Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review
Abstract
:1. Introduction
2. The Poisoning Mechanism of Mn-Based Catalysts
2.1. The Poisoning Mechanism of H2O
2.2. The Poisoning Mechanism of SO2
2.3. The Effect on N2 Selectivity
3. Research Progress of Mn-Based Catalysts for Water and Sulfur Resistance
3.1. Single MnOx Catalysts
3.2. Mn-Based Multi-Metal Oxide Catalysts
3.2.1. Mn-Based Binary Metal Oxide Catalysts
3.2.2. Mn-Based Ternary Metal Oxide Catalysts
3.3. Supported Mn-Based Catalysts
3.3.1. TiO2 Supported Mn-Based Catalysts
3.3.2. Carbon Materials Supported Mn-Based Catalysts
3.3.3. Other Supported Mn-Based Catalysts
4. Strategies to Reduce the Poisoning Effect
4.1. Metal Modification
4.2. Proper Support
4.3. Combination of Metal Modification and Support
4.4. Rational Design of Structure and Morphology
4.5. Monolithic Catalysts
5. Conclusions and Perspectives
- (1)
- The exploration of novel Mn-based catalysts with excellent resistance to SO2 and H2O is still worthwhile. Resistance to SO2 and H2O directly decides whether this catalyst can be commercialized. Up to now, mixing (or doping) MnOx with suitable metal oxides and loading Mn-based active components on a suitable support are considered an efficient strategy. Discovering new doping elements and novel supports may be promising research directions.
- (2)
- The actual effect of every specific doping element on tolerance promotion needs to be explained. To date, many works have been done to test the tolerance of Mn-based catalysts to H2O and SO2. However, the reasons why the tolerance of Mn-based catalysts to H2O and SO2 can be enhanced by mixing (or doping) them with other suitable elements need to be further explored in detail.
- (3)
- The role of support ought to be further analyzed. Does support only provide a higher specific surface area and a good dispersion of Mn? Is the support involved in SCR reaction? Such questions need to be answered.
- (4)
- Long-term tolerance tests need to be conducted. Most tests only last for several hours, and it is hard to predict the long-term performance of the catalyst under H2O and SO2 poison.
- (5)
- N2 selectivity is an important indicator for the commercialization of SCR catalysts, which is closely related to the yield of N2O. However, there is currently a lack of research on the effect of SO2 on N2 selectivity over Mn-based catalysts. Therefore, it is necessary to carry out this research in the near future.
- (6)
- Most studies focus on powder catalysts. From a commercial perspective, monolithic catalysts should be given more consideration.
Acknowledgments
Conflicts of Interest
References
- Kapteijn, F.; Rodriguez-Mirasol, J.; Moulijn, J.A. Heterogeneous catalytic decomposition of nitrous oxide. Appl. Catal. B Environ. 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Grange, P.; Delmon, B. Catalytic removal of NO. Catal. Today 1998, 46, 233–316. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Boningari, T.; Koirala, R.; Smirniotis, P.G. Low-temperature selective catalytic reduction of NO with NH3 over V/ZrO2 prepared by flame-assisted spray pyrolysis: Structural and catalytic properties. Appl. Catal. B Environ. 