The Role of Non-Framework Lewis Acidic Al Species of Alkali-Treated HZSM-5 in Methanol Aromatization
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Characterization
3.3. Reactivity Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Musilová, Z.; Žilková, N.; Park, S.E.; Čejka, J. Aromatic transformations over mesoporous zsm-5: Advantages and disadvantages. Top. Catal. 2010, 53, 1457–1469. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, K.; Christensen, C.H.; Kustova, M.; Christensen, C.H. Templating mesoporous zeolites. Chem. Mater. 2008, 20, 946–960. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C.H. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Verboekend, D.; Perez-Ramirez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef]
- Mitchell, S.; Pinar, A.B.; Kenvin, J.; Crivelli, P.; Kärger, J.; Pérez-Ramírez, J. Structural analysis of hierarchically organized zeolites. Nat. Commun. 2015, 6, 8633. [Google Scholar] [CrossRef] [PubMed]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; de Jong, K.P.; Zecevic, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, J.; Mitchell, S.; Verboekend, D.; Milina, M.; Michels, N.-L.; Krumeich, F.; Marti, N.; Erdmann, M. Expanding the horizons of hierarchical zeolites: Beyond laboratory curiosity towards industrial realization. ChemCatChem 2011, 3, 1731–1734. [Google Scholar] [CrossRef]
