1. Introduction
Worldwide environmental regulations regarding emissions of nitrogen oxides (NO
) from automotives are becoming more and more stringent. This poses a continuous need to improve existing technologies and develop new concepts that can meet prescribed and upcoming emission standards. Selective catalytic reduction (SCR) of NO
using ammonia is presently one of the most efficient technologies for abatement of NO
from combustion engines, in particular from diesel engines. Besides vanadia, Fe- and Cu-based zeolites are considered to be the most suitable catalyst formulations for NH
-SCR. In general Cu-zeolites show higher low-temperature (<250
C) SCR performance than Fe-zeolites, and vice versa, Fe-based zeolites are more active at high temperatures (>400
C) than Cu-zeolites [
1]. Amongst the different zeolite structures that have been proposed as NH
-SCR catalysts, zeolites with CHA and *BEA framework topologies have received much attention thanks to high low-temperature activity and hydrothermal stability [
2,
3].
It has been shown that the performance of Fe- and Cu-based zeolites as SCR catalysts depends on the type iron- and copper species that are formed during the ion-exchange of the zeolite [
4,
5,
6]. The most common methods for ion-exchange reported in the open literature are aqueous ion-exchange (AIE) and solid-state ion-exchange (SSIE) [
5,
7,
8,
9,
10,
11,
12,
13,
14]. These methods, however, have several substantial drawbacks. Among these are, as in the case of AIE, the need of using numerous operation steps and arising difficulties to overcome steric hindrance caused by the formation of large solvation shells for some multivalent metal ions [
3], and, as in the case of SSIE, the need of high temperatures that may cause partial or complete zeolite framework destruction. Recently, however, it was shown that in the presence of ammonia, zeolites can be functionalized with copper by SSIE at low temperatures [
15,
16]. In contrast to conventional SSIE, which requires high temperatures, typically above 700
C, Shwan et al. showed that copper-exchanged zeolites (*BEA, MFI and CHA) can be prepared by exposing physical mixtures of zeolite and copper oxide to NH
or NH
and NO already at 250
C [
16]. It was suggested that copper becomes mobile at low temperature, in the presence of NH
, owing to the formation of [Cu(NH
)
]
complexes. By including NO during the exposure to NH
, the migration rate of Cu into the porous structure of the zeolite is enhanced and it was suggested that NO assists the reduction of Cu
in CuO to Cu
, which with ammonia forms the mobile [Cu(NH
)
]
complexes [
16,
17]. The so-called [NO+NH
]-SSIE method is simple as it only involves two operation steps (mixing the CuO and zeolite powders, and flushing ammonia and NO over the mixture), both in the absence of aqueous media, and can be performed at considerably lower temperatures compared to conventional solid-state ion-exchange in air.
Recently, several examples of one-pot synthesis methods to functionalize zeolite structures with Fe and/or Cu have been reported, e.g., [
18,
19,
20,
21]. As an example, one can use a one-pot synthesis of Cu- or Fe-zeolite, where either copper or iron occupies T-atom positions in the zeolite framework structure, followed by ion-exchange using AIE or wet impregnation (WI) of the other metal. For instance, Zhang et al. synthesized Cu-SSZ-13 in a direct route and ion-exchanged it with Fe [
18]. Although the authors claimed an improvement of the NH
-SCR activity and a broadening of the active temperature window, the improvement was eventually observed only at high temperatures while the low-temperature activity of the (Fe + Cu)-SSZ-13 was considerably lower compared to the corresponding SSZ-13 ion-exchanged only with copper. Keeping in mind that the exhaust gas temperatures gradually become lower as engine development progresses, both high and stable SCR activity at low temperatures is required. For coated monolith substrates, Cu-zeolite and Fe-zeolite catalysts can be sequentially placed in an exhaust gas system. Furthermore, dual layer configurations, where separate washcoat slurries with Cu- and Fe-zeolites are deposited sequentially on the same substrate or just a physical mixture of Fe- and Cu-zeolites in the slurry, can be used [
1,
22].