2012, 127, 255–264. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Wang, H.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Chi Tsang, S. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y.; Wang, H.; Jin, R. DRIFT study of Manganese/Titania-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2007, 41, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Xu, W.; Li, J. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Tian, W.; Yang, H.; Fan, X.; Zhang, X. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature. J. Hazard. Mater. 2011, 188, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.; Niu, C. Rationally designed porous MnOx–FeOx nanoneedles for low-temperature selective catalytic reduction of NOx by NH3. ACS Appl. Mater. Interfaces 2017, 9, 16117–16127. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Shi, J.-W.; Fan, Z.; Gao, C.; Niu, C. MnM2O4 microspheres (M = Co, Cu, Ni) for selective catalytic reduction of NO with NH3: Comparative study on catalytic activity and reaction mechanism via in-situ diffuse reflectance infrared Fourier transform spectroscopy. Chem. Eng. J. 2017, 325, 91–100. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.-W.; Liu, C.; Gao, C.; Fan, Z.; Niu, C. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.-W.; Fan, Z.; Yu, Y.; Chen, J.; Li, Z.; Niu, C. Eu–Mn–Ti mixed oxides for the SCR of NOx with NH3: The effects of Eu-modification on catalytic performance and mechanism. Fuel Process. Technol. 2017, 167, 322–333. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.; Shi, J.; Li, B.; Gao, C.; Niu, C. MnOx–TiO2 and Sn doped MnOx–TiO2 selective reduction catalysts prepared using MWCNTs as the pore template. Chem. Eng. J. 2017, 327, 1–8. [Google Scholar] [CrossRef]
- Shi, J.-W.; Gao, C.; Liu, C.; Fan, Z.; Gao, G.; Niu, C. Porous MnOx for low-temperature NH3–SCR of NOx: The intrinsic relationship between surface physicochemical property and catalytic activity. J. Nanopart. Res. 2017, 19, 194–205. [Google Scholar] [CrossRef]
- Liu, C.; Gao, G.; Shi, J.-W.; He, C.; Li, G.; Bai, N.; Niu, C. MnOx–CeO2 shell-in-shell microspheres for NH3–SCR de-NOx at low temperature. Catal. Commun. 2016, 86, 36–40. [Google Scholar] [CrossRef]
- Loiland, J.A.; Lobo, R.F. Low temperature catalytic NO oxidation over microporous materials. J. Catal. 2014, 311, 412–423. [Google Scholar] [CrossRef]
- Kang, M.; Yeon, T.H.; Park, E.D.; Yie, J.E.; Kim, J.M. Novel MnOx catalysts for NO reduction at low temperature with ammonia. Catal. Lett. 2006, 106, 77–80. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. A superior catalyst for low-temperature NO reduction with NH3. Chem. Commun. 2003, 848–849. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn–Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Yao, X.; Kong, T.; Chen, L.; Ding, S.; Yang, F.; Dong, L. Enhanced low-temperature NH3–SCR performance of MnOx/CeO2 catalysts by optimal solvent effect. Appl. Surf. Sci. 2017, 420, 407–415. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T.; Chang, R. Low temperature selective catalytic reduction (SCR) of NO with NH3 over Fe–Mn based catalysts. Chem. Commun. 2002, 452–453. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-temperature selective catalytic reduction of NOx with NH3 over Fe–Mn mixed-oxide catalysts containing Fe3Mn3O8 Phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.; Huang, L.; Zhang, J.; Gao, R.; Zhang, D. Rational design of high-performance deNOx catalysts based on MnxCo3−xO4nanocages derived from metal–organic frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D. Low-temperature selective catalytic reduction of NO with NH3 over ordered mesoporous MnxCo3−xO4 catalyst. Catal. Commun. 2015, 62, 107–111. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D.; Wang, S. Facile preparation of ordered mesoporous MnCo2O4 for low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal. Today 2006, 111, 236–241. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm–MnOx for the NH3–SCR of NOx at low temperature: Promotional role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.-T.; Liu, S.-M.; Wang, S.-X.; Pan, W.-G.; Li, M.-Y. The enhanced performance of MnOx catalyst for NH3–SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx–CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Chen, X.; Ma, L.; Yang, S.; Schwank, J.W.; Hao, J. Effect of Sn on MnOx–CeO2 catalyst for SCR of NOx by ammonia: Enhancement of activity and remarkable resistance to SO2. Catal. Commun. 2012, 27, 54–57. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of activity and SO2 tolerance of Sn-modified MnOx–CeO2 catalysts for NH3–SCR at low temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx–CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Li, Y.; Su, H.; Ma, L. WO3 promoted Mn–Zr mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2016, 283, 1044–1050. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Xiong, S.; Dang, H.; Liao, Y.; Wong, P.K.; Li, J. MnOx supported on Fe–Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B Environ. 2016, 181, 570–580. [Google Scholar] [CrossRef]
- Shen, B.; Liu, T.; Zhao, N.; Yang, X.; Deng, L. Iron-doped Mn–Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2010, 22, 1447–1454. [Google Scholar] [CrossRef]
- Shen, B.; Ma, H.; Yao, Y. Mn–CeOx/Ti–PILCs for selective catalytic reduction of NO with NH3 at low temperature. J. Environ. Sci. 2012, 24, 499–506. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, K.H.; Hong, S.C. MnOx/CeO2–TiO2 mixed oxide catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chem. Eng. J. 2012, 195–196, 323–331. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn–Ce–Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, R.; Li, Z.; Yuan, F.; Niu, X.; Zhu, Y. Effect of Ni doping in NixMn1−xTi10 (x = 0.1–0.5) on activity and SO2 resistance for NH3–SCR of NO studied with in situ DRIFTS. Catal. Sci. Technol. 2017, 7, 3243–3257. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx–CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Hu, H.; Li, H.; Shi, L.; Zhang, D. Design of multi-shell Fe2O3@MnOx@CNTs for the selective catalytic reduction of NO with NH3: Improvement of catalytic activity and SO2 tolerance. Nanoscale 2016, 8, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Song, C.; Chang, C.-C.; Teng, Y.; Tong, Z.; Tang, X. Manganese oxides supported on TiO2–graphene nanocomposite catalysts for selective catalytic reduction of NOx with NH3 at low temperature. Ind. Eng. Chem. Res. 2014, 53, 11601–11610. [Google Scholar] [CrossRef]