- Liang, T.; Chen, J.; Qin, Z.; Li, J.; Wang, P.; Wang, S.; Wang, G.; Dong, M.; Fan, W.; Wang, J. Conversion of methanol to olefins over h-zsm-5 zeolite: Reaction pathway is related to the framework aluminum siting. ACS Catal. 2016, 6, 7311–7325. [Google Scholar] [CrossRef]
- Lietz, G.; Schnabel, K.H.; Peuker, C.; Gross, T.; Storek, W.; Völter, J. Modifications of H-ZSM-5 catalysts by naoh treatment. J. Catal. 1994, 148, 562–568. [Google Scholar] [CrossRef]
- Holm, M.S.; Svelle, S.; Joensen, F.; Beato, P.; Christensen, C.H.; Bordiga, S.; Bjørgen, M. Assessing the acid properties of desilicated zsm-5 by ftir using co and 2,4,6-trimethylpyridine (collidine) as molecular probes. Appl. Catal. A 2009, 356, 23–30. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, X.; Song, Y.; Wang, Q.; Xu, L. An effective method to enhance the stability on-stream of butene aromatization: Post-treatment of zsm-5 by alkali solution of sodium hydroxide. Appl. Catal. A 2006, 302, 69–77. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Effect of desilication of h-zsm-5 by alkali treatment on catalytic performance in hexane cracking. Appl. Catal. A 2012, 449, 188–197. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Spangsberg Holm, M.; Olsbye, U.; Lillerud, K.-P.; Svelle, S. Methanol to gasoline over zeolite h-zsm-5: Improved catalyst performance by treatment with naoh. Appl. Catal. A 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Lai, P.