Is Selective Heating of the Sulfonic Acid Catalyst AC-SO3H by Microwave Radiation Crucial in the Acid Hydrolysis of Cellulose to Glucose in Aqueous Media?
Abstract
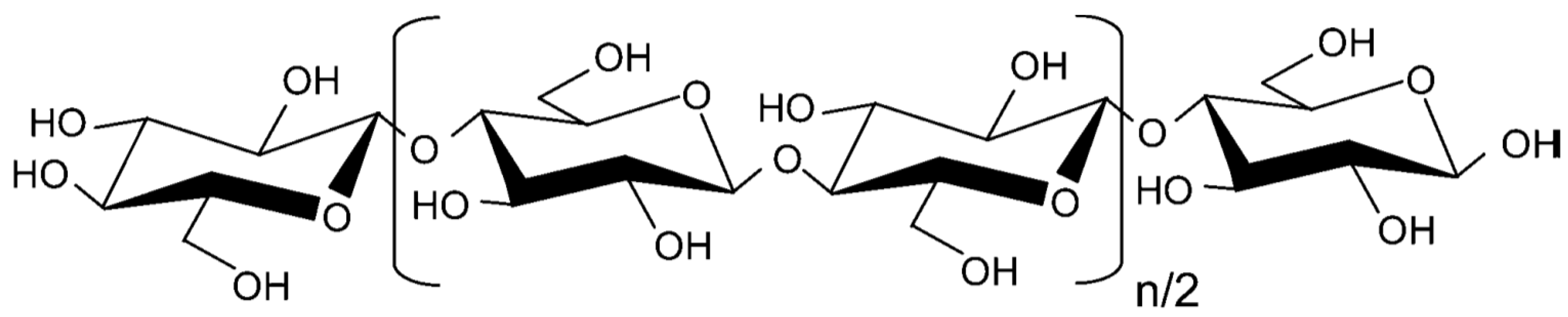
:1. Introduction
2. Results and Discussion
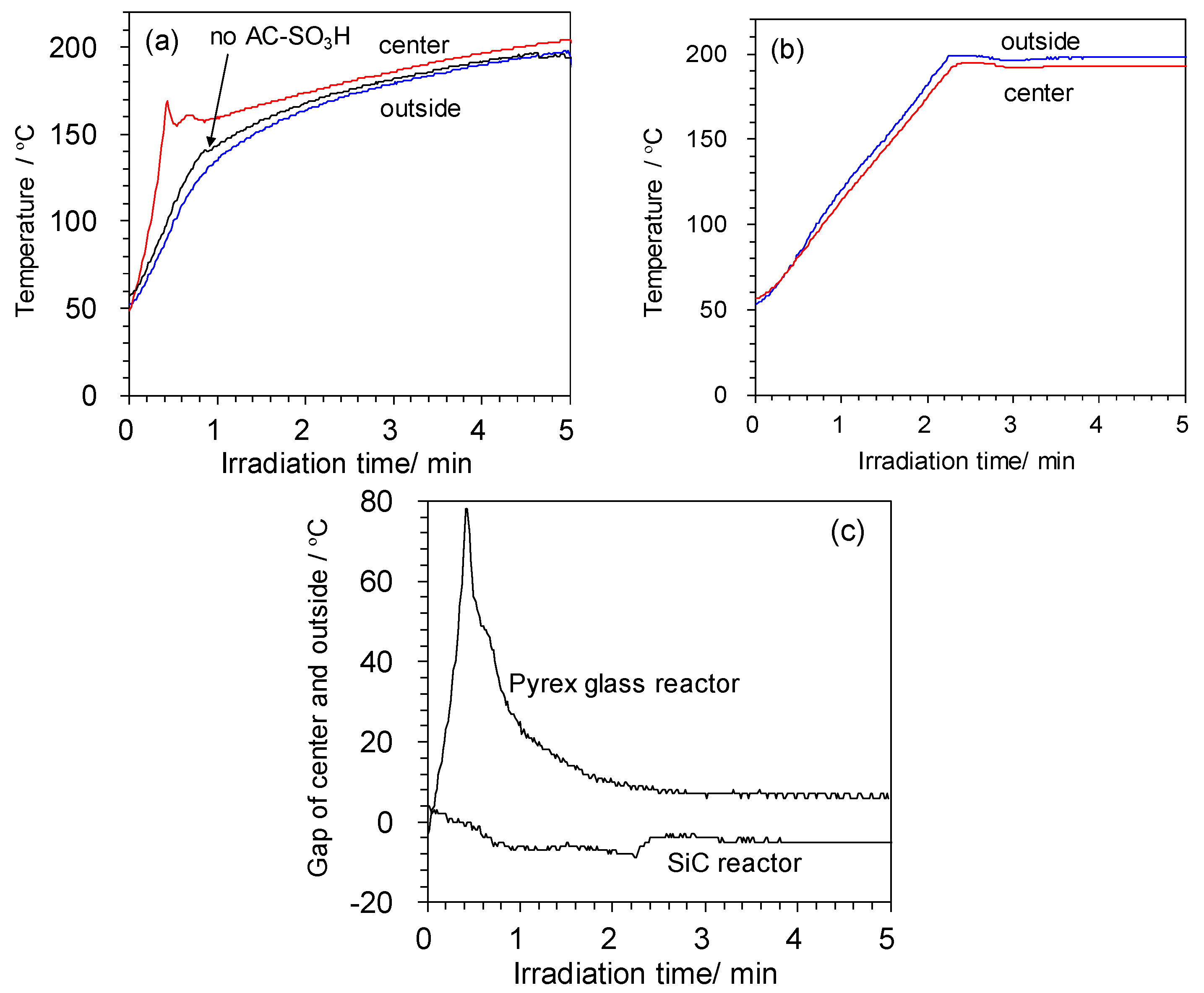
2.1. Reactor Consideration for Conventional Heating
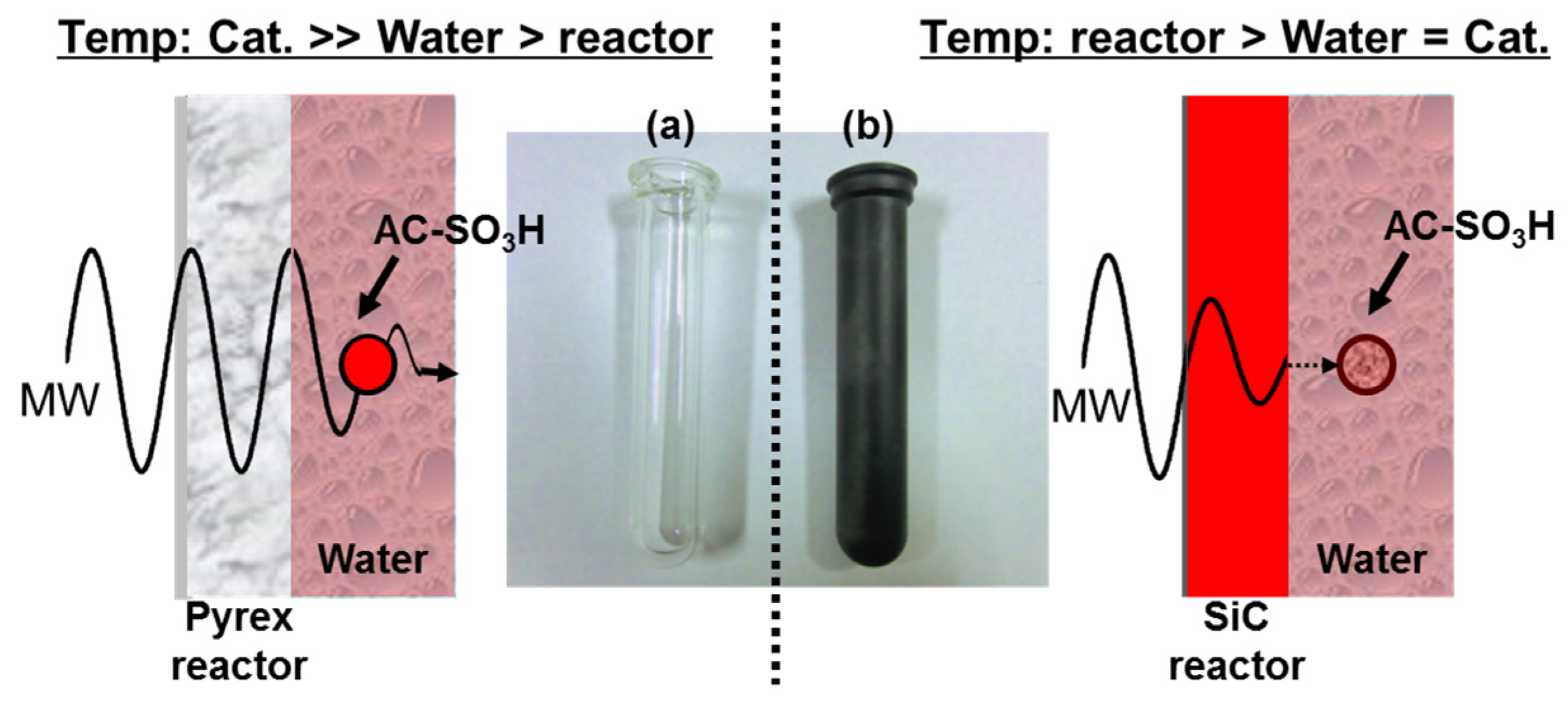
2.2. Temperature Changes in Different Reactors and Selective Heating
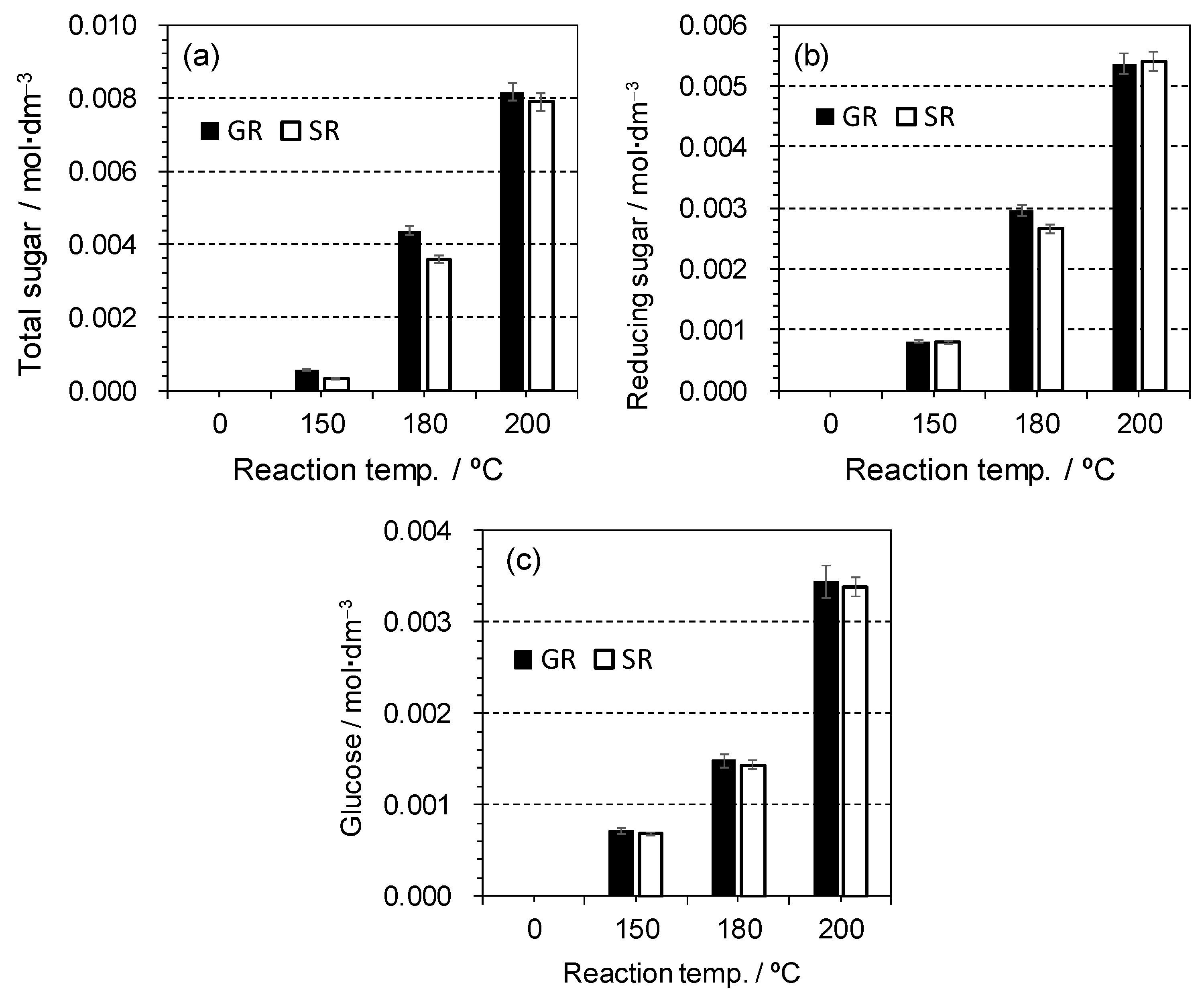
2.3. Acid Hydrolysis of Cellulose in the Presence of AC-SO3H
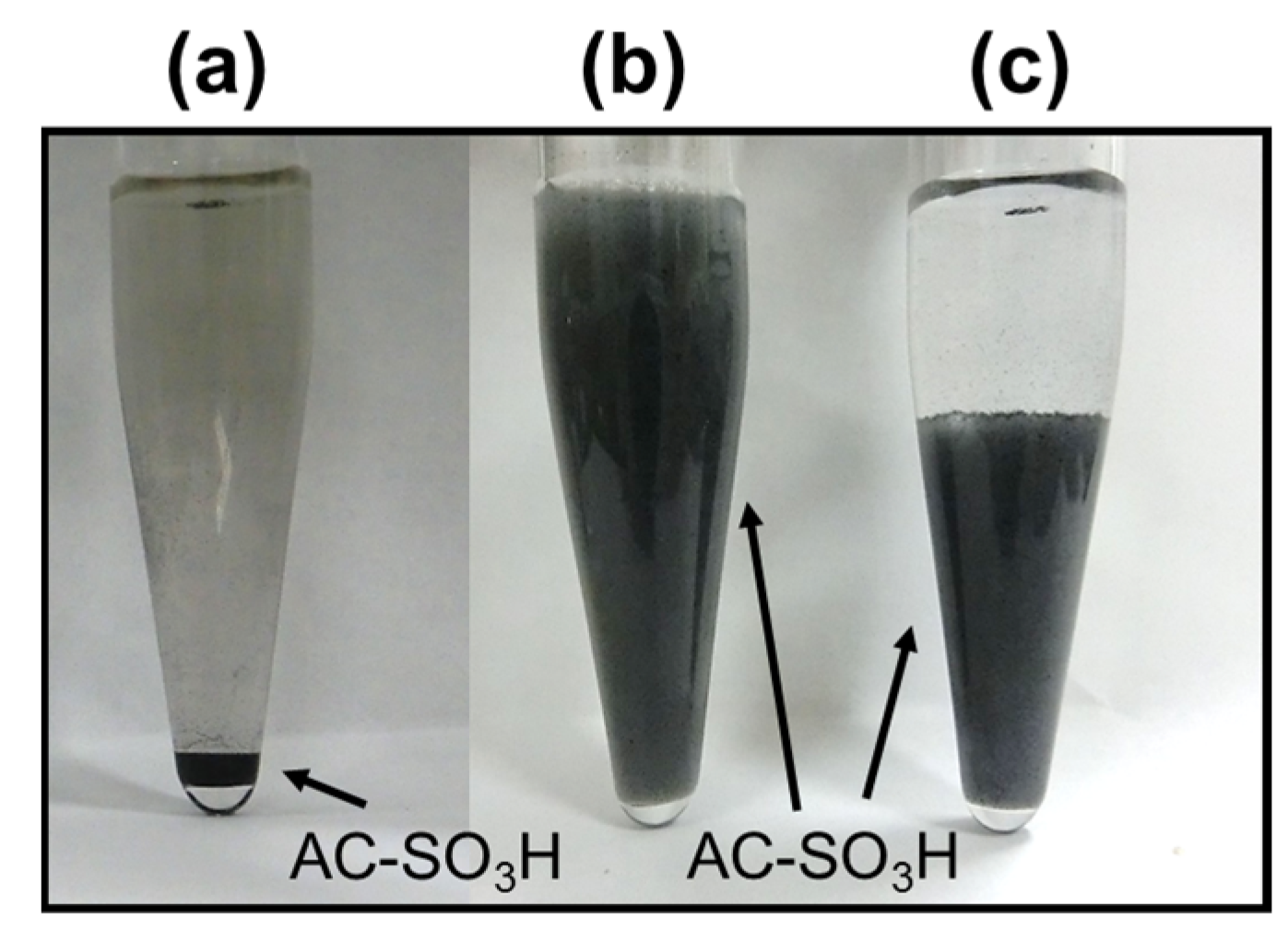
2.4. Changes in the Dispersion of AC-SO3H Catalyst Particulates in the Cellulose Gel
2.5. Other Characteristics of the Microwave Heating Method Compared to Conventional Heating
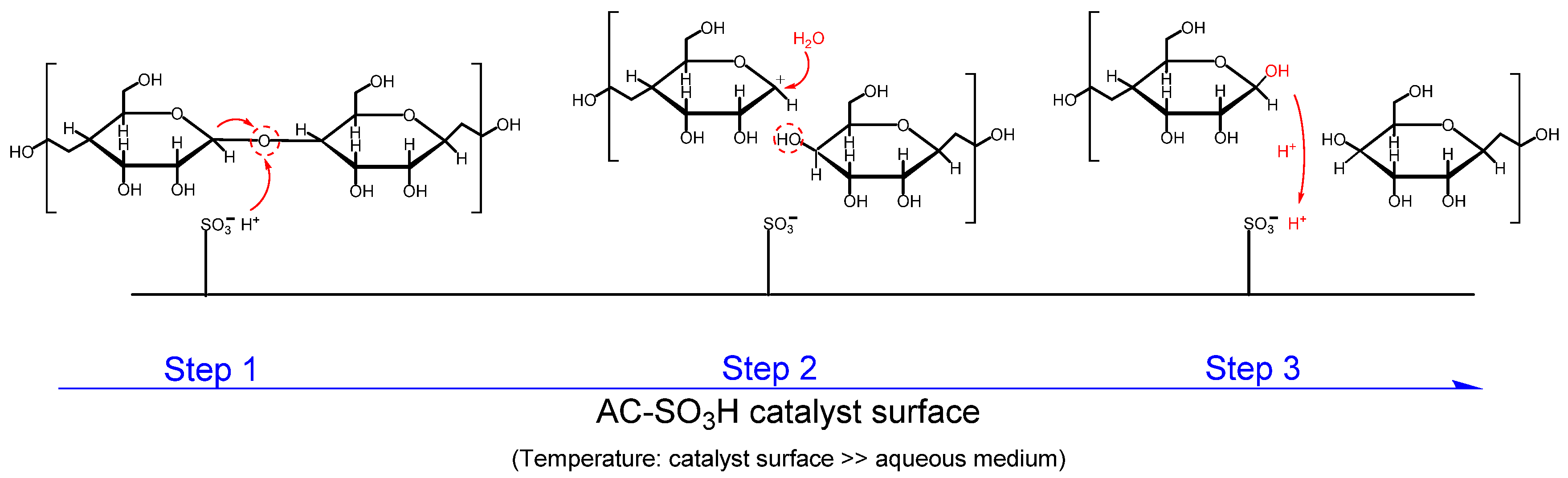
