Synthesis of NaOH-Modified TiOF2 and Its Enhanced Visible Light Photocatalytic Performance on RhB
Abstract
:1. Introduction
2. Results and Discussion
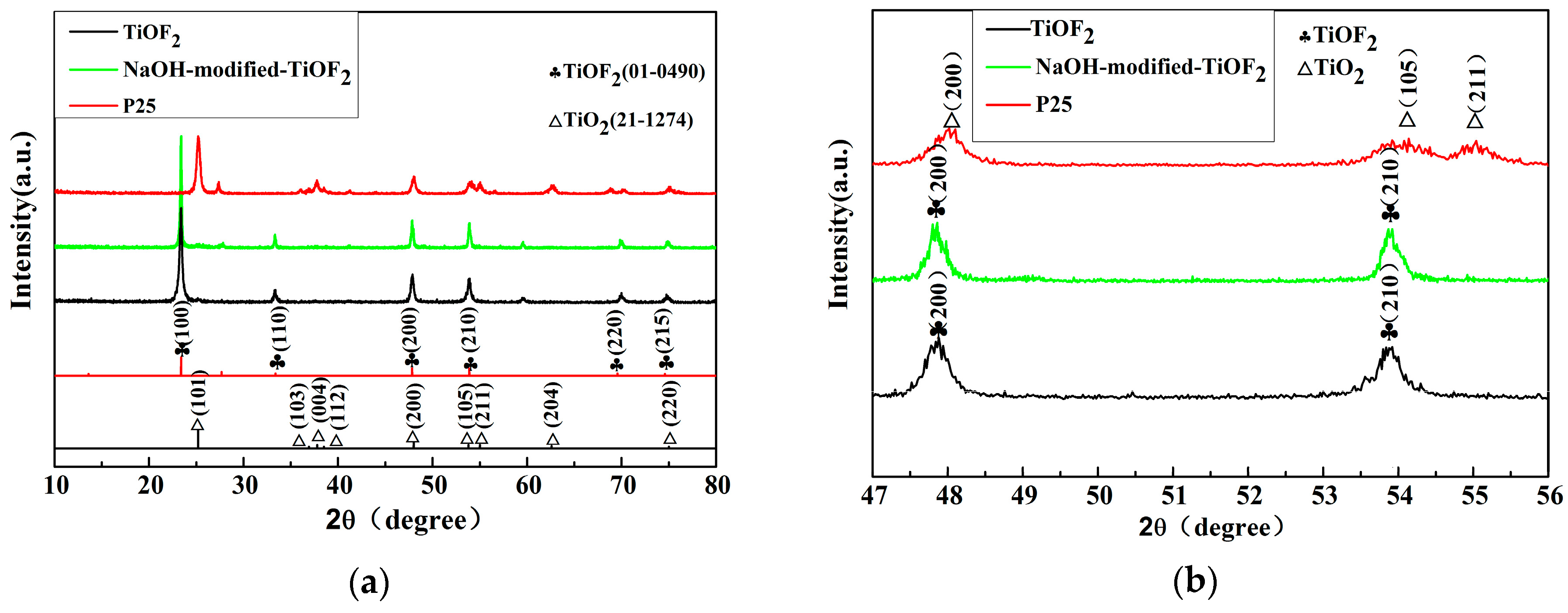
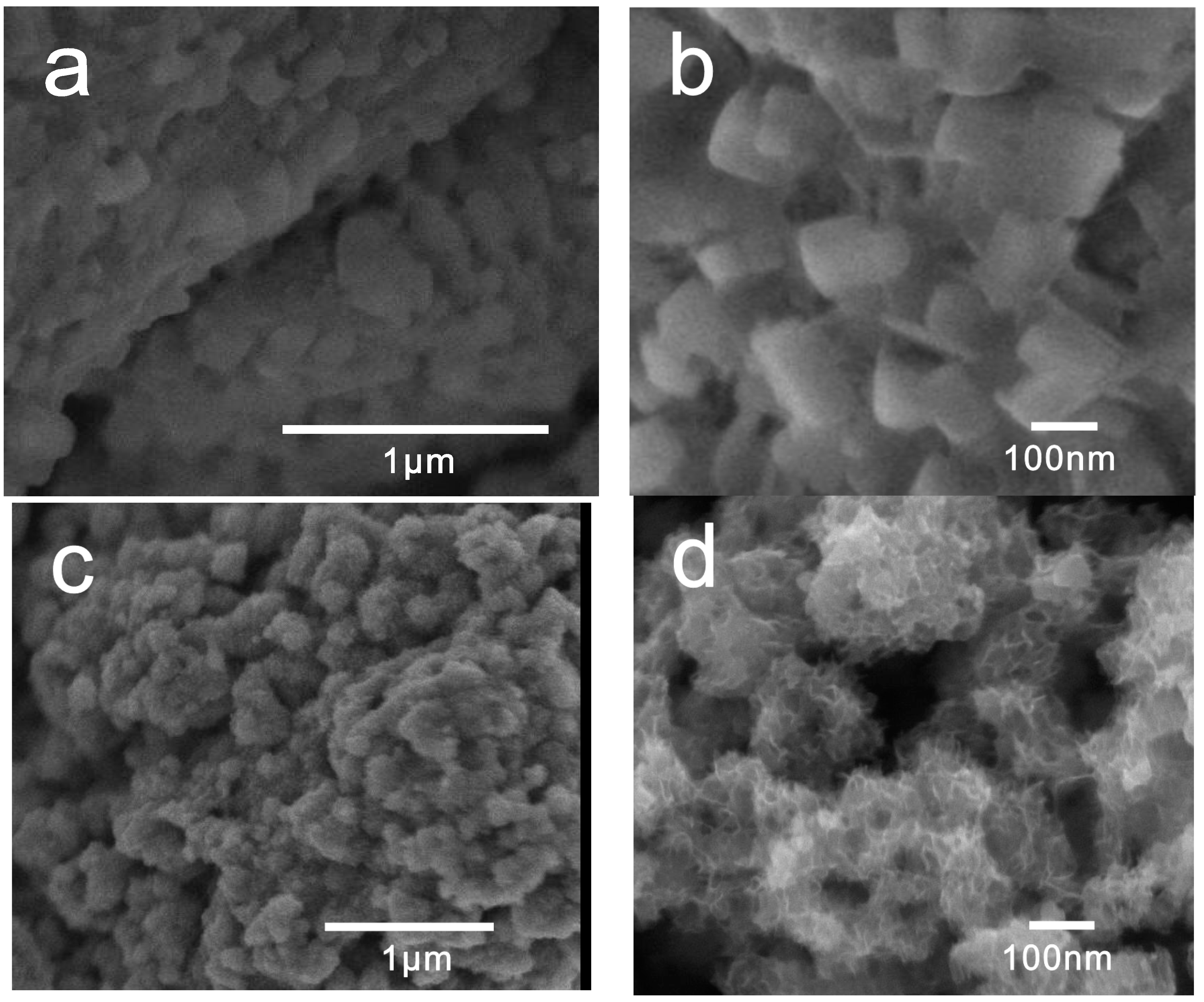
2.1. Phase Structures and Morphology
2.2. FTIR Analysis
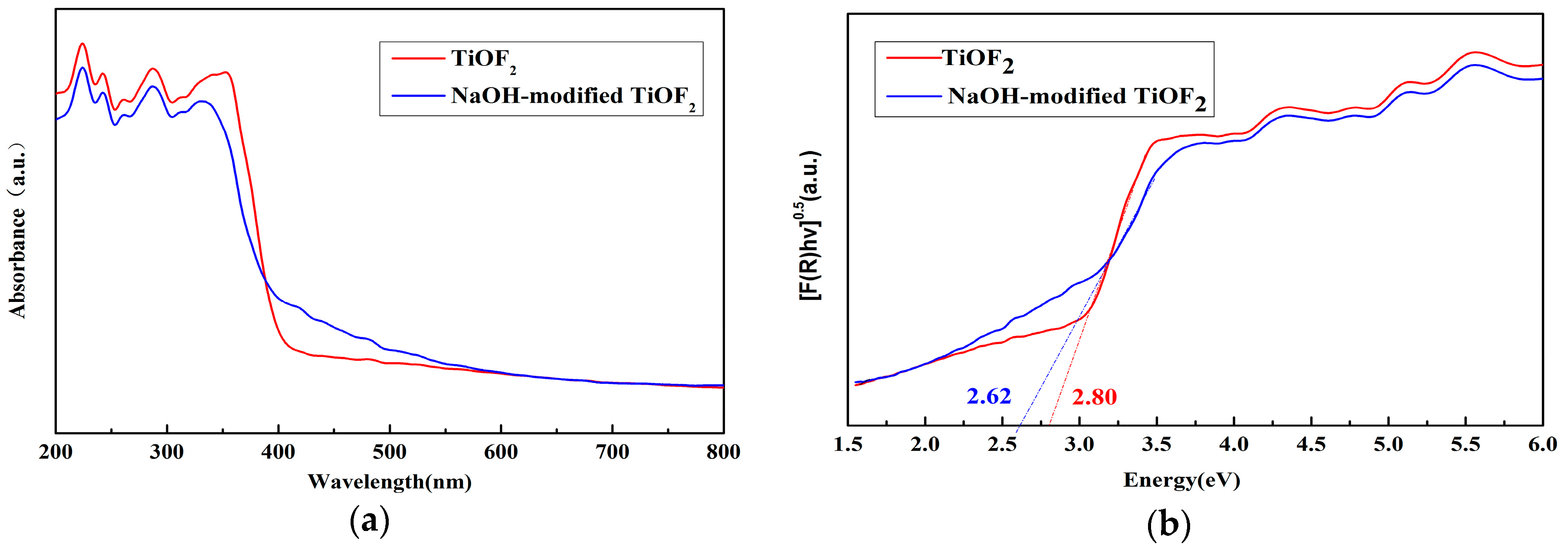
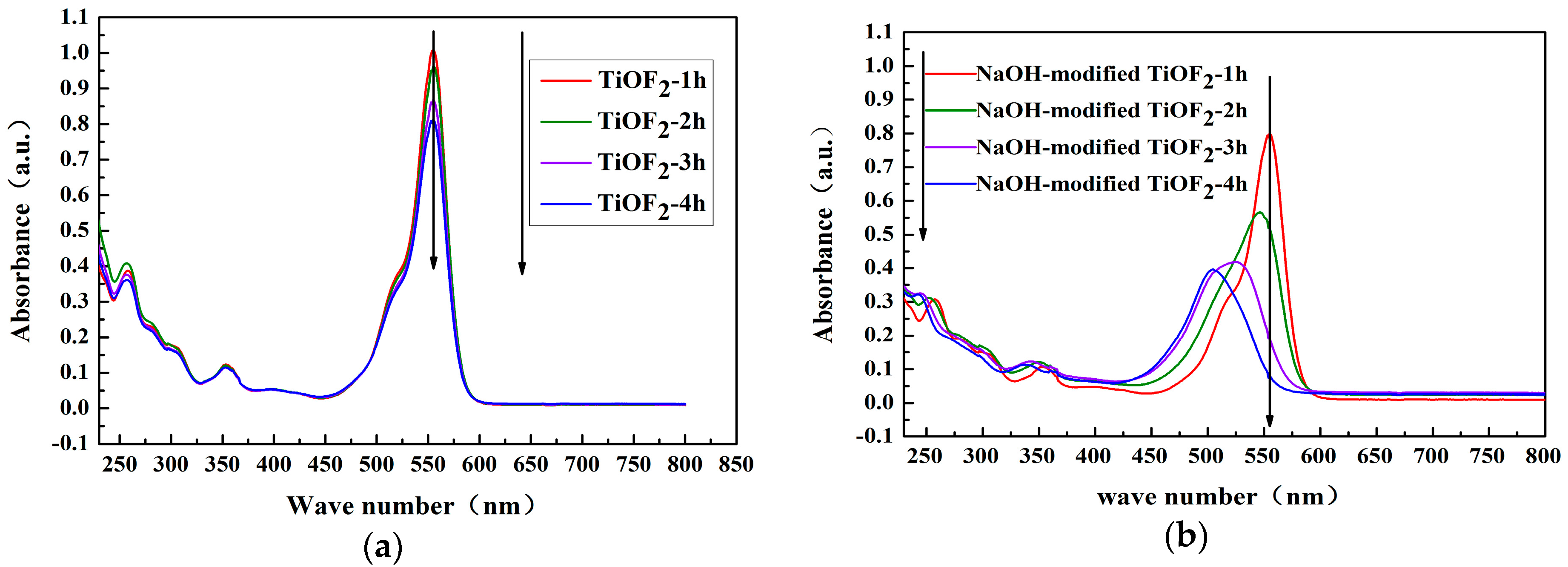
2.3. UV–Vis Analysis
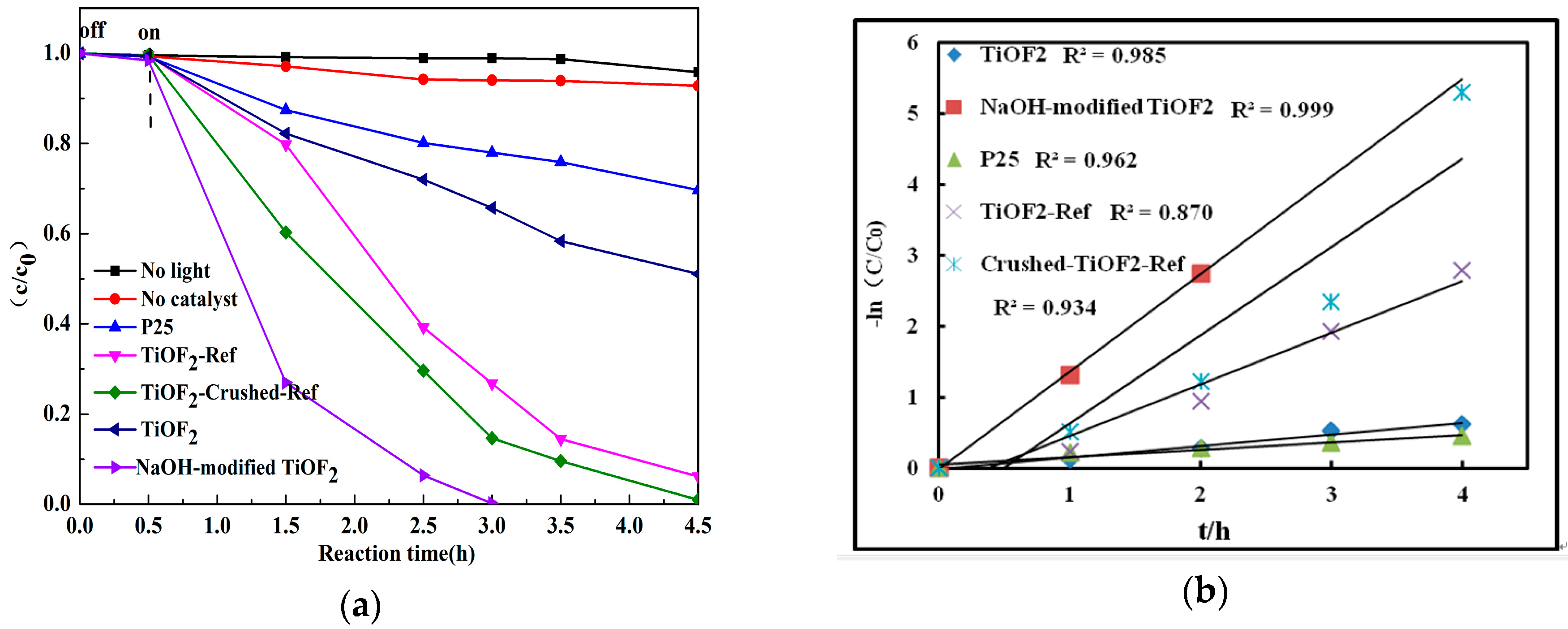
2.4. Catalytic Activity
2.5. Effect of the Sensitization Mechanism
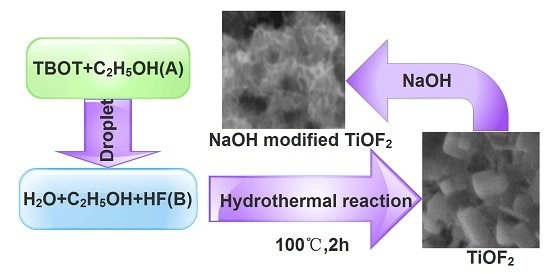
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Zuo, S.; Jiang, Z.; Liu, W.; Yao, C.; Chen, Q.; Liu, X. Synthesis and Characterization of Urchin-like Mischcrystal TiO2 and Its Photocatalysis. Mater. Charact. 2014, 96, 177–182. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.; Shi, J.; Niu, C. Highly Crystallized C-Doped MesoporousAnatase TiO2 with Visible Light Photocatalytic Activity. Catalysts 2016, 6, 117. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Magdaleno, T.; Sow, C.-H.; Ramanujachary, K.V.; Rao, G.V.S.; Chowdari, B.V.R. (N,F)-Co-doped TiO2: Synthesis, anatase-rutile conversion and Li-cycling properties. CrystEngComm 2012, 14, 978–986. [Google Scholar] [CrossRef]
- Ramasami, A.K.; Reddy, M.V.; Balakrishna, G.R. Combustion synthesis and characterization of NiO nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 194–202. [Google Scholar] [CrossRef]