-C.; Chen, C.-H.; Lee, C.-H.; Lin, Y.-C. Methanol conversion to aromatics over ga–supported hzsm-5 with evolved meso- and microporosities by desilication. ChemistrySelect 2016, 1, 6335–6344. [Google Scholar] [CrossRef]
- Lai, P.-C.; Chen, C.-H.; Hsu, H.-Y.; Lee, C.-H.; Lin, Y.-C. Methanol aromatization over ga-doped desilicated hzsm-5. RSC Adv. 2016, 6, 67361–67371. [Google Scholar] [CrossRef]
- Groen, J.C.; Pérez-Ramı́rez, J. Critical appraisal of mesopore characterization by adsorption analysis. Appl. Catal. A 2004, 268, 121–125. [Google Scholar] [CrossRef]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Pérez-Ramírez, J. Full compositional flexibility in the preparation of mesoporous mfi zeolites by desilication. J. Phys. Chem. C 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Shiralkar, V.P.; Kotasthanc, A.N.; Borade, R.B.; Ratnasamy, P. Studies in the synthesis of zsm-5 zeolites. Zeolites 1982, 2, 313–318. [Google Scholar] [CrossRef]
- Brunner, E.; Ernst, H.; Freude, D.; Fröhlich, T.; Hunger, M.; Pfeifer, H. Magic-angle-spinning nmr studies of acid sites in zeolite H-ZSM-5. J. Catal. 1991, 127, 34–41. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez-Ramírez, J. Mechanism of hierarchical porosity development in mfi zeolites by desilication: The role of aluminium as a pore-directing agent. Chem. Eur. J. 2005, 11, 4983–4994. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Pérez-Ramírez, J. Desilication mechanism revisited: Highly mesoporous all-silica zeolites enabled through pore-directing agents. Chem. Eur. J. 2011, 17, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M. Dealuminated zeolites. J. Mol. Catal. 1984, 27, 59–69. [Google Scholar] [CrossRef]
- Fanchiang, W.-L.; Lin, Y.-C. Catalytic fast pyrolysis of furfural over H-ZSM-5 and ZN/H-ZSM-5 catalysts. Appl. Catal. A 2012, 419, 102–110. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Microporous Mesoporous Mater. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Meshram, N.R.; Hegde, S.G.; Kulkarni, S.B. Active sites on ZSM-5 zeolites for toluene disproportionation. Zeolites 1986, 6, 434–438. [Google Scholar] [CrossRef]
- Woolery, G.L.; Kuehl, G.H.; Timken, H.C.; Chester, A.W.; Vartuli, J.C. On the nature of framework brønsted and Lewis acid sites in ZSM-5. Zeolites 1997, 19, 288–296. [Google Scholar] [CrossRef]
- Hunger, B.; Matysik, S.; Heuchel, M.; Einicke, W.-D. Adsorption of methanol on ZSM-5 zeolites. Langmuir 1997, 13, 6249–6254. [Google Scholar] [CrossRef]