2.6. Brief Mechanistic Considerations
3. Materials and Methods
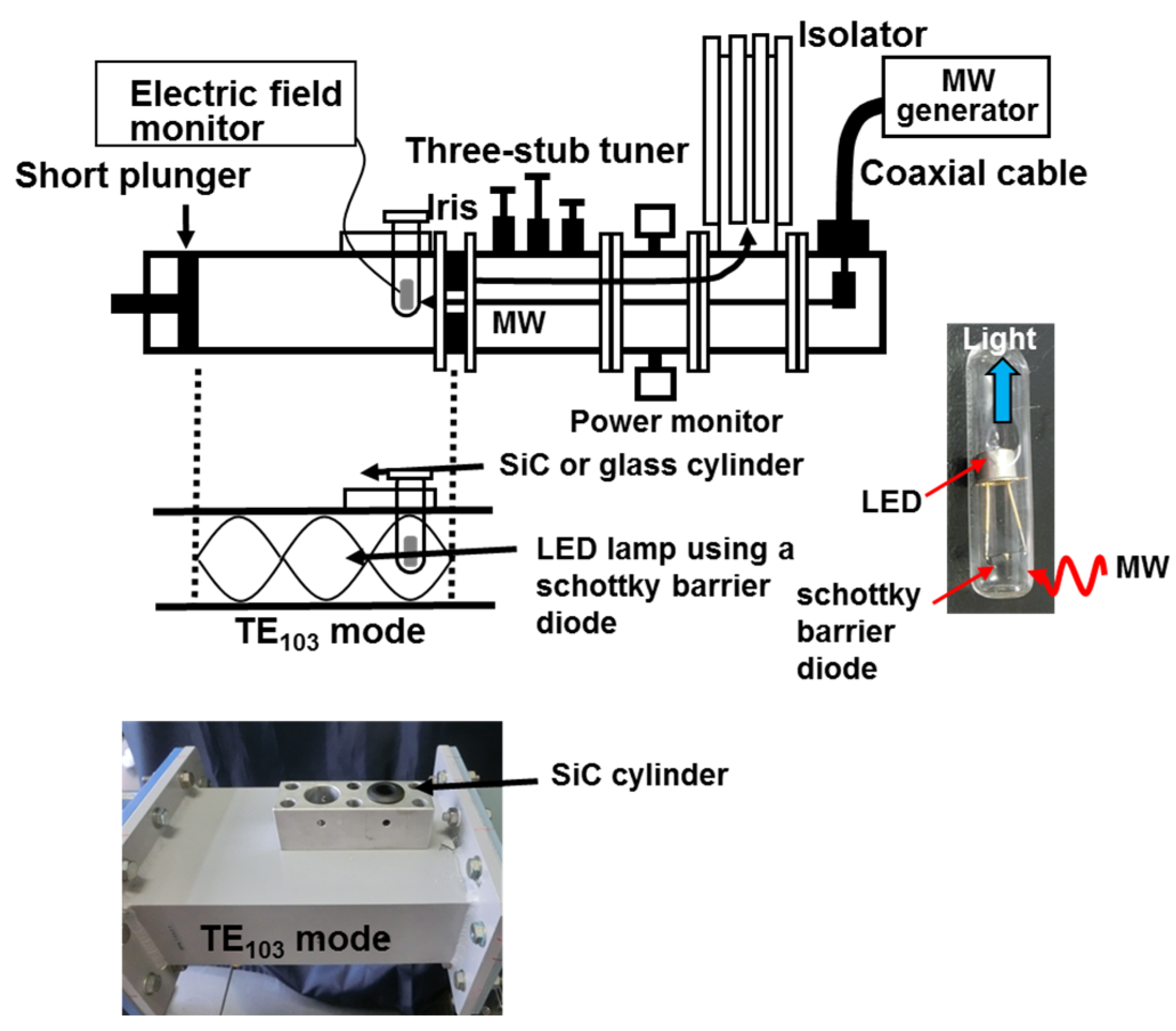
3.1. Microwave Transparency of SiC Reactor Using a LED Lamp
3.2. Preparation of the AC-SO3H Heterogeneous Catalyst Particulates
3.3. Acid Hydrolysis of Cellulose
3.4. Analytical Procedures
3.5. Simulation of the Heat Transfer Process
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Microwaves in Organic Synthesis, 3rd ed.; De la Hoz, A.; Loupy, A. (Eds.) Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Pratical Microwave Synthesis for Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Pedersen, S.L.; Tofteng, A.P.; Malik, L.; Jensen, K.J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 2012, 41, 1826–1844. [Google Scholar] [CrossRef] [PubMed]