- Lu, X.; Song, C.; Jia, S.; Tong, Z.; Tang, X.; Teng, Y. Low-temperature selective catalytic reduction of NOx with NH3 over cerium and manganese oxides supported on TiO2–graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Wang, F.; Liu, T. The effect of Ce–Zr on NH3–SCR activity over MnOx(0.6)/Ce0.5Zr0.5O2 at low temperature. Chem. Eng. J. 2014, 236, 171–180. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chang, H.; Peng, Y.; Li, J. Novel W-modified SnMnCeOx catalyst for the selective catalytic reduction of NOx with NH3. Catal. Commun. 2017, 100, 117–120. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Song, L.; Lu, T.; Zhao, J.; Liu, Z. Highly dispersed MnOx nanoparticles supported on three-dimensionally ordered macroporous carbon: A novel nanocomposite for catalytic reduction of NOx with NH3 at low temperature. RSC Adv. 2015, 5, 29577–29588. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, Q.; Sun, W.; Tade, M.; Liu, S. Improved activity of W-modified MnOx–TiO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2016, 288, 216–222. [Google Scholar] [CrossRef]
- Apostolescu, N.; Geiger, B.; Hizbullah, K.; Jan, M.; Kureti, S.; Reichert, D.; Schott, F.; Weisweiler, W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts. Appl. Catal. B Environ. 2006, 62, 104–114. [Google Scholar] [CrossRef]
- Lee, T.; Bai, H. Low temperature selective catalytic reduction of NOx with NH3 over Mn-based catalyst: A review. AIMS Environ. Sci. 2016, 3, 261–289. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, S.-L.; Zhong, Q.; Liu, X.-X. Low-temperature selective catalytic reduction of NO over manganese supported on TiO2 nanotubes. J. Fuel Chem. Technol. 2011, 39, 694–701. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Shan, W.; Shi, X. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Qu, L.; Li, C.; Zeng, G.; Zhang, M.; Fu, M.; Ma, J.; Zhan, F.; Luo, D. Support modification for improving the performance of MnOx–CeOy/γ-Al2O3 in selective catalytic reduction of NO by NH3. Chem. Eng. J. 2014, 242, 76–85. [Google Scholar] [CrossRef]
- Schill, L.; Putluru, S.S.R.; Jensen, A.D.; Fehrmann, R. MnFe/Al2O3 catalyst synthesized by deposition precipitation for low-temperature selective catalytic reduction of NO with NH3. Catal. Lett. 2015, 145, 1724–1732. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Liu, X.; Shi, L.; Maitarad, P.; Li, H.; Zhang, J.; Cao, W. Enhanced catalytic performance of V2O5–WO3/Fe2O3/TiO2 microspheres for selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2013, 3, 191–199. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Yu, Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT study of the SO2 effect on low-temperature SCR reaction over Fe−Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Superior Fe-ZSM-5 catalyst for selective catalytic reduction of nitric oxide by ammonia. J. Am. Chem. Soc. 1999, 121, 5595–5596. [Google Scholar] [CrossRef]