Besides studies on sequential [
23] and dual layer configurations of combined (Cu + Fe)-zeolite catalysts for NH
-SCR, a few studies focused on single layer Fe/Cu-zeolite catalysts prepared by two-step post-synthesis and conventional WI procedures have recently been reported by Borón et al. [
24]. The authors reported a widening of the temperature window of the catalyst being active for NH
-SCR. Still, however, there is a significant need for improvement of the properties of the catalyst, for instance, by modifying the method of ion-exchange while keeping a two-step post-synthesis method. From this perspective, we consider the [NH
+NO]-SSIE method [
16] to have high potential to produce catalyst formulations in which both copper and iron are exchanged in the microporous structure of the zeolite, facilitating potential synergistic effects between the two metals that are beneficial for the catalytic properties of the zeolite. Moreover, by varying the order of Fe- or Cu-exchange and/or Fe or Cu content, one would form iron and copper species of different nature and hence it would be possible to control the catalytic properties towards the desired temperature window.
The objective of the present study is to investigate if functionalization of SSZ-13 and Fe-beta with copper using NH- and NO-facilitated solid-state ion-exchange ([NH+NO]-SSIE) is a viable route to prepare Cu-SSZ-13 and (Cu + Fe)-beta catalysts active for NH-SCR, starting from H-SSZ-13 and Fe-beta, respectively. The physicochemical properties of the prepared samples are characterized by X-ray diffraction (XRD), ultraviolet-visible (UV-Vis) spectroscopy and