- Schulz, H.B.; Böhringer, W.; Zhao, S. Comparison of shape selectivity of coke formation in different zeolites. In Proceedings of the 9th International Zeolite Conference, Montreal, QC, Canada, 5–10 July 1992; von Ballmoos, R., Higgins, J.B., Treacy, M.M.J., Eds.; Butterworth-Heinemann: Montreal, QC, Canada, 1993; p. 567. [Google Scholar]
- Ono, Y.; Mori, T. Mechanism of methanol conversion into hydrocarbons over ZSM-5 zeolite. J. Chem. Soc. Faraday Trans. 1 1981, 77, 2209–2221. [Google Scholar] [CrossRef]
- Schulz, H.; Siwei, Z.; Kusterer, H. Autocatalysis, retardation, reanimation and deactivation during methanol conversion on zeolite HZSM-5. Stud. Surf. Sci. Catal. 1991, 60, 281–290. [Google Scholar]
- Sun, X.; Mueller, S.; Liu, Y.; Shi, H.; Haller, G.L.; Sanchez-Sanchez, M.; van Veen, A.C.; Lercher, J.A. On reaction pathways in the conversion of methanol to hydrocarbons on HZSM-5. J. Catal. 2014, 317, 185–197. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef] [PubMed]
- Teketel, S.; Westgard Erichsen, M.; Lonstad Bleken, F.; Svelle, S.; Petter Lillerud, K.; Olsbye, U. Shape Selectivity in Zeolite Catalysis. The Methanol to Hydrocarbons (mth) Reaction; The Royal Society of Chemistry: London, UK, 2014; Volume 26, pp. 179–217. [Google Scholar]
- Ilias, S.; Khare, R.; Malek, A.; Bhan, A. A descriptor for the relative propagation of the aromatic- and olefin-based cycles in methanol-to-hydrocarbons conversion on H-ZSM-5. J. Catal. 2013, 303, 135–140. [Google Scholar] [CrossRef]
