Exploiting the Versatility of Aminated Supports Activated with Glutaraldehyde to Immobilize β-galactosidase from Aspergillus oryzae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization of β-gal on MANAE-Agarose at Different pH Values
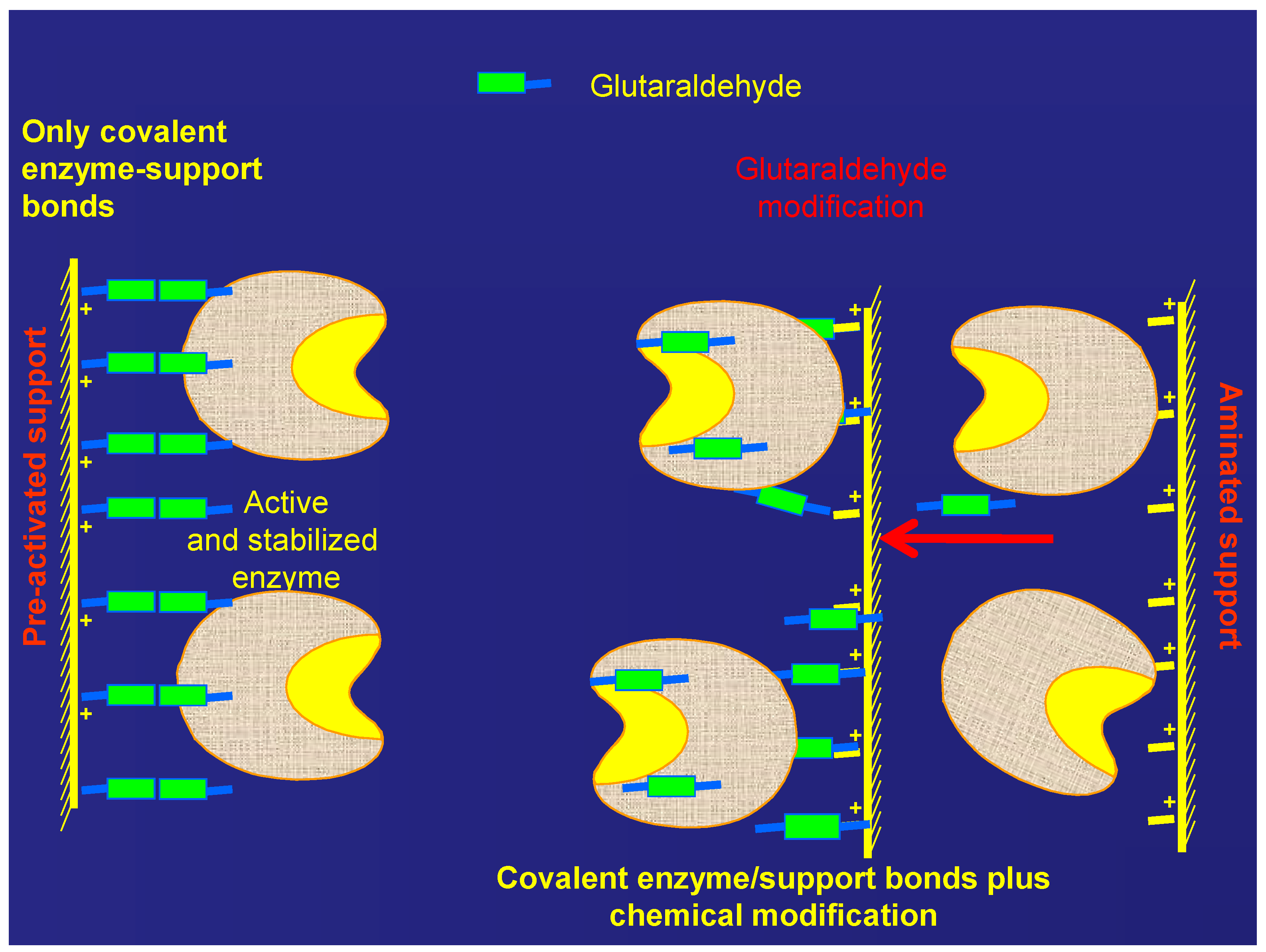
2.2. Treatment of the MANAE-Agarose Immobilized Enzyme with Glutaraldehyde
2.3. Immobilization of β-gal on Glutaraldehyde Pre-Activated Supports
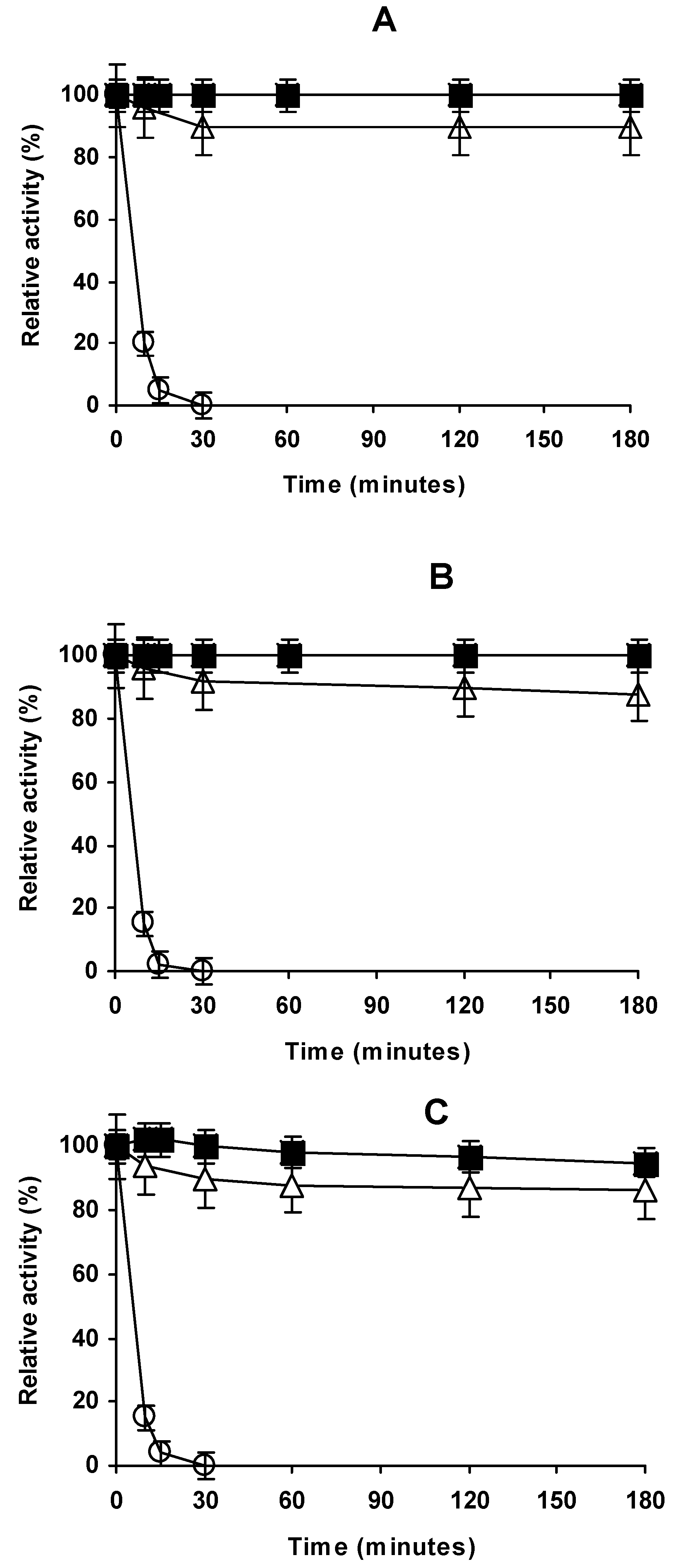
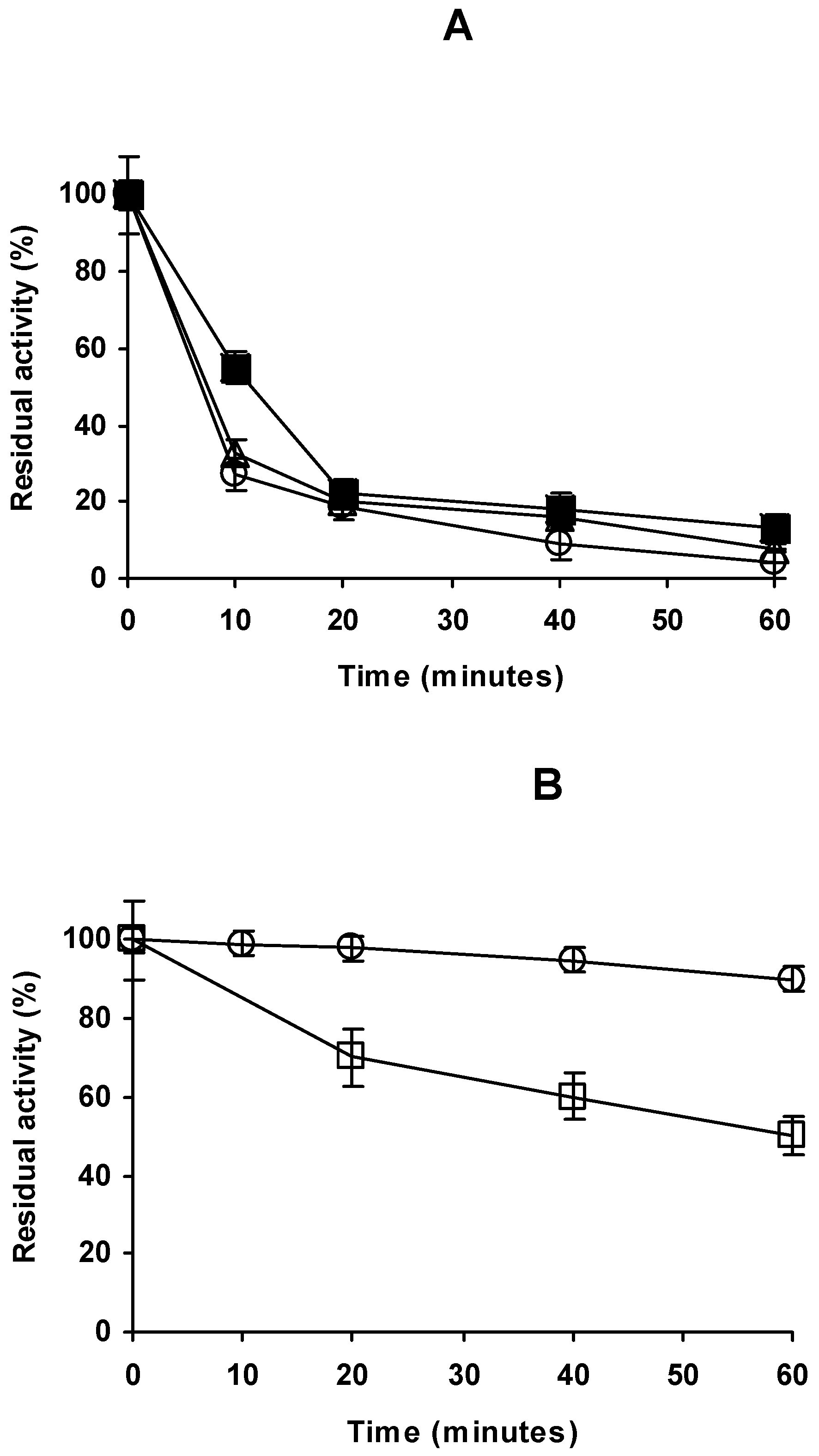
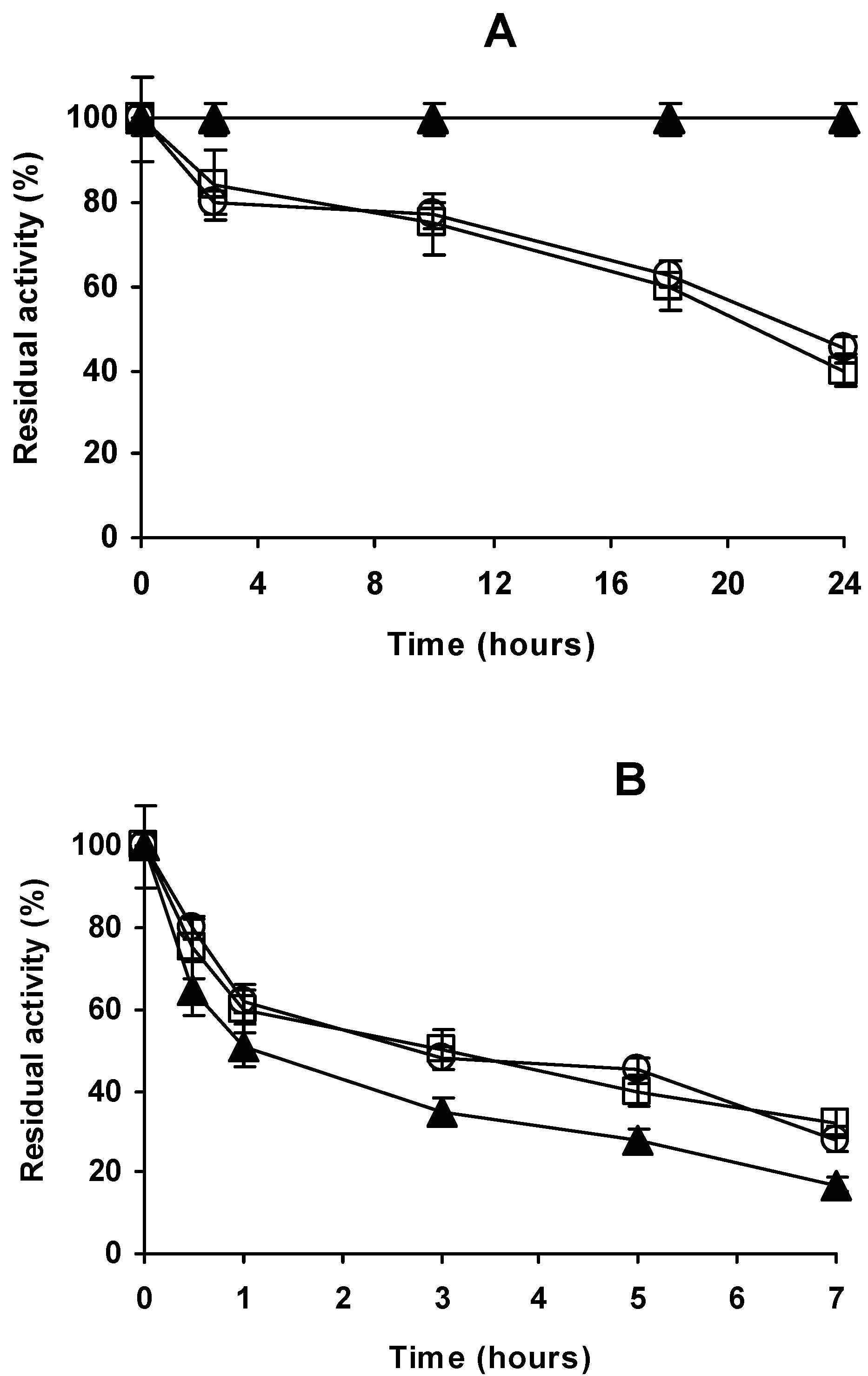
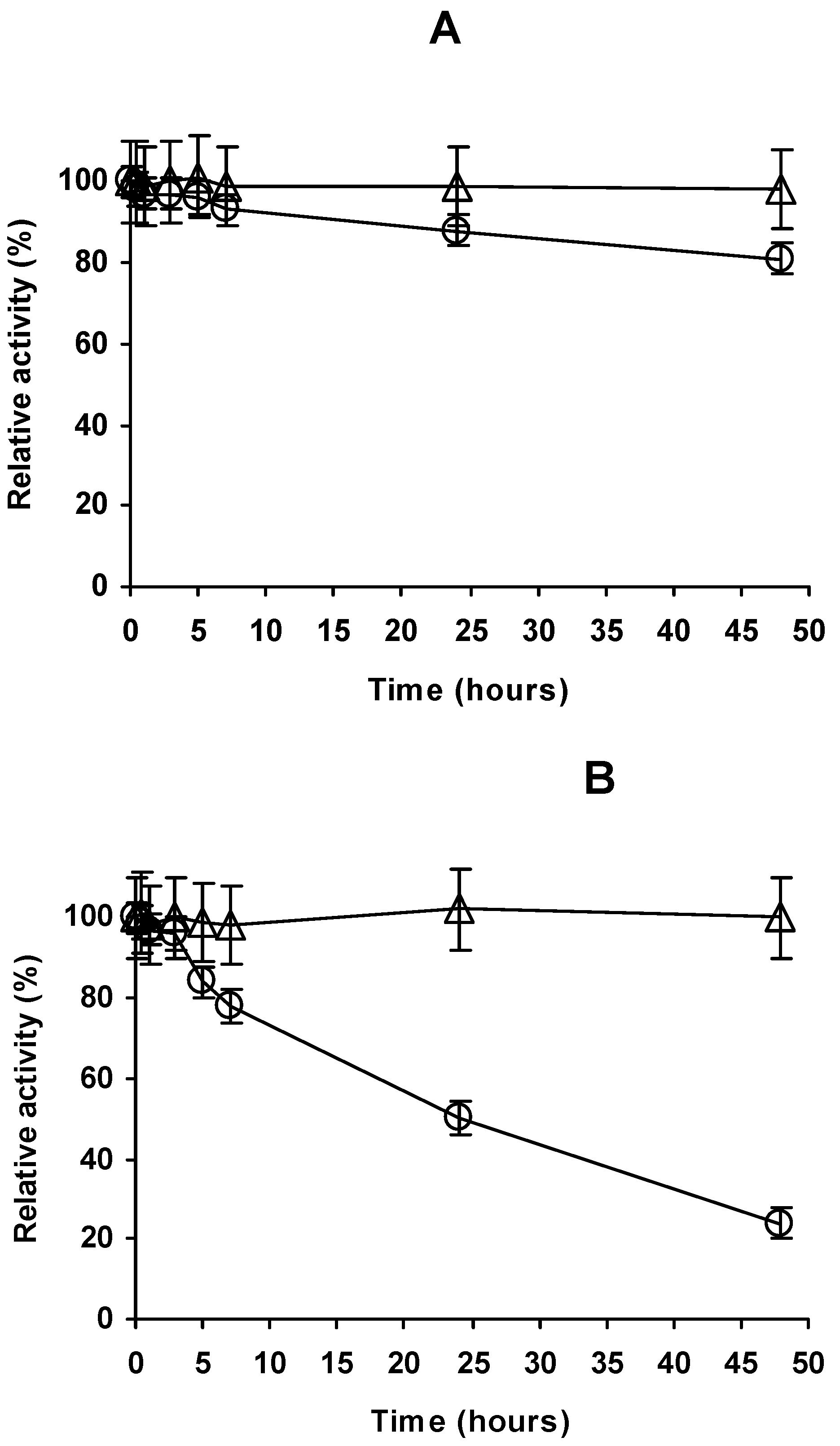
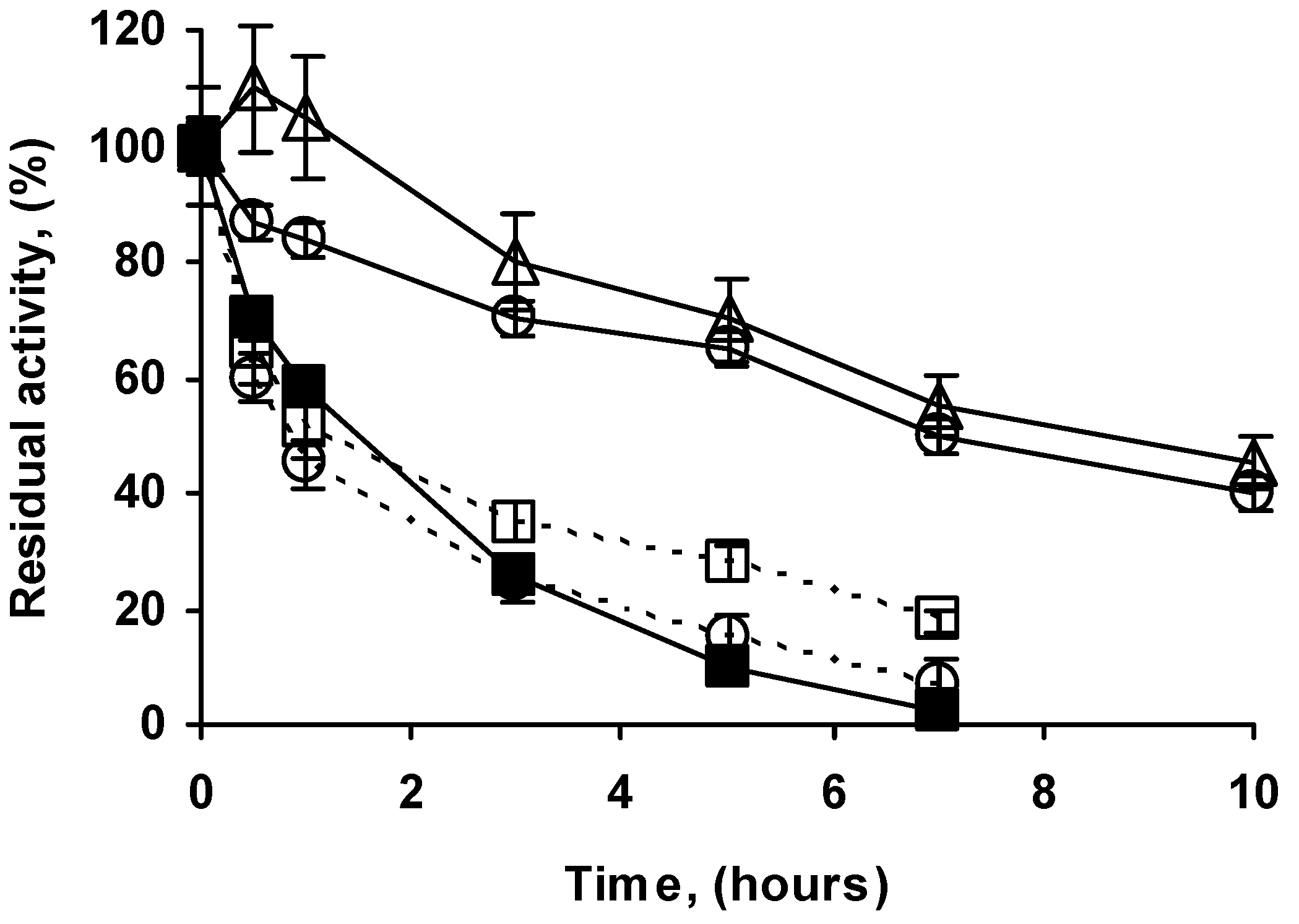
2.4. Stability of the Different Preparations
3. Materials and Methods
3.1. Materials
3.2. Preparation of Glutaraldehyde Agarose Beads
3.3. Standard Determination of Enzyme Activity
3.4. Immobilization of β-Galactosidase on Different Supports