scanning transmission electron microscopy equipped with energy-dispersive X-ray spectroscopy (STEM-EDS) in order to confirm that copper originally present in the physical mixture of CuO and H-SSZ-13, and CuO and Fe-beta is inserted into the micropores of SSZ-13 and Fe-beta, respectively. Furthermore, the activity of the prepared samples for NH-SCR is measured in gas-flow reactor experiments.
2. Results and Discussion
In
Table 1, the crystal size of CuO determined by XRD of physical mixtures of CuO and H-SSZ-13 with different CuO content is shown before and after [NH
+NO]-SSIE. The mean crystal sizes were calculated using the Scherrer equation taking into account the broadening of the CuO peaks in the XRD patterns positioned at 2
= 36.2 and 39.6
. The CuO crystal size increases with increasing Cu loading after the [NH
+NO]-SSIE indicating crystallite agglomeration. However, the CuO peak intensity is found to decrease for each sample after the [NH
+NO]-SSIE, which is in accordance with the finding by Shwan et al. that CuO is consumed while treating the samples with NO and NH
[
16].
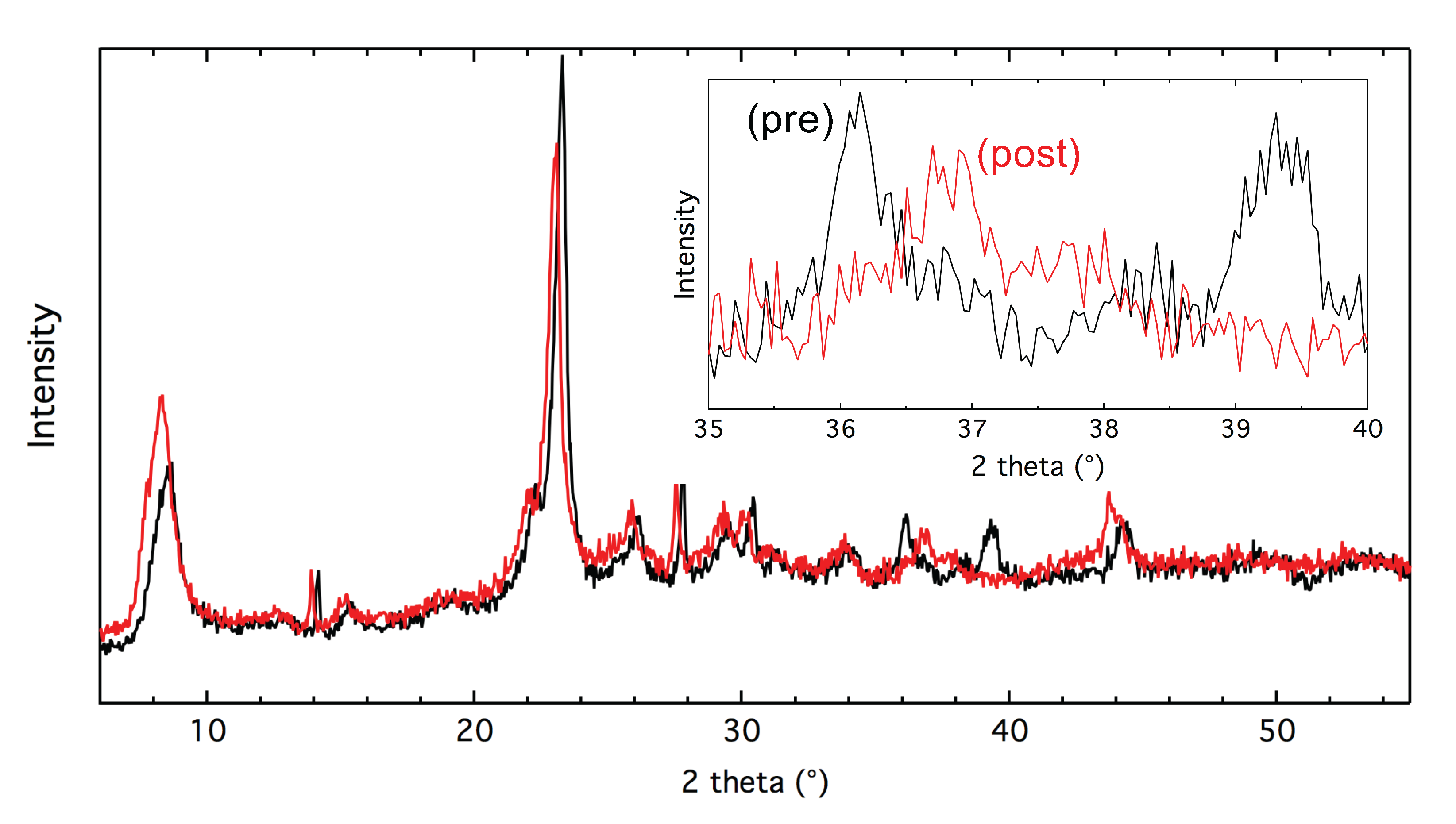
In
Figure 1, the X-ray diffractograms for the 2% (Cu + Fe)-beta sample before and after the [NH
+NO]-SSIE are shown. The clearly pronounced CuO reflections at 2
= 36.2 and 39.6
for the physical mixture of CuO and Fe-beta before the [NH
+NO]-SSIE, disappear after the [NH
+NO]-SSIE. It means that for Fe-beta, CuO is most likely completely consumed for ion-exchange as the result of the [NH
+NO]-SSIE, thanks to lack of steric restrictions for copper to enter larger pores of zeolite beta, compared to SSZ-13.