- NIST Chemistry Webbook. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C67561&Units=SI&Mask=200 (accessed on 20 July 2017).
- Derouane, E.G.; Abdul Hamid, S.B.; Ivanova, I.I.; Blom, N.; Højlund-Nielsen, P.-E. Thermodynamic and mechanistic studies of initial stages in propane aromatisation over ga-modified H-ZSM-5 catalysts. J. Mol. Catal. 1994, 86, 371–400. [Google Scholar] [CrossRef]
- Schulz, H. “Coking” of zeolites during methanol conversion: Basic reactions of the mto-, mtp- and mtg processes. Catal. Today 2010, 154, 183–194. [Google Scholar] [CrossRef]
- Yun, J.H. Structure and Reactivity of Dehydroxylated Bronsted Acid Sites in H-ZSM-5 Zeolite: Generation of Stable Organic Radical Cation and Catalytic Activity for Isobutane Conversion. Mater’s Thesis, University of Delaware, Newark, NJ, USA, 2011. [Google Scholar]
- Khare, R.; Bhan, A. Mechanistic studies of methanol-to-hydrocarbons conversion on diffusion-free mfi samples. J. Catal. 2015, 329, 218–228. [Google Scholar] [CrossRef]
- Stöcker, M. Methanol-to-hydrocarbons: Catalytic materials and their behavior. Microporous Mesoporous Mater. 1999, 29, 3–48. [Google Scholar] [CrossRef]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K.; et al. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Cai, D.; Wang, Q.; Jia, Z.; Ma, Y.; Cui, Y.; Muhammad, U.; Wang, Y.; Qian, W.; Wei, F. Equilibrium analysis of methylbenzene intermediates for a methanol-to-olefins process. Catal. Sci. Technol. 2016, 6, 1297–1301. [Google Scholar] [CrossRef]