- Xiong, S.; Liao, Y.; Xiao, X.; Dang, H.; Yang, S. The mechanism of the effect of H2O on the low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel. Catal. Sci. Technol. 2015, 5, 2132–2140. [Google Scholar] [CrossRef]
- Xiong, S.; Liao, Y.; Xiao, X.; Dang, H.; Yang, S. Novel Effect of H2O on the low temperature selective catalytic reduction of NO with NH3 over MnOx–CeO2: Mechanism and kinetic study. J. Phys. Chem. C 2015, 119, 4180–4187. [Google Scholar] [CrossRef]
- Marban, G. Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4. Role of surface NH3 species: SCR mechanism. J. Catal. 2004, 226, 138–155. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Sun, L.; Hao, J. Origination of N2O from NO reduction by NH3 over β–MnO2 and α–Mn2O3. Appl. Catal. B Environ. 2010, 99, 156–162. [Google Scholar] [CrossRef]
- Wang, C.; Sun, L.; Cao, Q.; Hu, B.; Huang, Z.; Tang, X. Surface structure sensitivity of manganese oxides for low-temperature selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2011, 101, 598–605. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D.; Ran, R.; Yu, J. Rare earth containing catalysts for selective catalytic reduction of NOx with ammonia: A review. J. Rare Earths 2014, 32, 907–917. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-temperature selective catalytic reduction (SCR) of NO with NH3 by using Mn, Cr, and Cu oxides supported on hombikat TiO2. Angew. Chem. Int. Ed. 2001, 40, 2479–2482. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Chen, L.; Niu, X.; Li, Z.; Dong, Y.; Zhang, Z.; Yuan, F.; Zhu, Y. Promoting catalytic performances of Ni–Mn spinel for NH3–SCR by treatment with SO2 and H2O. Catal. Commun. 2016, 85, 48–51. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Chang, H.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Wang, C.; Li, J. Novel effect of SO2 on the SCR reaction over CeO2: Mechanism and significance. Appl. Catal. B Environ. 2013, 136–137, 19–28. [Google Scholar] [CrossRef]
- Liu, Y.; Cen, W.; Wu, Z.; Weng, X.; Wang, H. SO2 poisoning structures and the effects on pure and Mn doped CeO2: A first principles investigation. J. Phys. Chem. C 2012, 116, 22930–22937. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporousMn-based catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhan, L.; Li, C.; Qiao, W.; Ling, L. Effect of SO2 on activated carbon honeycomb supported CeO2–MnOx catalyst for NO removal at low temperature. Ind. Eng. Chem. Res. 2015, 54, 2274–2278. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, Y.; Su, W.; Liu, J. MnO2 doped CeO2 with tailored 3-D channels exhibits excellent performance for NH3–SCR of NO. RSC Adv. 2015, 5, 26231–26235. [Google Scholar] [CrossRef]
- Park, E.; Kim, M.; Jung, H.; Chin, S.; Jurng, J. Effect of sulfur on Mn/Ti catalysts prepared using chemical vapor condensation (CVC) for low-temperature NO reduction. ACS Catal. 2013, 3, 1518–1525. [Google Scholar] [CrossRef]
- Lu, P.; Li, C.; Zeng, G.; He, L.; Peng, D.; Cui, H.; Li, S.; Zhai, Y. Low temperature selective catalytic reduction of NO by activated carbon fiber loading lanthanum oxide and ceria. Appl. Catal. B Environ. 2010, 96, 157–161. [Google Scholar] [CrossRef]