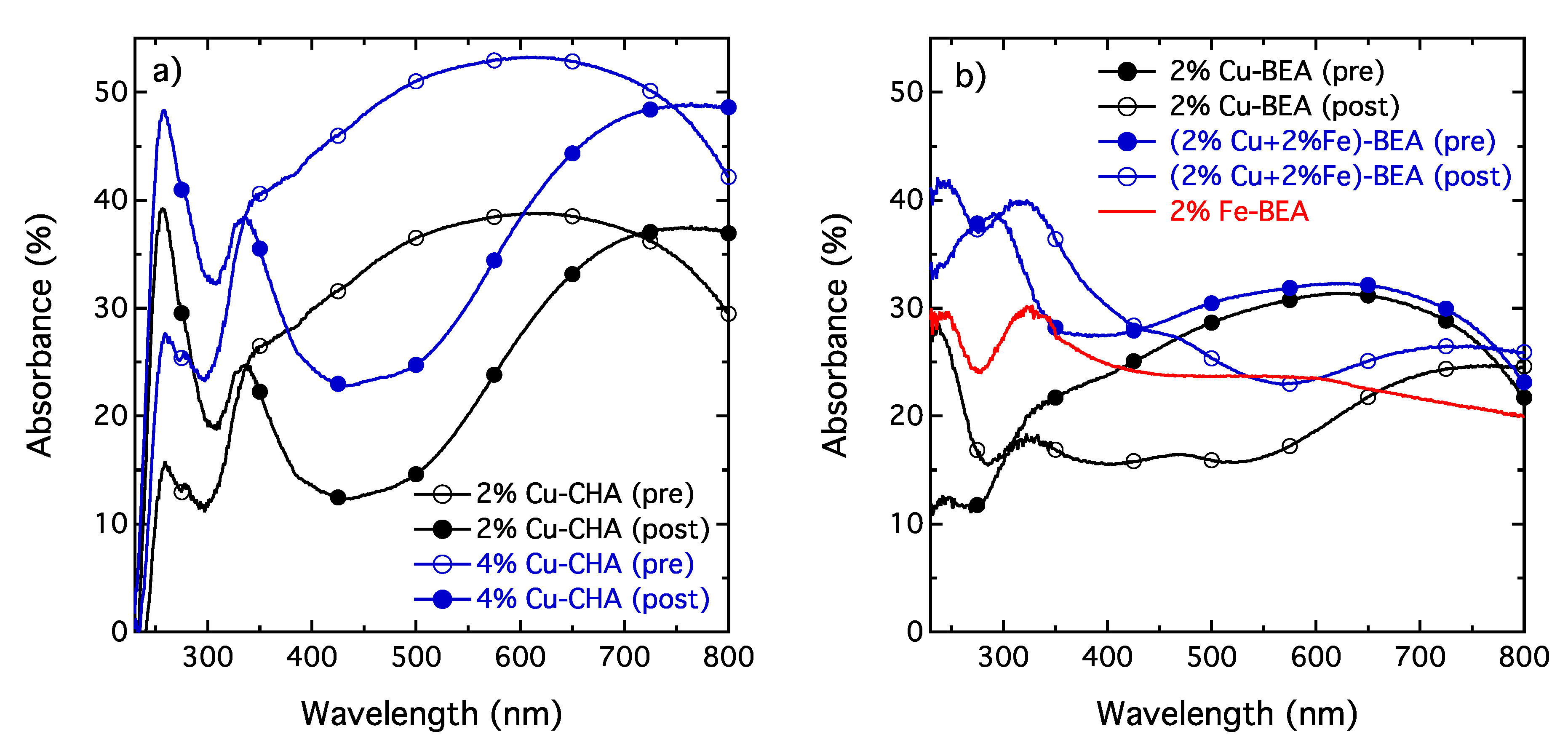
The results from the UV-Vis spectroscopy measurements for the 2% and 4% Cu-SSZ-13 samples are shown in
Figure 2a and the corresponding results for the 2% Fe-beta, 2% Cu-beta and (2% Cu + 2% Fe)-beta samples are shown in
Figure 2b. One can observe a higher number of absorption peaks after the [NH
+NO]-SSIE than before this treatment. The peaks around 260 and 340 nm can be assigned to isolated Cu
and Cu
ions located in the micropores of the zeolite [
7]. In contrast, the presence of CuO typically results in a broad absorption over the visible range of the spectrum as observed for the samples before the [NH
+NO]-SSIE. This is an additional indication of the migration of copper from the physical mixture of CuO and H-SSZ-13 to the zeolite pores, forming Cu
and Cu
species. For the ion-exchanged zeolite beta samples, the tendency is somewhat the same, although the presence of iron complicates the spectra. However, the peaks at 260 and 340 nm become more pronounced, indicating formation of Cu
and Cu
species in the zeolite pores.