- Wang, J.; Cao, F.; Bian, Z.; Leung, M.K.H.; Li, H. Ultrafine single-crystal TiOF2 nanocubes with mesoporous structure, high activity and durability in visible light driven photocatalysis. Nanoscale 2014, 6, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ana, C.; Zhang, J. Synthesis of heterostructured Pd@TiO2/TiOF2 nanohybrids with enhanced photocatalytic performance. Mater. Res. Bull. 2016, 80, 337–343. [Google Scholar]
- Dongn, P.; Cui, E.; Hou, G.; Guan, R.; Zhang, Q. Synthesis and photocatalytic activity of Ag3PO4/TiOF2 composites with enhanced stability. Mater. Lett. 2015, 143, 20–23. [Google Scholar] [CrossRef]
- Lv, K.; Yu, J.; Cui, L.; Chen, S.; Li, M. Preparation of thermally stable anatase TiO2 photocatalyst from TiOF2 precursor and its photocatalytic activity. J. Alloys Compd. 2011, 509, 4557–4562. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Dai, Y.; Zhang, X.; Qin, X.; Li, Z.; Zheng, Z.; Cheng, H.; Guo, L. Topotactic transformation of single-crystalline TiOF2 nanocubes to ordered arranged 3D hierarchical TiO2 nanoboxes. CrystEngComm 2012, 14, 4578–4581. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Lv, K.; Zheng, Y.; Deng, K. Transformation of TiOF2 cube to a hollow nanobox assembly from anatase TiO2 nanosheets with exposed {001} facets via solvothermal strategy. ACS Appl. Mater. Interfaces 2013, 5, 8663–8669. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Z.; Hu, Q.H.; Guo, Y.N.; Gong, X.Q.; Qiao, S.Z.; Yang, H.G. From titanium oxydifluoride (TiOF2) to titania (TiO2): Phase transition and non-metal doping with enhanced photocatalytic hydrogen (H2) evolution properties. Chem. Commun. 2011, 47, 6138–6140. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kim, Y.; Lee, Y. Enhancement of the electrochemical capacitance of TiOF2 obtained via control of the crystal structure. J. Ind. Eng. Chem. 2017, 47, 187–193. [Google Scholar] [CrossRef]
- Louvain, N.; Karkar, Z.; El-Ghozzi, M.; Bonnet, P.; Gúerin, K.; Willmann, P. Fluorinationof anatase TiO2 towards titanium oxyfluoride TiOF2: A novel synthesis approach and proof of the Li-insertion mechanism. J. Mater. Chem. A 2014, 2, 15308–15315. [Google Scholar] [CrossRef]
- Li, W.; Body, M.; Legein, C.; Dambournet, D. Identify OH groups in TiOF2 and their impact on the lithium intercalation properties. J. Solid State Chem. 2017, 246, 113–118. [Google Scholar] [CrossRef]
- Chen, L.; Shen, L.; Nie, P.; Zhang, X.; Li, H. Facile hydrothermal synthesis of single crystalline TiOF2 nanocubes and their phase transitions to TiO2 hollow nanocages as anode materials for lithium-ion battery. Electrochim. Acta 2012, 62, 408–415. [Google Scholar] [CrossRef]