- Chang, C.D. Hydrocarbons from methanol. Catal. Rev. 1983, 25, 1–118. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified ZN/P/ZSM-5 catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
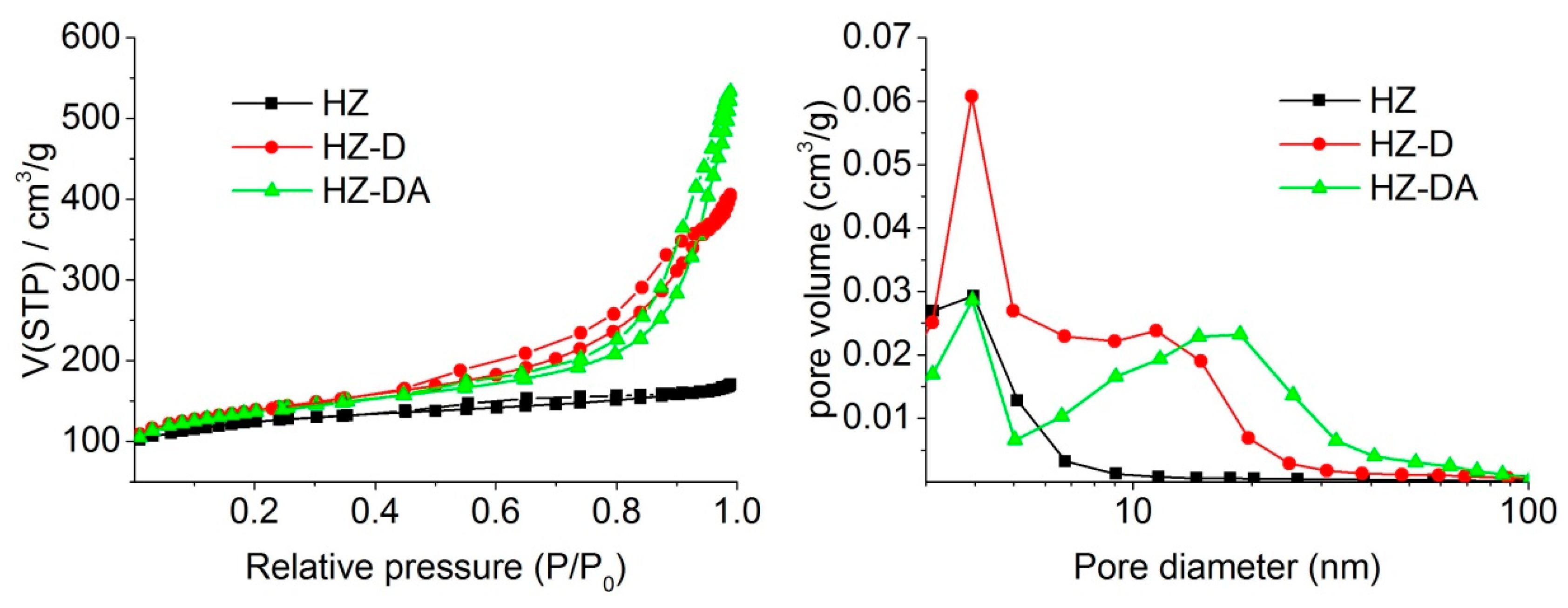
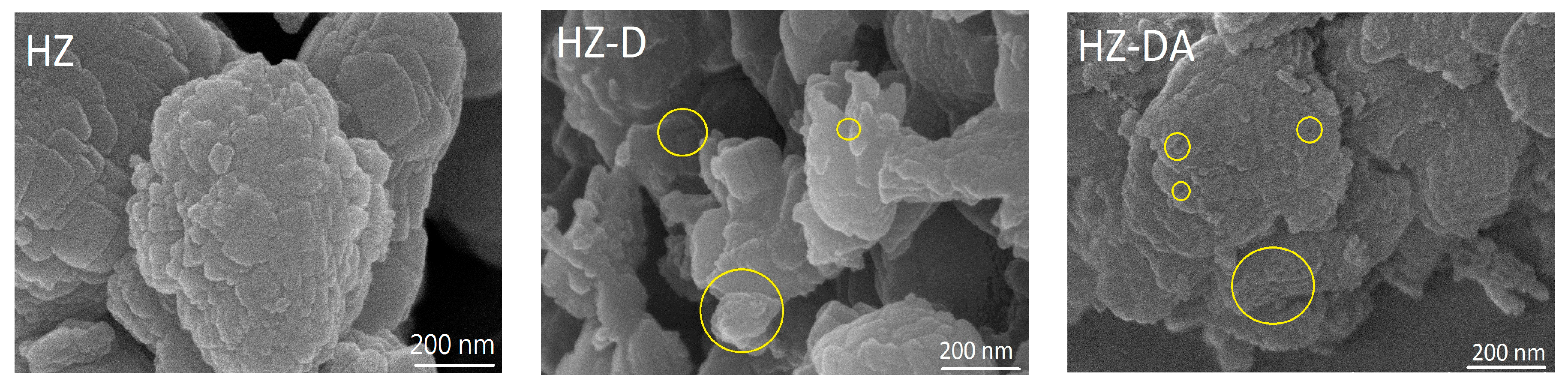
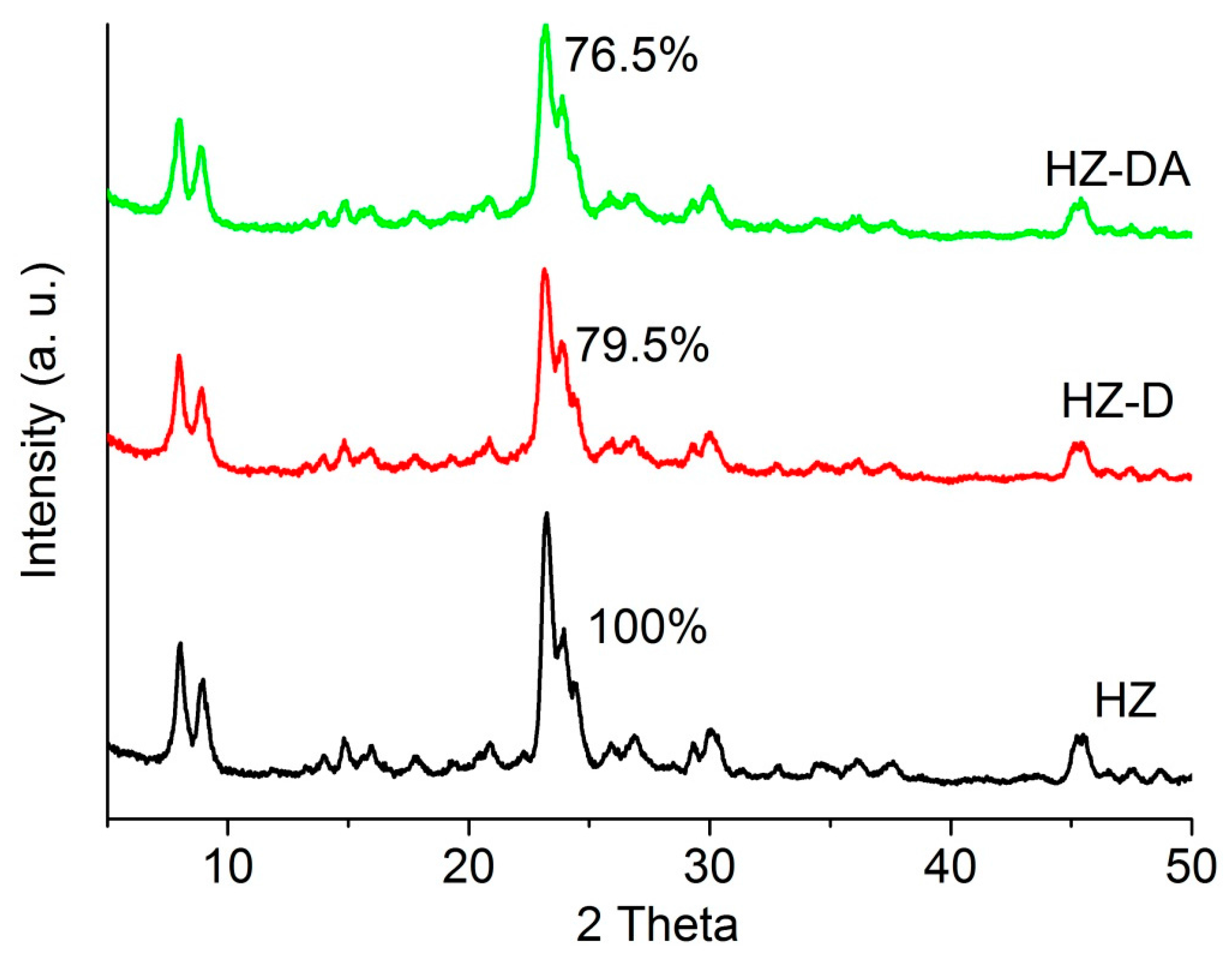
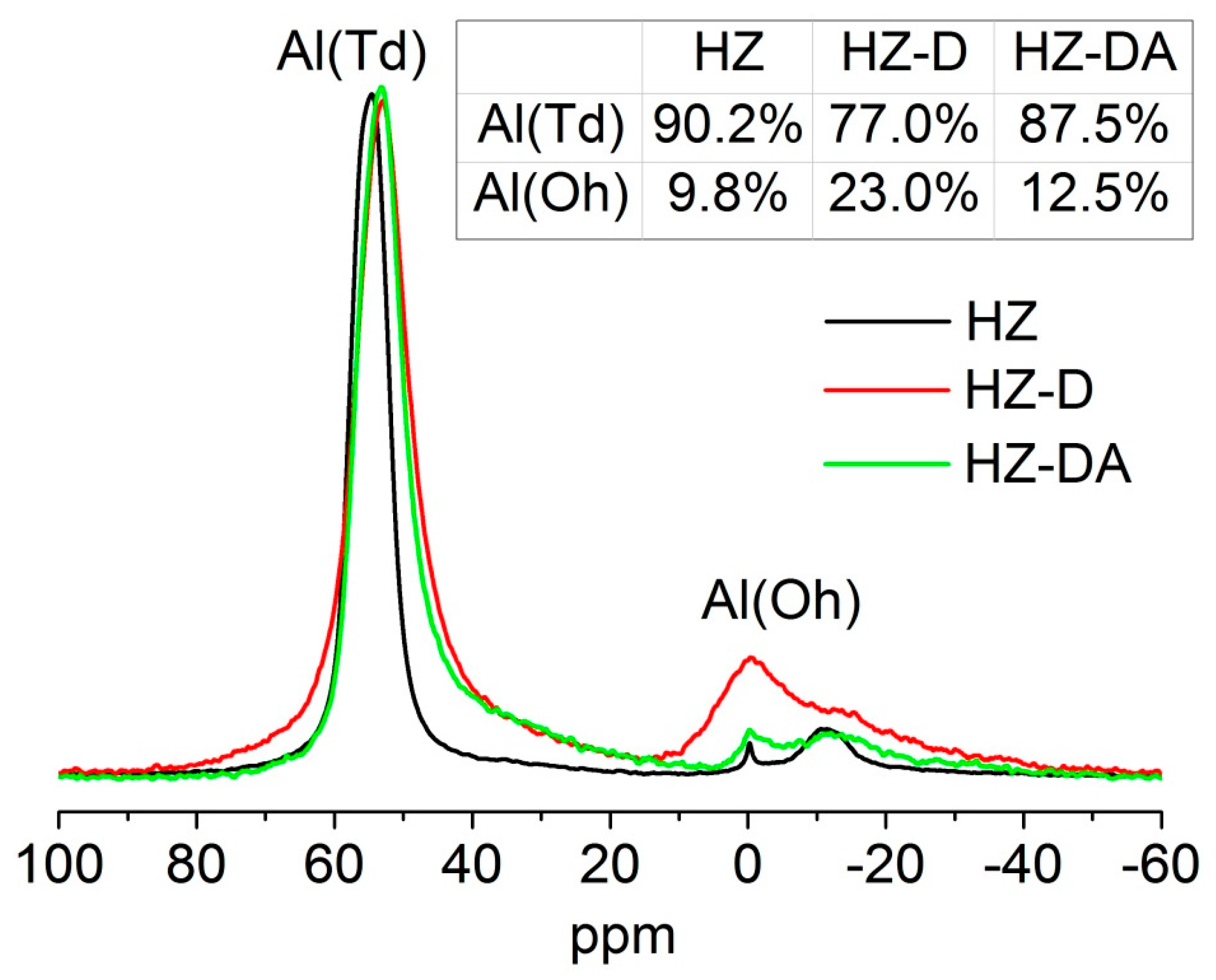
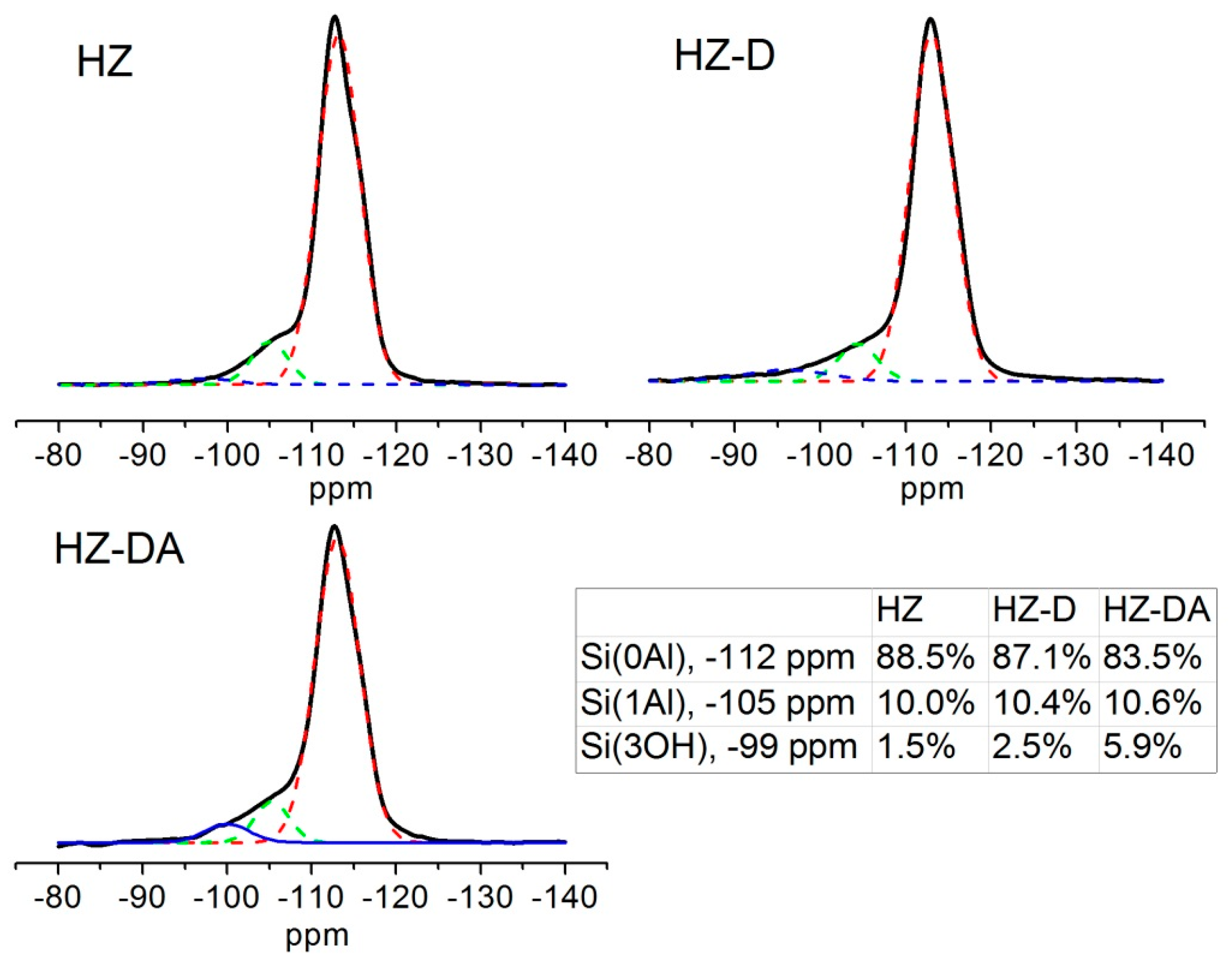
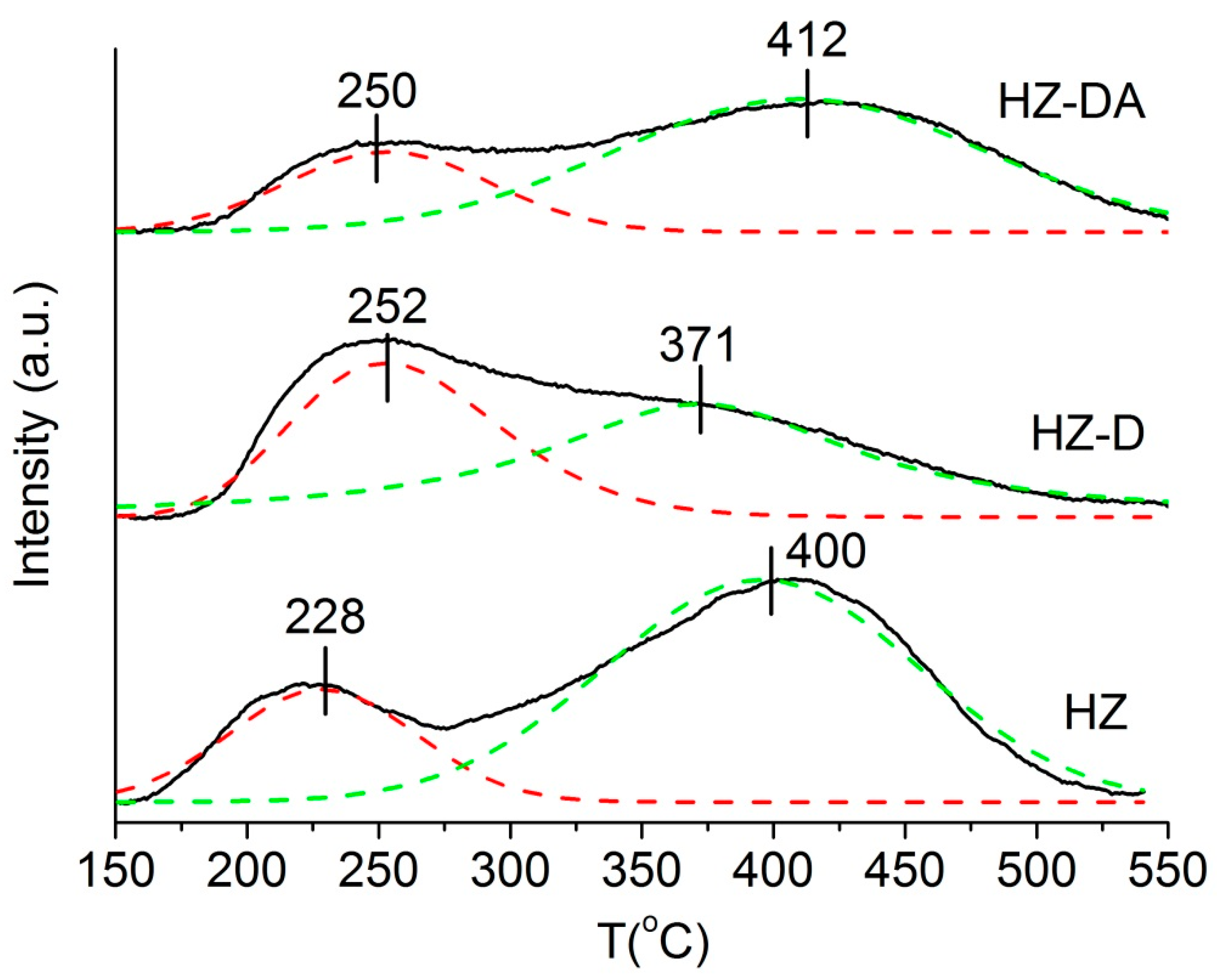
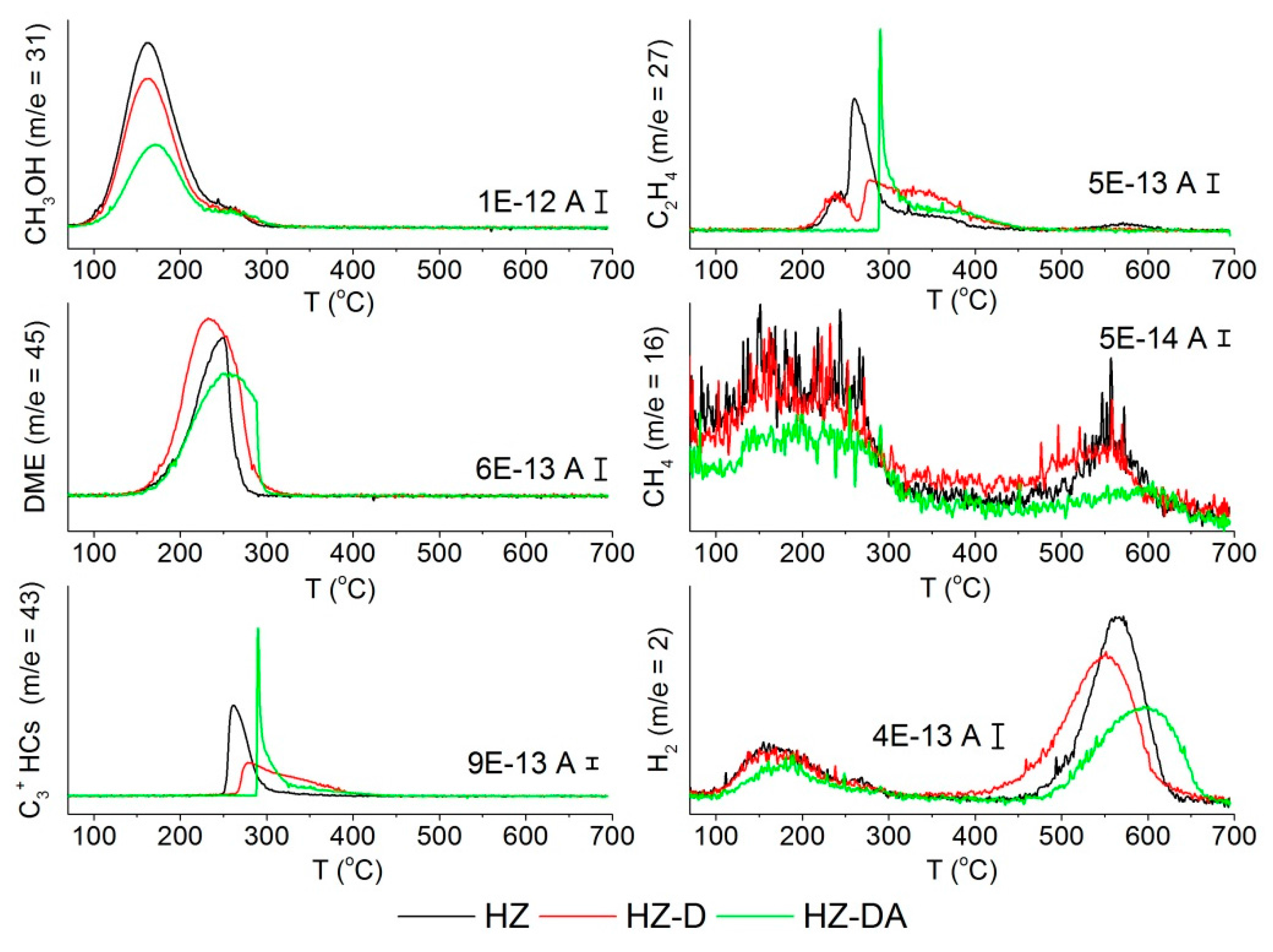
| Catalyst | SBET (m2/g) | Smicro 1 (m2/g) | Smeso 2 (m2/g) | Vtotal 3 (cm3/g) | Vmicro 1 (cm3/g) | Vmeso 4 (cm3/g) |
|---|---|---|---|---|---|---|
| HZ | 519.4 | 296.3 | 223.2 | 0.299 | 0.123 | 0.176 |
| HZ-D | 502.2 | 252.2 | 250.0 | 0.610 | 0.105 | 0.505 |
| HZ-DA | 493.4 | 241.5 | 251.9 | 0.769 | 0.103 | 0.666 |
| Catalyst | (Si/Al)F 1 | (Si/Al)B 2 | Lewis (mmol/g) | Brønsted (mmol/g) |
|---|---|---|---|---|
| HZ | 41 | 39 | 0.08 | 0.26 |
| HZ-D | 39 | 24 | 0.10 | 0.14 |
| HZ-DA | 38 | 37 | 0.05 | 0.15 |
| Catalyst | HZ | HZ-D | HZ-DA | |||
|---|---|---|---|---|---|---|
| W/F (kgcat * min/mol) | 0.24 | 0.49 | 0.24 | 0.49 | 0.17 | 0.49 |
| Conversion (%) | 99 | 100 | 99 | 100 | 99 | 100 |
| C yield (%) | ||||||