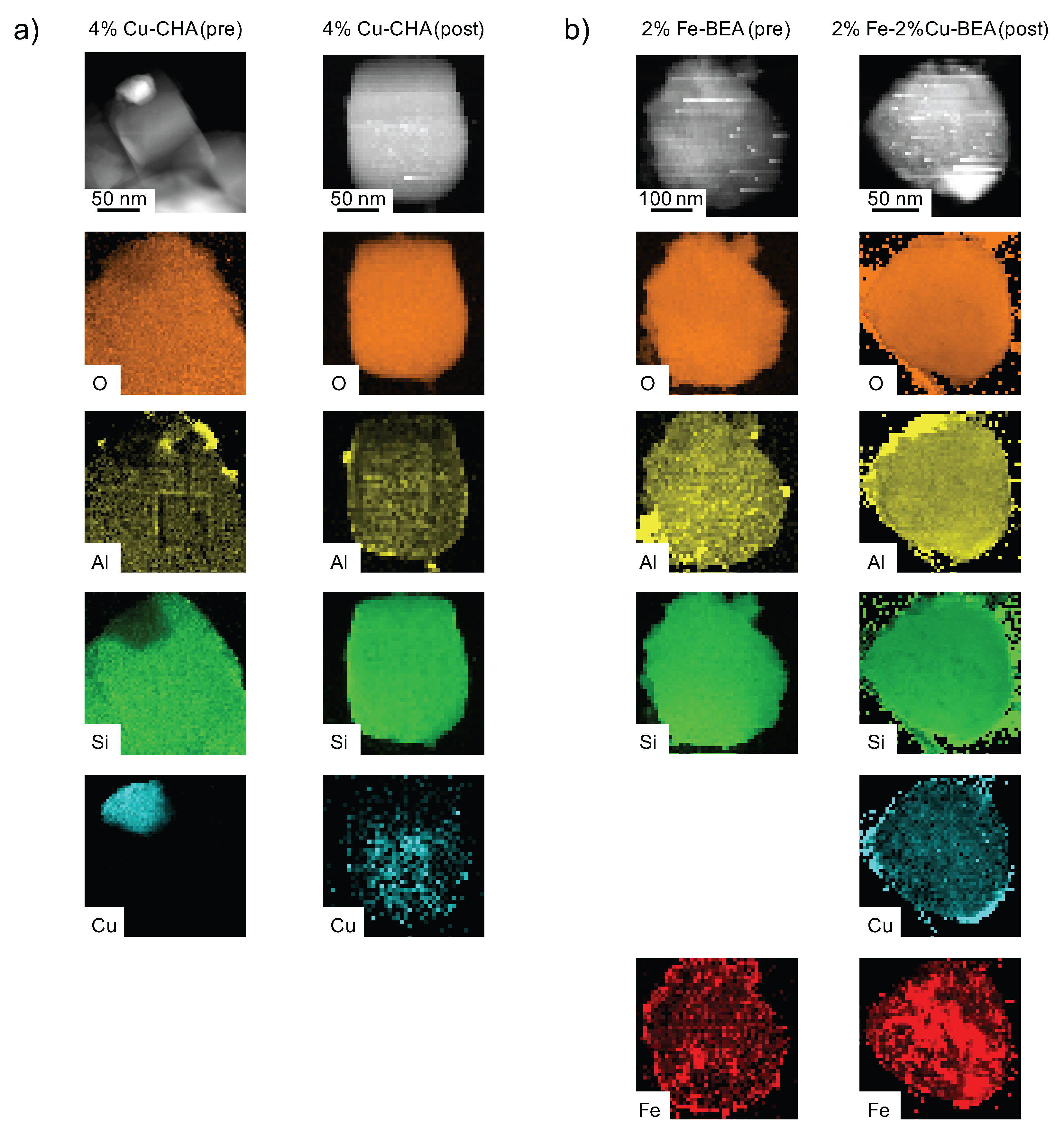
In order to monitor the copper distribution in the zeolite crystals and to further confirm the insertion of copper into the micropores of the zeolite as a result of the [NH
+NO]-SSIE, STEM-EDS elemental mapping was performed (see
Figure 3). In the EDS map recorded before the [NH
+NO]-SSIE, the copper is observed to be present only in the form of copper oxide outside the zeolite crystal, whereas after the [NH
+NO]-SSIE, copper is found to be distributed quite evenly inside the zeolite crystal. For the copper- and iron-exchanged zeolite beta sample, it is similar, after the [NH
+NO]-SSIE the copper is evenly distributed inside the zeolite crystal. Also, the iron is evenly distributed after the wet-impregnation procedure. In addition, the NH
-SCR activity can be used as an indicator of a successful copper ion exchange because only copper exchanged into the micropores of the zeolite is active for NH
-SCR [
25].