- Reddy, M.V.; Madhavi, S.; Rao, G.V.S.; Chowdari, B.V.R. Metal oxyfluorides TiOF2 and NbO2F as anodes for Li-ion batteries. J. Power Sources 2006, 162, 1312–1321. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Nie, L.; Jaroniec, M. Dual-dehydrogenation-promoted catalytic oxidation of formaldehyde on alkali-treated Pt clusters at room temperature. J. Mater. Chem. A 2015, 3, 10432–10438. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Li, X.; Cheng, B.; Liu, G.; Jaroniec, M. Enhanced Performance of NaOH-Modified Pt/TiO2 toward Room Temperature Selective Oxidation of Formaldehyde. Environ. Sci. Technol. 2013, 47, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Zampieri, A.; Schwieger, W. Mesoporous ZSM-5 zeolites via alkali treatment for the direct hydroxylation of benzene to phenol with N2O. J. Catal. 2008, 260, 193–197. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, F.; Liu, J.; Song, D.; Jia, C.; Chen, Y. Enhanced catalytic isomerization of α-pinene over mesoporous zeolite beta of low Si/Al ratio by NaOH treatment. Microporous Mesoporous Mater. 2012, 162, 168–174. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, K.; Wang, G.; Deng, K.; Tang, D. Study on the shape control and photocatalytic activity of high-energy anatasetitania. Appl. Catal. B Environ. 2010, 100, 378–385. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, Q.; Liu, J.; Qian, J.; Zhou, X. TiO2 nanoparticles embedded in hollow cube with highly exposed {001} facets: Facile synthesis and photovoltaic applications. J. Alloys Compd. 2016, 656, 863–870. [Google Scholar] [CrossRef]