3.5. Thermal Stability of the Enzyme Preparations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dicosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, R.A.; Van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F. Conformational changes of enzymes upon immobilization. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Maksimainen, M.M.; Lampio, A.; Mertanen, M.; Turunen, O.; Rouvinen, J. The crystal structure of acidic β-galactosidase from Aspergillus oryzae. Int. J. Biol. Macromol. 2013, 60, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Guidini, C.Z.; Santana, L.N.S.; de Resende, M.M.; Cardoso, V.L.; Ribeiro, E.J. Optimization and modeling of lactose hydrolysis in a packed bed system using immobilized β-galactosidase from Aspergillus oryzae. J. Mol. Catal. B Enzym. 2013, 85–86, 178–186. [Google Scholar] [CrossRef]
- Urrutia, P.; Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Wilson, L.; Illanes, A.; Plou, F.J. Detailed analysis of galactooligosaccharides synthesis with β-galactosidase from Aspergillus oryzae. J. Agric. Food Chem. 2013, 61, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Guerrero, C.; Illanes, A.; Conejeros, R. Fed-batch synthesis of galacto-oligosaccharides with Aspergillus oryzae β-galactosidase using optimal control strategy. Biotechnol. Prog. 2014, 30, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Carević, M.; Veličković, D.; Stojanović, M.; Milosavić, N.; Rogniaux, H.; Ropartz, D.; Bezbradica, D. Insight in the regioselective enzymatic transgalactosylation of salicin catalyzed by β-galactosidase from Aspergillus oryzae. Process Biochem. 2015, 50, 782–788. [Google Scholar] [CrossRef]
- Porciúncula González, C.; Castilla, A.; Garófalo, L.; Soule, S.; Irazoqui, G.; Giacomini, C. Enzymatic synthesis of 2-aminoethyl β-d-galactopyranoside catalyzed by Aspergillus oryzae β-galactosidase. Carbohydr. Res. 2013, 368, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Guerrero, C.; Wilson, L.; Illanes, A. Synthesis of propyl-β-d-galactoside with free and immobilized β-galactosidase from Aspergillus oryzae. Process Biochem. 2017, 53, 162–171. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzyme Microb. Technol. 2007, 39, 877–882. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisán, J.M.; Fernández-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Monsan, P. Optimization of glutaraldehyde activation of a support for enzyme immobilization. J. Mol. Catal. 1978, 3, 371–384. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernández-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Wine, Y.; Cohen-Hadar, N.; Freeman, A.; Frolow, F. Elucidation of the mechanism and end products of glutaraldehyde crosslinking reaction by X-ray structure analysis. Biotechnol. Bioeng 2007, 98, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernández-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Guisán, J.M. Strategies for enzyme stabilization by intramolecular crosslinking with bifunctional reagents. Enzyme Microb. Technol. 1995, 17, 517–523. [Google Scholar] [CrossRef]
- De Albuquerque, T.L.; Peirce, S.; Rueda, N.; Marzocchella, A.; Gonçalves, L.R.B.; Rocha, M.V.P.; Fernández-Lafuente, R. Ion exchange of β-galactosidase: The effect of the immobilization pH on enzyme stability. Process Biochem. 2016, 51, 875–880. [Google Scholar] [CrossRef]
- Grazú, V.; López-Gallego, F.; Montes, T.; Abian, O.; González, R.; Hermoso, J.A.; García, J.L.; Mateo, C.; Guisán, J.M. Promotion of multipoint covalent immobilization through different regions of genetically modified penicillin G acylase from E. coli. Process Biochem. 2010, 45, 390–398. [Google Scholar] [CrossRef]
- Mansfeld, J.; Vriend, G.; Van den Burg, B.; Eijsink, V.G.H.; Ulbrich-Hofmann, R. Probing the unfolding region in a thermolysin-like protease by site- specific immobilization. Biochemistry 1999, 38, 8240–8245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfeld, J.; Ulbrich-Hofmann, R. Site-specific and random immobilization of thermolysin-like proteases reflected in the thermal inactivation kinetics. Biotechnol. Appl. Biochem. 2000, 32, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich-Hofmann, R.; Arnold, U.; Mansfeld, J. The concept of the unfolding region for approaching the mechanisms of enzyme stabilization. J. Mol. Catal. B Enzym. 1999, 7, 125–131. [Google Scholar] [CrossRef]