- Microwaves in Nanoparticle Synthesis—Fundamentals and Applications; Horikoshi, S.; Serpone, N. (Eds.) Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Adlington, K.; McSweeney, R.; Dimitrakis, G.; Kingman, S.W.; Robinson, J.P.; Irvine, D.J. Enhanced in situ catalysis via microwave selective heating: Catalytic chain transfer polymerization. RSC Adv. 2014, 4, 16172–16180. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Role of microwaves in heterogeneous catalytic systems. Catal. Sci. Technol. 2014, 4, 1197–1210. [Google Scholar] [CrossRef]
- Microwaves in Catalysis—Methodology and Applications; Horikoshi, S.; Serpone, N. (Eds.) Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 10-0198502346. [Google Scholar]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hotspots. Part V. Mechanisms of hotspot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. Appl. Catal. A Gen. 2013, 460–461, 52–60. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Sumi, T.; Serpone, N. Selective heating of Pd/AC catalyst in heterogeneous systems for the microwave-assisted continuous hydrogen evolution from organic hydrides: Temperature distribution in the fixed-bed reactor. Int. J. Hydrog. Energy 2016, 41, 12029–12037. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Abe, M.; Serpone, N. On the generation of hotspots by microwave electric and magnetic fields and their impact on a microwave-assisted heterogeneous reaction in the presence of metallic Pd nanoparticles on an activated carbon support. J. Phys. Chem. C 2011, 115, 23030–23035. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Deuss, P.J.; Barta, K.; de Vries, J.G. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal. Sci. Technol. 2014, 4, 1174–1196. [Google Scholar] [CrossRef]