- He, M.; Wang, Z.; Yan, X.; Tian, L.; Liu, G.; Chen, X. Hydrogenation effects on the lithium ion battery performance of TiOF2. J. Power Sources 2016, 306, 309–316. [Google Scholar] [CrossRef]
- Iwata, T.; Watanabe, A.; Iseki, M.; Watanabe, M.; Kandor, H. Strong Donation of the Hydrogen Bond of Tyrosine during Photoactivation of the BLUF Domain. J. Phys. Chem. Lett. 2011, 2, 1015–1019. [Google Scholar] [CrossRef]
- Ning, Y. Structural Identification of Organic Compounds and Organic Spectroscopy; Science Press: Beijing, China, 2000; ISBN 978-7-03-0074-126. [Google Scholar]
- Zhang, Y.; Zhang, Q.; Xia, T.; Zhu, D.; Chen, Y.; Chen, X. The influence of reaction temperature on the formation and photocatalytic hydrogen generation of (001) faceted TiO2 nanosheets. ChemNanoMat 2015, 1, 270–275. [Google Scholar] [CrossRef]
- Vaiano, V.; Sannino, D.; Almeida, A.R.; Mul, G.; Ciambelli, P. Investigation of the Deactivation Phenomena Occurring in the Cyclohexane Photocatalytic Oxidative Dehydrogenation on MoOx/TiO2 through Gas Phase and in situ DRIFTS Analyses. Catalysts 2013, 3, 978–997. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A.; Basirun, W.J.; Centi, G. Synergetic effects in novel hydrogenated F-doped TiO2 photocatalysts. Appl. Surf. Sci. 2016, 370, 380–393. [Google Scholar] [CrossRef]