Figure 4 shows the conversion of NO during NH
-SCR for the Cu-SSZ-13 samples prepared by [NH
+NO]-SSIE and additionally one Cu-SSZ-13 sample, with identical SAR, prepared by conventional AIE [
10]. For the catalysts prepared by [NH
+NO]-SSIE, the activity for NO reduction generally increases with increasing copper content and complete NO conversion is reached for the samples with 2, 4, and 6 wt. % Cu. Only a slight decrease in NO conversion is observed for the samples with the highest copper loadings of 4 and 6 wt. % Cu at temperatures above 450
C. The decreased high-temperature SCR activity is most likely due to direct NH
oxidation by O
, which also has been observed by Shwan et al. after [NH
+NO]-SSIE of physical mixtures of CuO and zeolite with high copper oxide content [
16]. Below 300
C, the activity of the 2 wt. % Cu-SSZ-13 sample is observed to be somewhat lower compared to the 4 and 6 wt. % Cu-SSZ-13 samples, while the high-temperature activity is maintained even at the highest investigated temperature of 500
C. Compared to the 3 wt. % Cu-SSZ-13 sample prepared by AIE, the 2 wt. % Cu-SSZ-13 sample prepared by [NH
+NO]-SSIE exhibits a higher NH
-SCR activity not only at low but also at high temperatures.
These results indicate that the [NH+NO]-SSIE method when using an optimal concentration of copper in the physical mixture of zeolite and copper oxide allows one to broaden the active temperature window for NH-SCR and, in particular, significantly promote the reaction at low temperatures.
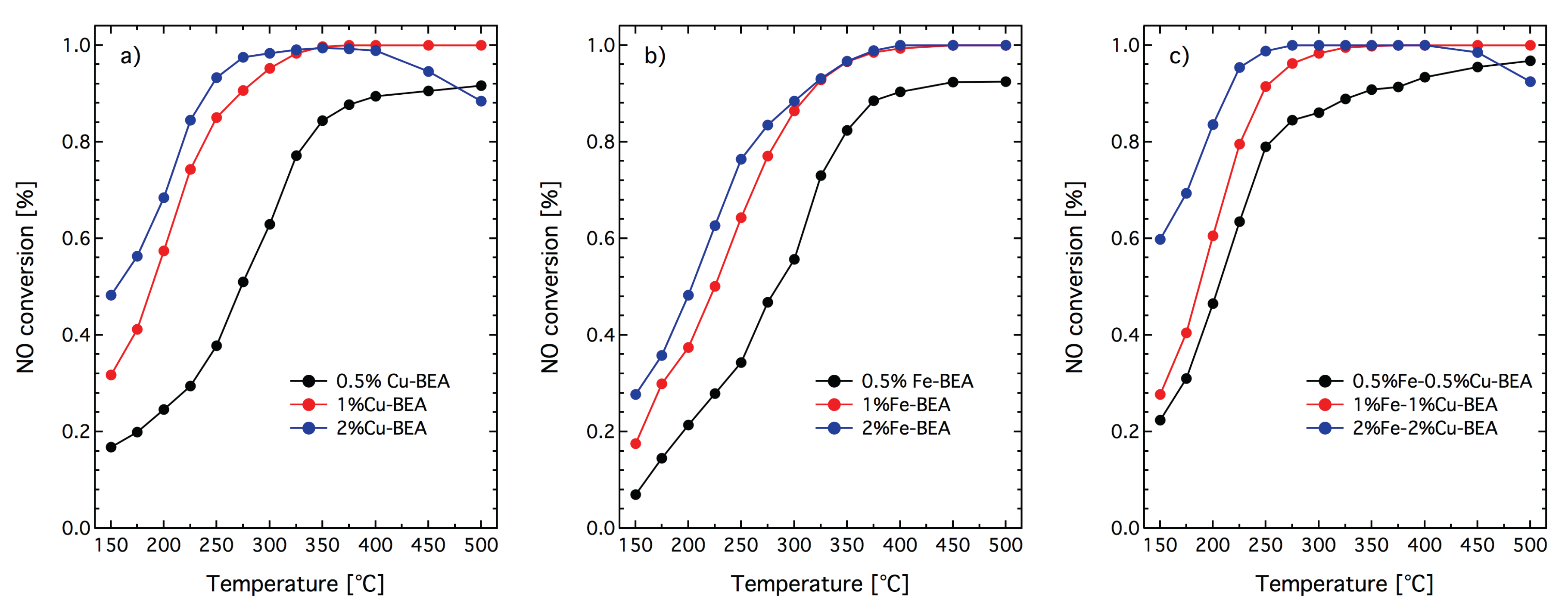
In order to further improve the performance for NH
-SCR, sequentially metal-exchanged Cu- and Fe-zeolites were studied. For these studies, zeolite beta was chosen as the prototype zeolite as both Cu-beta and Fe-beta have shown high NH
-SCR activity [
3,
26,
27,