- Torres, R.; Mateo, C.; Fernández-Lorente, G.; Ortiz, C.; Fuentes, M.; Palomo, J.M.; Guisán, J.M.; Fernández-Lafuente, R. A novel heterofunctional epoxy-amino sepabeads for a new enzyme immobilization protocol: Immobilization-stabilization of β-galactosidase from Aspergillus oryzae. Biotechnol. Prog. 2003, 19, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Fernández-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.M.; Guisán, J.M. Protecting effect of competitive inhibitors during very intense insolubilized enzyme-activated support multipoint attachments: trypsin (amine)-agarose (aldehyde) system. Enzyme Microb. Technol. 1988, 10, 227–232. [Google Scholar] [CrossRef]
- Blanco, R.M.; Guisán, J.M. Stabilization of enzymes by multipoint covalent attachment to agarose-aldehyde gels. Borohydride reduction of trypsin-agarose derivatives. Enzyme Microb. Technol. 1988, 11, 360–366. [Google Scholar] [CrossRef]
- Zaak, H.; Kornecki, J.F.; Siar, E.H.; Fernández-Lopez, L.; Corberán, V.C.; Sassi, M.; Fernández-Lafuente, R. Coimmobilization of enzymes in bilayers using PEI as a glue to reuse the most stable enzyme: Preventing PEI release during inactivated enzyme desorption. Process Biochem. 2017. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernández-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernández-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazú, V.; Lopez-Gallego, F.; Pessela, B.C.C.; Hidalgo, A.; Fernández-Lorente, G.; Fernández-Lafuente, R.; et al. Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Fernández-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Santana, C.; Soler, G.; Bastida, A.; Guisán, J.M. Preparation of activated supports containing low pK amino groups. A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb. Technol 1993, 15, 546–550. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Jordá, J.L.; Rey, F.; Fernandez-Lafuente, R.; Guisan, J.M.; Mateo, C. Electrostatic and covalent immobilisation of enzymes on ITQ-6 delaminated zeolitic materials. Chem. Commun. 2001, 5, 419–420. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaak, H.; Peirce, S.; De Albuquerque, T.L.; Sassi, M.; Fernandez-Lafuente, R. Exploiting the Versatility of Aminated Supports Activated with Glutaraldehyde to Immobilize β-galactosidase from Aspergillus oryzae. Catalysts 2017, 7, 250. https://doi.org/10.3390/catal7090250
Zaak H, Peirce S, De Albuquerque TL, Sassi M, Fernandez-Lafuente R. Exploiting the Versatility of Aminated Supports Activated with Glutaraldehyde to Immobilize β-galactosidase from Aspergillus oryzae. Catalysts. 2017; 7(9):250. https://doi.org/10.3390/catal7090250
Chicago/Turabian StyleZaak, Hadjer, Sara Peirce, Tiago L. De Albuquerque, Mohamed Sassi, and Roberto Fernandez-Lafuente. 2017. "Exploiting the Versatility of Aminated Supports Activated with Glutaraldehyde to Immobilize β-galactosidase from Aspergillus oryzae" Catalysts 7, no. 9: 250. https://doi.org/10.3390/catal7090250