- Ding, X.; Hong, Z.; Wang, Y.; Lai, R.; Wei, M. Synthesis of square-like anatase TiO2 nanocrystals based on TiOF2 quantum dots. J. Alloys Compd. 2013, 550, 475–478. [Google Scholar] [CrossRef]
- Nishikiori, H.; Hayashibe, M.; Fujii, T. Visible Light-Photocatalytic Activity of Sulfate-Doped Titanium Dioxide Prepared by the Sol−Gel Method. Catalysts 2013, 3, 363–377. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of Titanium Dioxide with Platinum Ions and Clusters: Application in Photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Photocatalytic Reactivities of Nafion-Coated TiO2 for the Degradation of Charged Organic Compounds under UV or Visible Light. J. Phys. Chem. B 2005, 109, 11667–11674. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Liu, W.; Zhu, J. Synthesis of NaOH-Modified TiOF2 and Its Enhanced Visible Light Photocatalytic Performance on RhB. Catalysts 2017, 7, 243. https://doi.org/10.3390/catal7080243
Hou C, Liu W, Zhu J. Synthesis of NaOH-Modified TiOF2 and Its Enhanced Visible Light Photocatalytic Performance on RhB. Catalysts. 2017; 7(8):243. https://doi.org/10.3390/catal7080243
Chicago/Turabian StyleHou, Chentao, Wenli Liu, and Jiaming Zhu. 2017. "Synthesis of NaOH-Modified TiOF2 and Its Enhanced Visible Light Photocatalytic Performance on RhB" Catalysts 7, no. 8: 243. https://doi.org/10.3390/catal7080243