- Sui, M.; Xing, S.; Sheng, L.; Huang, S.; Guo, H. Heterogeneous catalytic ozonation of ciprofloxacin in water with carbon nanotube supported manganese oxides as catalyst. J. Hazard. Mater. 2012, 227–228, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, B.; Su, Y.; Zhou, G.; Wang, K.; Luo, H.; Ye, D. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: Catalytic activity and characterization. Chem. Eng. J. 2012, 192, 232–241. [Google Scholar] [CrossRef]
- Yao, X.; Kong, T.; Yu, S.; Li, L.; Yang, F.; Dong, L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3–SCR at low temperature. Appl. Surf. Sci. 2017, 402, 208–217. [Google Scholar] [CrossRef]
- Huang, J.; Tong, Z.; Huang, Y.; Zhang, J. Selective catalytic reduction of NO with NH3 at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl. Catal. B Environ. 2008, 78, 309–314. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2015, 166–167, 37–44. [Google Scholar] [CrossRef]
- Xiao, X.; Xiong, S.; Shi, Y.; Shan, W.; Yang, S. Effect of H2O and SO2 on the selective catalytic reduction of NO with NH3 over Ce/TiO2 catalyst: Mechanism and kinetic Study. J. Phys. Chem. C 2016, 120, 1066–1076. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Yi, H.; Li, J. Low-temperature SCR of NO with NH3 over AC/C supported manganese-based monolithic catalysts. Catal. Today 2007, 126, 406–411. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. Influence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. A Gen. 2015, 497, 160–166. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Guo, R.-T.; Wang, Q.-S.; Pan, W.-G.; Wang, W.-H.; Yang, N.-Z.; Lu, C.-Z.; Wang, S.-X. The catalytic performance of Mn/TiWOx catalyst for selective catalytic reduction of NOx with NH3. Fuel 2016, 181, 852–858. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Characterization and FTIR studies of MnOx−CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. J. Phys. Chem. B 2004, 108, 15738–15747. [Google Scholar] [CrossRef]
- Guo, F.; Yu, J.; Chu, M.; Xu, G. Interaction between support and V2O5 in the selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2014, 4, 2147–2155. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Xu, Z.; Liu, X.; Zhang, Y. Low-temperature selective catalytic reduction of NO over MnOx/CNTs catalysts. Catal. Commun. 2014, 50, 34–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Wang, X.; Lu, X. Preparation of Mn–FeOx/CNTs catalysts by redox co-precipitation and application in low-temperature NO reduction with NH3. Catal. Commun. 2015, 62, 57–61. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Guo, X.; Cao, L.; Xu, T.; Chen, Z.; Wu, X.; Si, Z.; Weng, D. Nb-modified Mn/Ce/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A Gen. 2017, 545, 64–71. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Sun, N.; Wang, H.; Zhong, L.; He, C.; Wei, W.; Sun, Y. Hollow MnOx–CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Li, Y.; Zhan, S.; Guan, Q.; Tian, Y. Low-temperature selective catalytic reduction of NO with NH3 over Mn2O3-doped Fe2O3 hexagonal microsheets. ACS Appl. Mater. Interfaces 2016, 8, 5224–5233. [Google Scholar] [CrossRef] [PubMed]