| C2= | 5 | 6 | 3 | 3 | 4 | 4 |
| C3= | 21 | 24 | 13 | 12 | 14 | 14 |
| C4–C7 | 32 | 38 | 31 | 29 | 36 | 33 |
| ABs | 40 | 31 | 52 | 55 | 45 | 48 |
| Others | 2 | 1 | 1 | 1 | 1 | 1 |
| Catalyst | HZ | HZ-D | HZ-DA | |||
|---|---|---|---|---|---|---|
| W/F (kgcat * min/mol) | 0.24 | 0.49 | 0.24 | 0.49 | 0.17 | 0.49 |
| Conversion (%) | 99 | 100 | 99 | 100 | 99 | 100 |
| Aromatic sel. (%) | ||||||
| Benzene | 2 | 3 | 0 | 2 | 2 | 2 |
| Toluene | 2 | 3 | 2 | 2 | 4 | 4 |
| p- + m-xylenes | 17 | 19 | 12 | 13 | 17 | 18 |
| o-xylene | 7 | 6 | 4 | 5 | 7 | 6 |
| C9 aromatics | 61 | 56 | 60 | 54 | 59 | 53 |
| Durene | 11 | 13 | 22 | 24 | 11 | 17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, P.-C.; Hsieh, C.-Y.; Chen, C.-H.; Lin, Y.-C. The Role of Non-Framework Lewis Acidic Al Species of Alkali-Treated HZSM-5 in Methanol Aromatization. Catalysts 2017, 7, 259. https://doi.org/10.3390/catal7090259
Lai P-C, Hsieh C-Y, Chen C-H, Lin Y-C. The Role of Non-Framework Lewis Acidic Al Species of Alkali-Treated HZSM-5 in Methanol Aromatization. Catalysts. 2017; 7(9):259. https://doi.org/10.3390/catal7090259
Chicago/Turabian StyleLai, Po-Chen, Chi-Ying Hsieh, Chao-Huang Chen, and Yu-Chuan Lin. 2017. "The Role of Non-Framework Lewis Acidic Al Species of Alkali-Treated HZSM-5 in Methanol Aromatization" Catalysts 7, no. 9: 259. https://doi.org/10.3390/catal7090259