- Kappe, C.O. Unraveling the mysteries of microwave chemistry using silicon carbide reactor technology. Acc. Chem. Res. 2013, 46, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Kingman, S.; Irvine, D.; Licence, P.; Smith, A.; Dimitrakis, G.; Obermayer, D.; Kappe, C.O. Electromagnetic simulations of microwave heating experiments using reaction vessels made out of silicon carbide. Phys. Chem. Chem. Phys. 2010, 12, 10793–10800. [Google Scholar] [CrossRef] [PubMed]
- Atwater, J.E.; Wheeler, R.R., Jr. Microwave permittivity and dielectric relaxation of a high surface area activated carbon. Appl. Phys. A 2004, 79, 125–129. [Google Scholar] [CrossRef]
- Fan, J.; de bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct Microwave-Assisted Hydrothermal Depolymerization of Cellulose. J. Am. Chem. Soc. 2013, 135, 11728–11731. [Google Scholar] [CrossRef] [PubMed]
- Suttisawat, Y.; Sakai, H.; Abe, M.; Rangsunvigit, P.; Horikoshi, S. Microwave effect in the dehydrogenation of tetralin and decalin with a fixed-bed reactor. Int. J. Hydrog. Energy 2012, 37, 3242–3250. [Google Scholar] [CrossRef]
- Onda, A. Selective hydrolysis of cellulose and polysaccharides into sugars by catalytic hydrothermal method using sulfonated activated-carbon. J. Jpn. Petroleum Inst. 2012, 55, 73–86. [Google Scholar] [CrossRef]
- Onda, A.; Ochi, T.; Yanagisawa, K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008, 10, 1033–1037. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Solid acid and microwave-assisted hydrolysis of cellulose in ionic liquid. Carbohydr. Res. 2009, 344, 2069–2072. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Sumi, T.; Serpone, N. Unusual effect of the magnetic field component of the microwave radiation on aqueous electrolyte solutions. J. Microw. Power Electromagn. Energy 2012, 46, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Takagaki, A.; Okumura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Green chemistry: Biodiesel made with sugar catalyst. Nature 2005, 438, 178. [Google Scholar] [CrossRef] [PubMed]


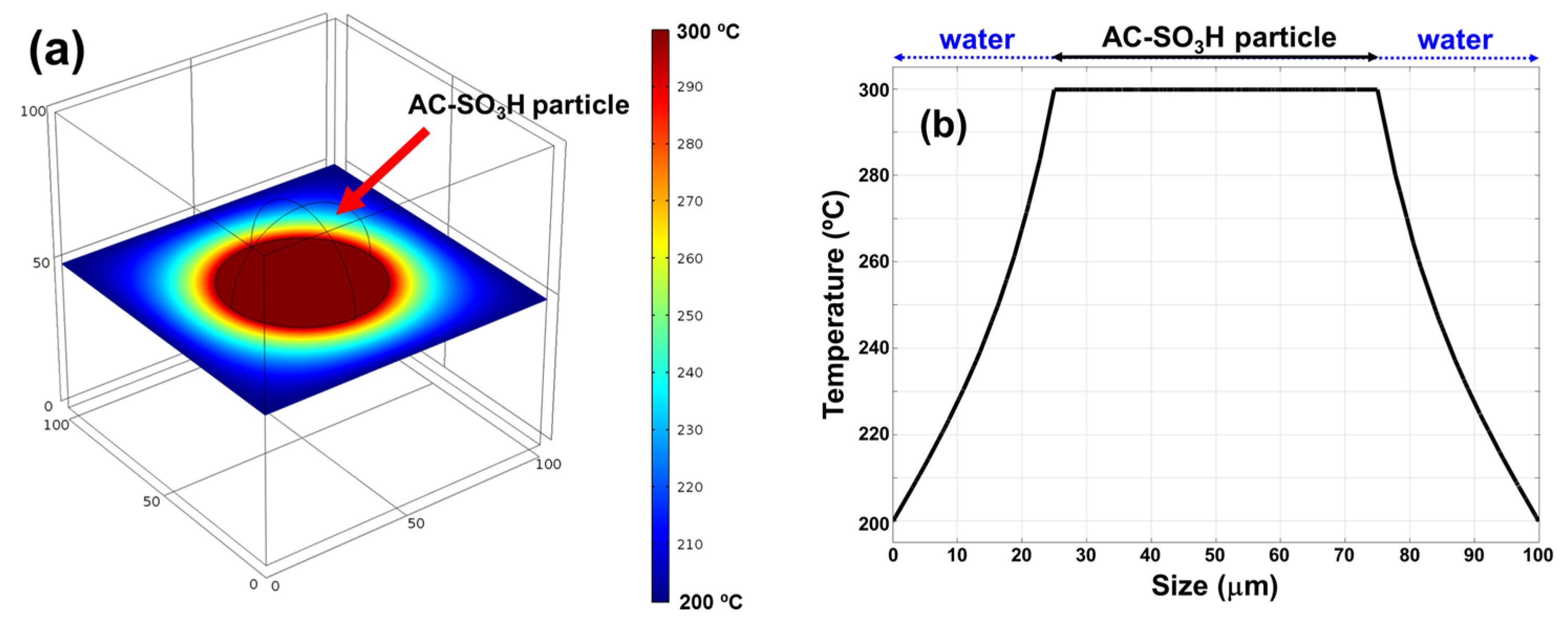
| Parameters | Water | AC-SO3H |
|---|---|---|
| Radius/μm | 100 × 100 × 100 | 25 |
| Specific heat capacity/kJ·kg‒1·K‒1 | 4.216 | 1.300 |
| Thermal conductivity/mW·m‒1·K‒1 | 679.1 | 140 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikoshi, S.; Minagawa, T.; Tsubaki, S.; Onda, A.; Serpone, N. Is Selective Heating of the Sulfonic Acid Catalyst AC-SO3H by Microwave Radiation Crucial in the Acid Hydrolysis of Cellulose to Glucose in Aqueous Media? Catalysts 2017, 7, 231. https://doi.org/10.3390/catal7080231
Horikoshi S, Minagawa T, Tsubaki S, Onda A, Serpone N. Is Selective Heating of the Sulfonic Acid Catalyst AC-SO3H by Microwave Radiation Crucial in the Acid Hydrolysis of Cellulose to Glucose in Aqueous Media? Catalysts. 2017; 7(8):231. https://doi.org/10.3390/catal7080231
Chicago/Turabian StyleHorikoshi, Satoshi, Takashi Minagawa, Shuntaro Tsubaki, Ayumu Onda, and Nick Serpone. 2017. "Is Selective Heating of the Sulfonic Acid Catalyst AC-SO3H by Microwave Radiation Crucial in the Acid Hydrolysis of Cellulose to Glucose in Aqueous Media?" Catalysts 7, no. 8: 231. https://doi.org/10.3390/catal7080231