28]. Moreover, zeolite beta is a large-pore zeolite that impose less steric hindrance to entering species. For small- and medium-pore zeolites, cations exchanged in the first stage may block the pores and prevent introduction of the second cation into the zeolite. In
Figure 5, the NO conversion during NH
-SCR over the prepared Fe-beta, Cu-beta and (Cu + Fe)-beta samples is shown. The figure shows that the addition of copper to the Fe-containing zeolite beta samples is beneficial for the catalytic properties. The Fe-exchanged zeolite beta samples show lower NH
-SCR activity at lower temperatures and higher NH
-SCR activity at higher temperatures. The Cu-beta samples, on the other hand, show higher NH
-SCR activity at lower temperatures and lower NH
-SCR activity at higher temperatures. By adding copper to Fe-beta using [NH
+NO]-SSIE, the NH
-SCR activity at low temperatures increases and the NH
-SCR activity at high temperatures decreases, however not as pronounced as for the pure Cu-beta samples. Despite different reaction conditions, the NH
-SCR activity results for the (Fe + Cu)-beta samples are in line with those of Borón et al. [
24] for (Fe + Cu)-beta with extra-framework Fe and Cu, prepared by conventional wet-impregnation.
These results presented in the present investigation show that the preparation method of sequentially exchanged (Cu + Fe)-beta catalysts, where iron-impregnation of H-beta is followed by solid-state copper-exchange facilitated by NH and NO, results in highly active NH-SCR catalysts. However, there might still be some room for improvement of this method for zeolite functionalization, and the catalytic activity can, potentially, be improved even further. For instance, varying the method of ion-exchange and/or the order of metal functionalization may result in even higher NH-SCR activity.
