | 11 |
| 10 | DME:H2O | 6.0 | 75 | 48 | 72 f | 47 | 34 | 10 | 9 |
| 11 | DME:H2O | 6.0 | 75 | 56 | >99 | 49 | 36 | 11 | 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendiran, S.; Park, G.; Yoon, S. Direct Conversion of Propylene Oxide to 3-Hydroxy Butyric Acid Using a Cobalt Carbonyl Ionic Liquid Catalyst. Catalysts 2017, 7, 228. https://doi.org/10.3390/catal7080228
Rajendiran S, Park G, Yoon S. Direct Conversion of Propylene Oxide to 3-Hydroxy Butyric Acid Using a Cobalt Carbonyl Ionic Liquid Catalyst. Catalysts. 2017; 7(8):228. https://doi.org/10.3390/catal7080228
Chicago/Turabian StyleRajendiran, Senkuttuvan, Gyoosoon Park, and Sungho Yoon. 2017. "Direct Conversion of Propylene Oxide to 3-Hydroxy Butyric Acid Using a Cobalt Carbonyl Ionic Liquid Catalyst" Catalysts 7, no. 8: 228. https://doi.org/10.3390/catal7080228