- Tomašić, V. Application of the monoliths in DeNOx catalysis. Catal. Today 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Heck, R.M.; Gulati, S.; Farrauto, R.J. The application of monoliths for gas phase catalytic reactions. Chem. Eng. J. 2001, 82, 149–156. [Google Scholar] [CrossRef]
- Shan, W.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Ouzzine, M.; Cifredo, G.A.; Gatica, J.M.; Harti, S.; Chafik, T.; Vidal, H. Original carbon-based honeycomb monoliths as support of Cu or Mn catalysts for low-temperature SCR of NO: Effects of preparation variables. Appl. Catal. A Gen. 2008, 342, 150–158. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J.; Cao, W. In situ synthesis of 3D flower-like NiMnFe mixed oxides as monolith catalysts for selective catalytic reduction of NO with NH3. Chem. Commun. 2012, 48, 10645–10647. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H. Selective catalytic reaction of NOx with NH3 over Ce–Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B Environ. 2014, 150–151, 630–635. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Liu, Y.; Huang, L.; Zhang, J.; Shi, L.; Zhang, D. In situ fabrication of porous MnCoxOy nanocubes on Ti mesh as high performance monolith de-NOx catalysts. RSC Adv. 2017, 7, 36319–36325. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Qiu, C.; Lin, T.; Gong, M.; Chen, Y. Tungsten modified MnOx–CeO2/ZrO2 monolith catalysts for selective catalytic reduction of NOx with ammonia. Chem. Eng. Sci. 2012, 76, 120–128. [Google Scholar] [CrossRef]












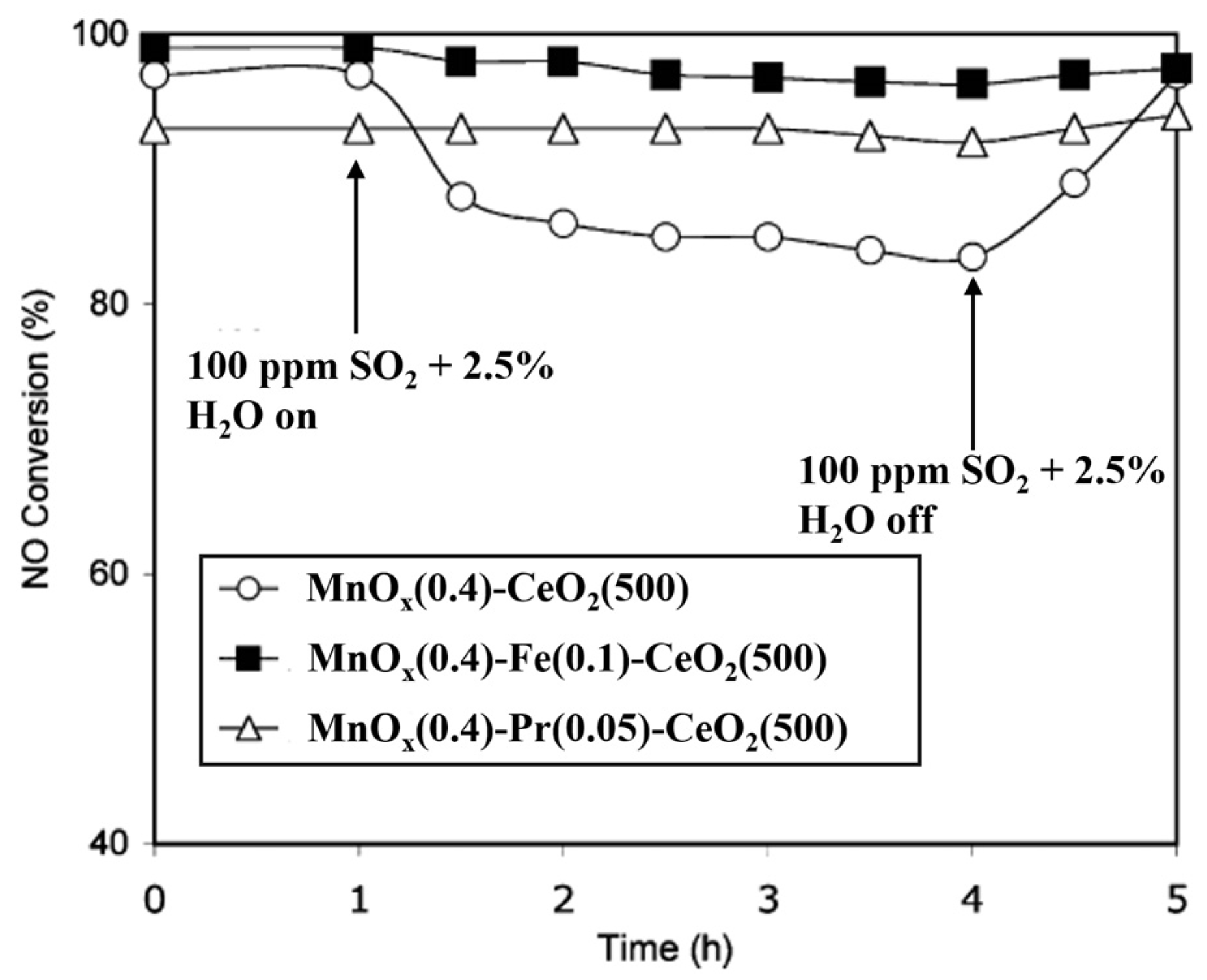









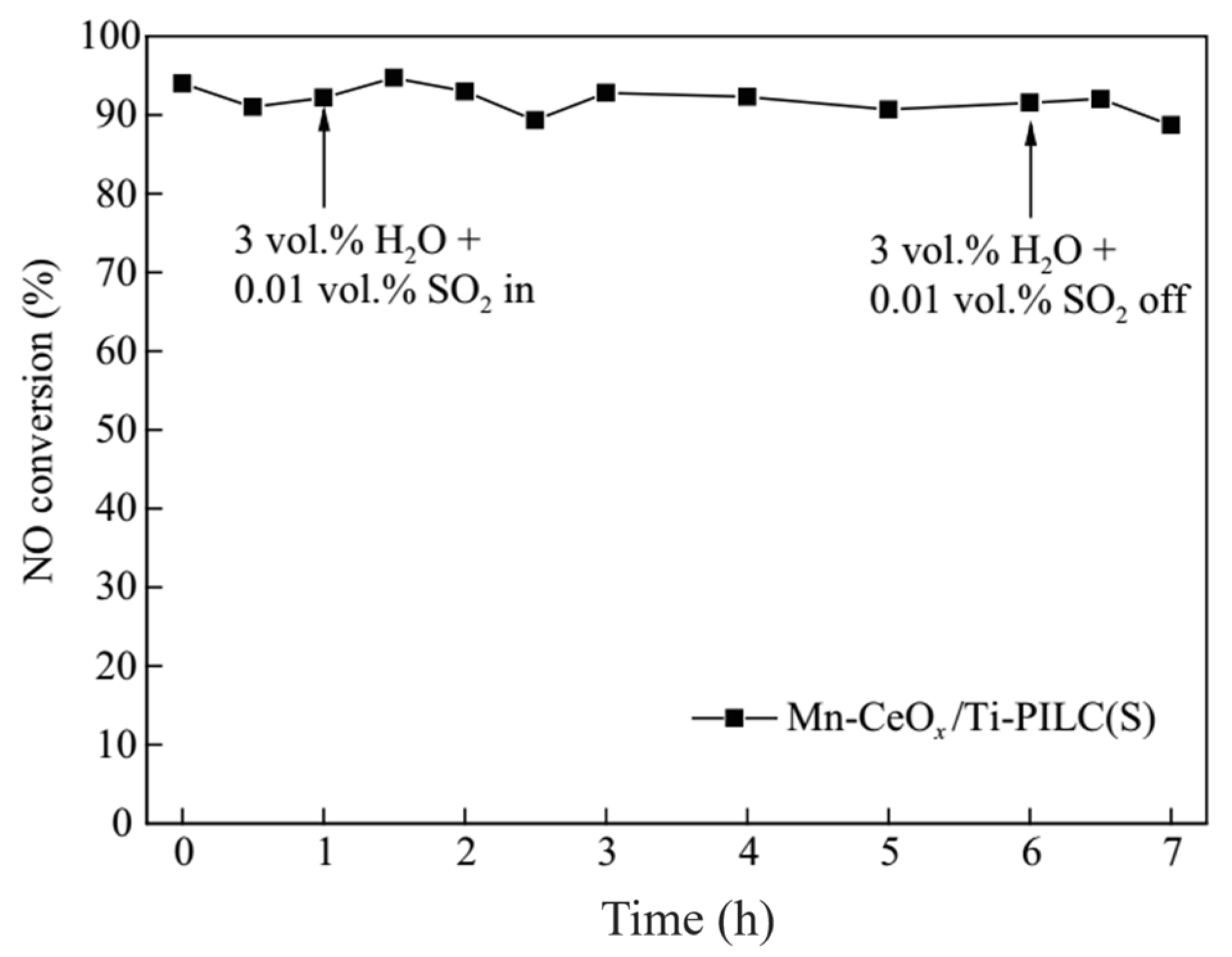

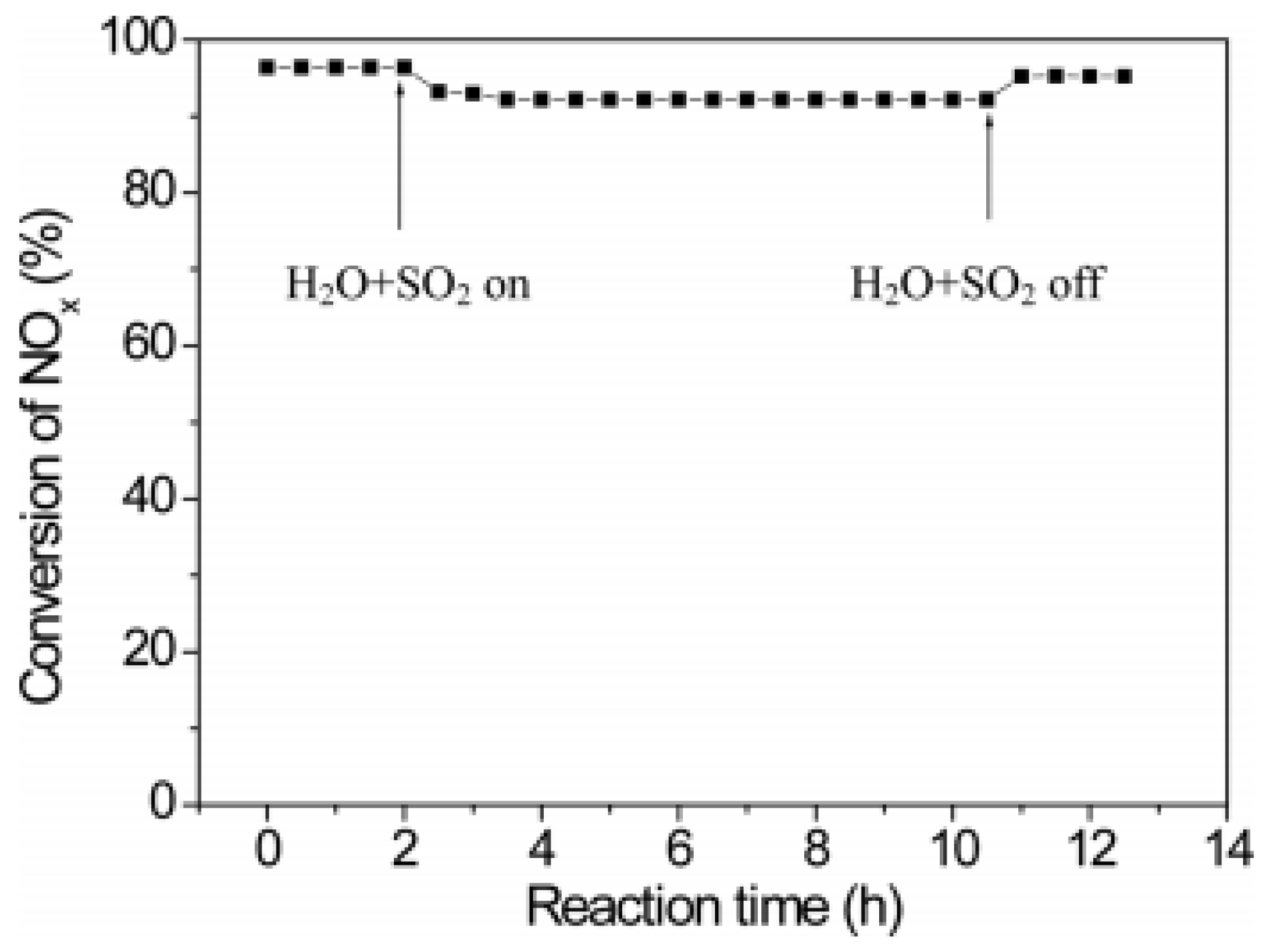







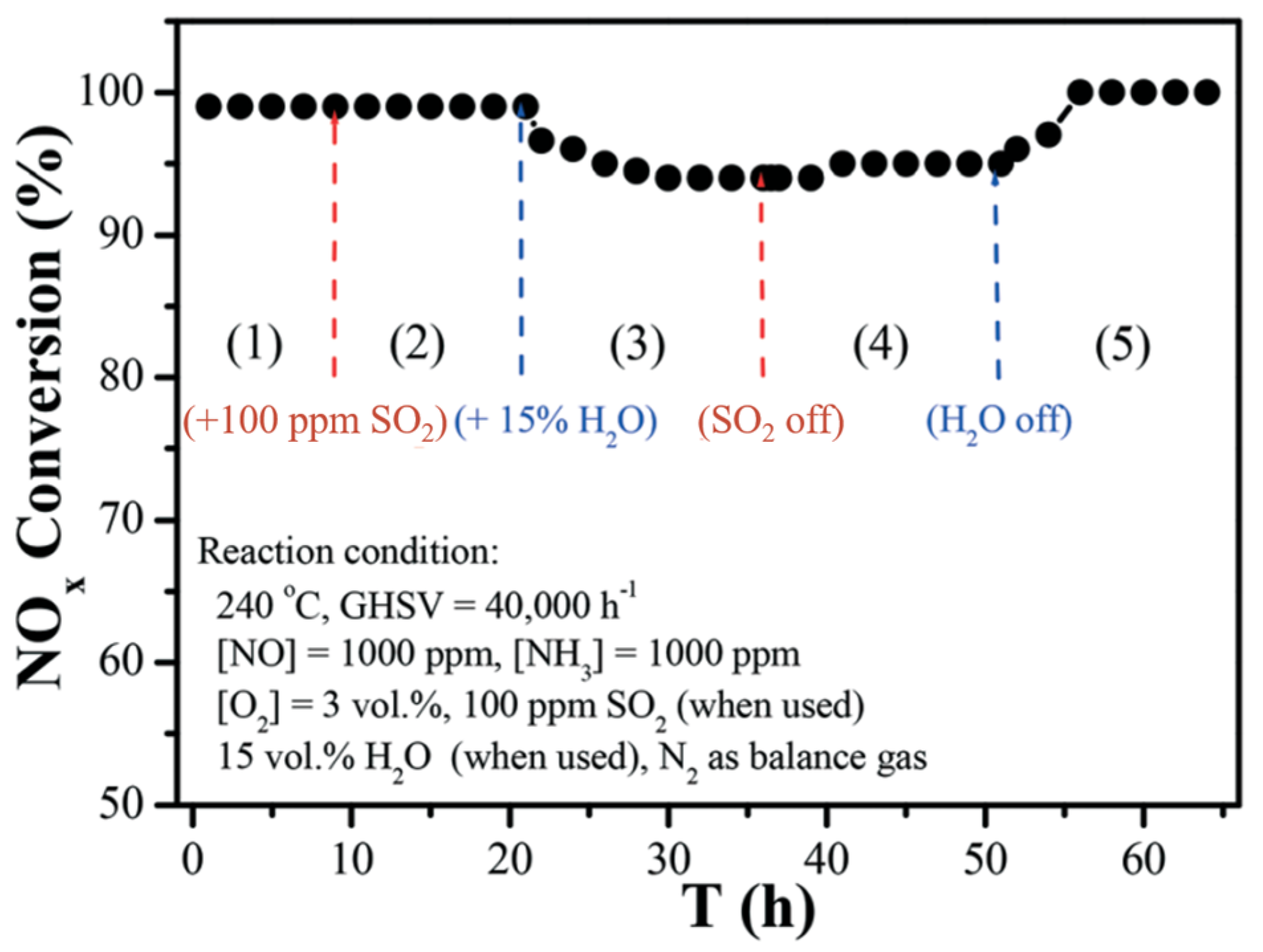
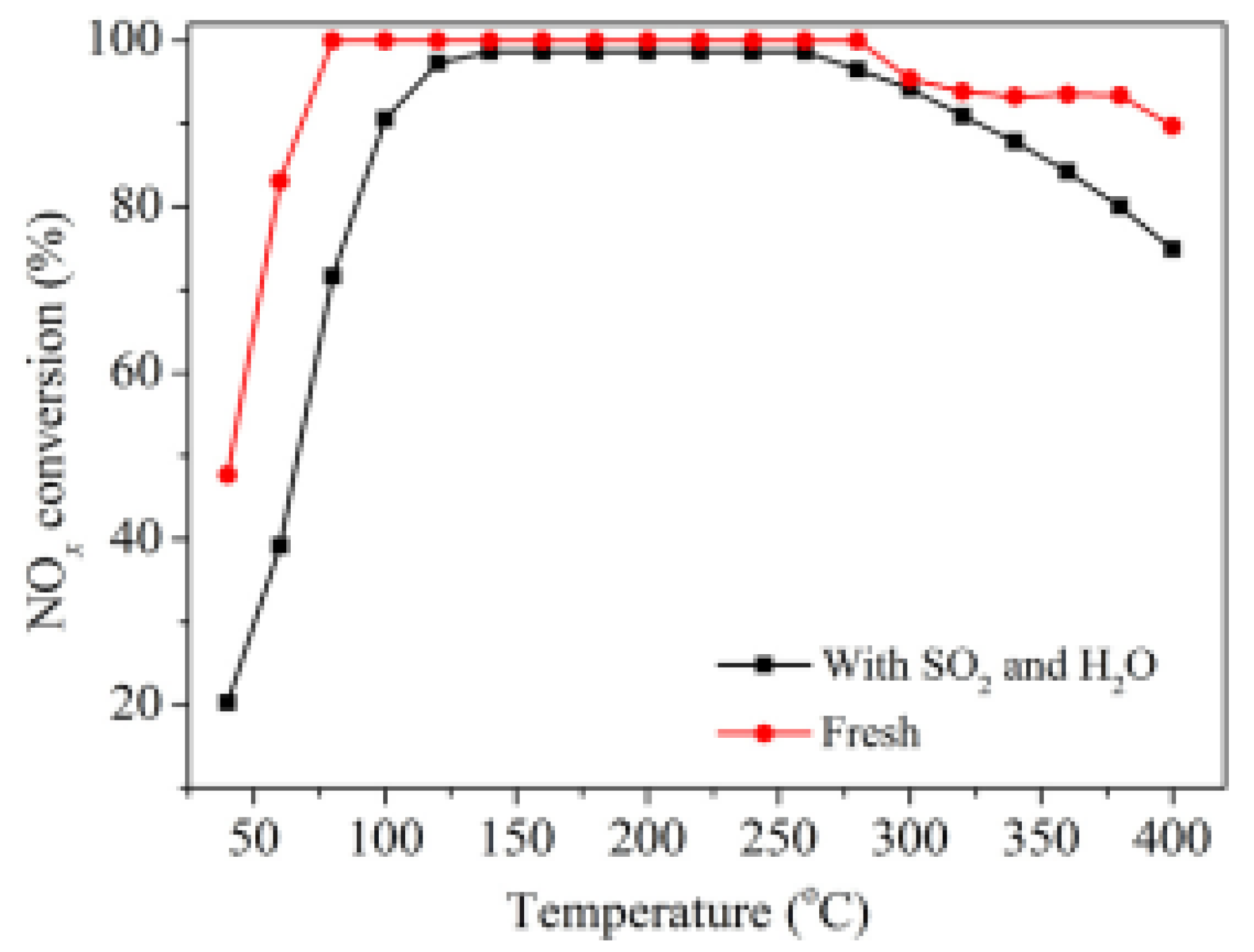
| Catalyst | Reaction Conditions | T/℃ | XNO | XNO-U | XNO-A | References |
|---|---|---|---|---|---|---|
| MnOx | 500 ppm NH3, 500 ppm NO, 3% O2, 10% H2O, 100 ppm SO2GHSV at 47,000 h−1 | 80 | 98% | 70% | 90% | [13] |
| MnOx | 500 ppm NH3, 500 ppm NO, 5% O2, 11% H2O, 100 ppm SO2GHSV at 50,000 h−1 | 120 | 100% | 94% | 100% | [23] |
| Mn–Ce | 1000 ppm NH3, 1000 ppm NO, 2% O2, 2.5% H2O, 100 ppm SO2GHSV at 42,000 h−1 | 120 | 100% | 95% | 100% | [24] |
| Mn–Ce | 500 ppm NH3, 500 ppm NO, 5% O2, 5% H2O, 50 ppm SO2GHSV at 64,000 h−1 | 150 | ~98% | ~95% | / | [25] |
| Mn–Ce | 500 ppm NH3, 500 ppm NO, 5% O2, 5% H2O, 100 ppm SO2 60,000 mL g−1 h−1 | 200 | ~97% | ~70% | ~85% | [26] |
| Mn–Fe | 1000 ppm NH3, 1000 ppm NO, 2% O2, 2.5% H2O, 37.5 ppm SO2GHSV at 15,000 h−1 | 160 | 100% | ~98% | / | [27] |
| Mn–Fe | 1000 ppm NH3, 1000 ppm NO, 3% O2, 5% H2O, 100 ppm SO2GHSV at 30,000 h−1 | 120 | 100% | 87% | 93% | [28] |
| Mn–Co | 500 ppm NH3, 500 ppm NO, 3% O2, 8% H2O, 200 ppm SO2GHSV at 38,000 h−1 | 175 | 100% | 90% | 100% | [29] |
| Mn–Co | 500 ppm NH3, 500 ppm NO, 5% O2, 5% H2O, 100 ppm SO2GHSV at 50,000 h−1 | 200 | 100% | 80% | 90% | [30,31] |
| Mn–Cu | 500 ppm NH3, 500 ppm NO, 5% O2, 11% H2O, 100 ppm SO2GHSV at 50,000 h−1 | 125 | 95% | 64% | ~90% | [32] |
| Mn–Sm | 500 ppm NH3, 500 ppm NO, 5% O2, 2% H2O, 100 ppm SO2GHSV at 49,000 h−1 | 100 | 100% | 91% | 97% | [33] |
| Mn–Eu | 600 ppm NH3, 600 ppm NO, 5% O2, 5% H2O, 100 ppm SO2GHSV at 108,000 h−1 | 350 | 100% | 90% | 95% | [34] |