3. Experimental Section
Zeolite SSZ-13 with an Si/Al-ratio (SAR) of 20 was synthesized in fluoride medium using aluminium isopropoxide (Sigma Aldrich, Schelldorf, Germany), tetraethylortosilicate (Sigma Aldrich, Schnelldorf, Germany), 25% water solution,
N,
N,
N-trimethyl adamantanammonium hydroxide (AlCheTech, Moscow, Russian Federation) as structural directing agent (SDA) and 48% HF (Merck, Darmstadt, Germany). The molar composition of the gel used for the zeolite synthesis was 1.0 SiO
:0.05 Al:0.5 SDA:0.5 HF. First, the silica and aluminium sources were mixed. After addition of SDA, the solution was stirred overnight. Then HF was added and the mixture was stirred for 30 min. The synthesis mixture was then left at elevated temperature to let the water evaporate to achieve the desired composition. Finally, the synthesized gel was transferred to a Teflon-lined autoclave and heated to 150
C for 3 days without agitation; afterwards, the product was recovered by centrifugation and washed excessively with Milli-Q water (18 M
cm). The CHA structure of the product was confirmed by XRD. The SAR of the zeolite beta used (H-beta, Zeolyst International) is 38. The ion-exchange of these zeolites with copper and/or iron was performed following four routes. For the [NH
+NO]-SSIE, the physical mixture of CuO and zeolite was placed in a flow reactor comprised of a quartz tube with a heating coil (described in detail in [
5]), and exposed to a gas atmosphere consisting of 850 vol.-ppm NO and 850 vol.-ppm NH
in Ar at 250
C for 5 h. For the Cu-SSZ-13 samples, H-SSZ-13 was thoroughly physically mixed with the certain amount of CuO corresponding to 0.5, 1, 2, 4 or 6 wt. % copper followed by [NH
+NO]-SSIE, in order to obtain 0.5%, 1%, 2%, 4% and 6% Cu-SSZ-13, respectively. For the 3% Cu-SSZ-13 sample functionalized by AIE, an 0.025 M copper nitrate solution was prepared by dissolving Cu(NO
)
2.5H
O in Milli-Q water. The pH of the solution was adjusted to 3.5 by addition of nitric acid with subsequent addition of NH
-SSZ-13. After heating at 80
C for one hour under stirring, the slurry was filtered, washed with Milli-Q water, dried at room temperature for 12 h and finally calcined at 550
C for 3 h in air [
10].
For the Cu-beta samples, H-beta was thoroughly physically mixed with the certain amount of CuO corresponding to 0.5, 1 or 2 wt. % copper followed by [NH+NO]-SSIE, in order to obtain 0.5%, 1% and 2% Cu-beta, respectively. For the Fe-beta samples, H-beta was wet-impregnated with the certain amount of aqueous solution of Fe(NO) corresponding to 0.5%, 1% or 2% iron, in order to obtain 0.5%, 1% and 2% Fe-beta, respectively. For the (Cu + Fe)-beta samples, the 0.5%, 1%, and 2% Fe-beta samples were mixed the certain amount of CuO corresponding to 0.5, 1 or 2 wt. % copper followed by [NH+NO]-SSIE, in order to obtain (0.5% Cu + 0.5% Fe)-beta, (1% Cu + 1% Fe)-beta, and (2% Cu + 2% Fe)-beta, respectively.
Characterization of the samples before and after [NH
+NO]-SSIE was performed by XRD, UV-Vis-spectroscopy and STEM-EDS. The crystal phases of the prepared samples were determined using a Siemens D5000 X-ray diffractometer scanning from 2
= 5 to 50
in the scan mode (0.02
, 1 s). Ni-filtered Cu K
radiation (
= 1.54187 Å) was used for the analysis. The ultraviolet and visible absorption of the samples was investigated by UV-Vis spectroscopy using a Varian Cary 5000 instrument equipped with an integrating sphere detector (Internal DRA 2500). By STEM-EDS (FEI Titan 80-300 instrument, Hillsboro, Oregon, USA) analysis, the oxygen, aluminum, silicon, copper and iron distributions in the zeolite crystallites were examined. The catalytic activity of the prepared samples for NH
-SCR was investigated using a quartz tube flow reactor, previously described in [
10]. The composition of the inlet gas flow was controlled by a set of mass-flow controllers (Bronkhorst Hi-Tech, Ruurlo, The Netherlands). Water was separately introduced to the reactor system via an evaporator mixer system (CEM, Bronkhorst Hi-Tech LOW-
P-FLOW). The effluent gas stream was analyzed by an MKS 2000 FTIR spectrometer. For each activity measurement, 60 mg of the catalyst with a particle size of 300–365
m was used. The samples were exposed to 400 vol.-ppm NO, 400 vol.-ppm NH
, 8 vol. % O
, 5 vol. % H
O and Ar (balance) at a gas hourly space velocity (GHSV) of 200,000 h
. The catalytic activity of each sample was measured at steady state conditions for one hour at several temperatures, ranging from 150 to 500
C with intervals of 25
C at low temperatures and 50
C at high temperatures.