| Mn–Fe–Ce | 1000 ppm NH3, 1000 ppm NO, 2% O2, 2.5% H2O, 100 ppm SO2GHSV at 42,000 h−1 | 150 | 98% | 95% | 98% | [35] |
| Mn–Ce–Fe | 1000 ppm NH3, 1000 ppm NO, 3% O2, 10% H2O, 100 ppm SO2GHSV at 30,000 h−1 | 120 | 100% | 75% | 95% | [36] |
| Mn–Sn–Ce | 1000 ppm NH3, 1000 ppm NO, 2% O2, 12% H2O, 100 ppm SO2GHSV at 35,000 h−1 | 110 | 100% | 70% | 90% | [37,38] |
| Mn–Ce–Ni | 500 ppm NH3, 500 ppm NO, 5% O2, 10% H2O, 150 ppm SO2GHSV at 48,000 h−1 | 175 | ~90% | ~78% | ~90% | [39] |
| Mn–Ce–Co | 500 ppm NH3, 500 ppm NO, 5% O2, 10% H2O, 150 ppm SO2GHSV at 48,000 h−1 | 175 | ~90% | ~72% | ~90% | [39] |
| Mn–W–Zr | 500 ppm NH3, 500 ppm NO 5% O2, 5% H2O, 50 ppm SO2 GHSV at 128,000 h−1 | 300 | 100% | ~90% | 100% | [40] |
| Mn–Fe/TiO2 | 1000 ppm NH3, 1000 ppm NO, 2% O2, 2.5% H2O, 100 ppm SO2GHSV at 15,000 h−1 | 150 | 100% | 90% | 100% | [41] |
| Mn/Fe–TiO2 | 500 ppm NH3, 500 ppm NO, 2% O2, 8% H2O, 60 ppm SO2GHSV at 12,000 h−1 | 200 | 100% | 83% | 100% | [42] |
| Mn–Ce/TiO2 | 1000 ppm NH3, 1000 ppm NO, 3% O2, 3% H2O, 100 ppm SO2GHSV at 30,000 h−1 | 150 | 100% | 84% | / | [9] |
| Mn–Fe–Ce/TiO2 | 600 ppm NH3, 600 ppm NO, 3% O2, 3% H2O, 100 ppm SO2GHSV at 50,000 h−1 | 180 | 100% | 84% | 90% | [43] |
| Mn–Ce/Ti–PILC | 600 ppm NH3, 600 ppm NO, 3% O2, 3% H2O, 100 ppm SO2GHSV at 50,000 h−1 | 200 | ~95% | ~90% | ~90% | [44] |
| Mn–Ce/TiO2 | 220 ppm NH3, 200 ppm NO, 8% O2, 6% H2O, 100 ppm SO2GHSV at 60,000 h−1 | 180 | 100% | 62% | 70% | [45] |
| Mn–Ce/TiO2 | 500 ppm NH3, 500 ppm NO, 5% O2, 5% H2O, 50 ppm SO2GHSV at 64,000 h−1 | 200 | ~95% | ~90% | ~93% | [46] |
| Ni–Mn/TiO2 | 1000 ppm NH3, 1000 ppm NO, 3% O2, 15% H2O, 100 ppm SO2GHSV at 40,000 h−1 | 240 | 100% | ~95% | 100% | [47] |
| MnCe@CNTs | 500 ppm NH3, 500 ppm NO, 3% O2, 4% H2O, 100 ppm SO2GHSV at 10,000 h−1 | 300 | 100% | 87% | 90% | [48] |
| Fe2O3@MnOx@CNTs | 550 ppm NH3, 550 ppm NO, 5% O2, 10% H2O, 100 ppm SO2GHSV at 20,000 h−1 | 240 | 97% | 91% | 95% | [49] |
| Mn–Ce/TiO2–graphene | 500 ppm NH3, 500 ppm NO, 7% O2, 10% H2O, 200 ppm SO2GHSV at 67,000 h−1 | 180 | 95% | 95% | 100% | [50,51] |
| MnOx(0.6)/Ce0.5Zr0.5O | 600 ppm NH3, 600 ppm NO, 3% O2, 3% H2O, 200 ppm SO2GHSV at 30,000 h−1 | 180 | 100% | ~92% | ~98% | [52] |
| WySnMnCeOx | 500 ppm NH3, 500 ppm NO, 5% O2, 5% H2O, 100 ppm SO2 60,000 mL g−1 h−1 | 200 | ~97% | ~90% | ~95% | [53] |
| MnOx/3DOMC | 1000 ppm NH3, 1000 ppm NO, 5% O2, 5% H2O, 200 ppm SO2GHSV at 36,000 h−1 | 190 | 100% | ~87% | ~95% | [54] |
| W0.25–Mn0.25–Ti0.5 | 1000 ppm NH3, 1000 ppm NO, 5% O2, 10% H2O, 100 ppm SO2GHSV at 25,000 h−1 | / | ~100% | ~100% | / | [55] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Shi, J.-W.; Fan, Z.; Gao, G.; Niu, C. Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catalysts 2018, 8, 11. https://doi.org/10.3390/catal8010011
Gao C, Shi J-W, Fan Z, Gao G, Niu C. Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catalysts. 2018; 8(1):11. https://doi.org/10.3390/catal8010011
Chicago/Turabian StyleGao, Chen, Jian-Wen Shi, Zhaoyang Fan, Ge Gao, and Chunming Niu. 2018. "Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review" Catalysts 8, no. 1: 11. https://doi.org/10.3390/catal8010011





