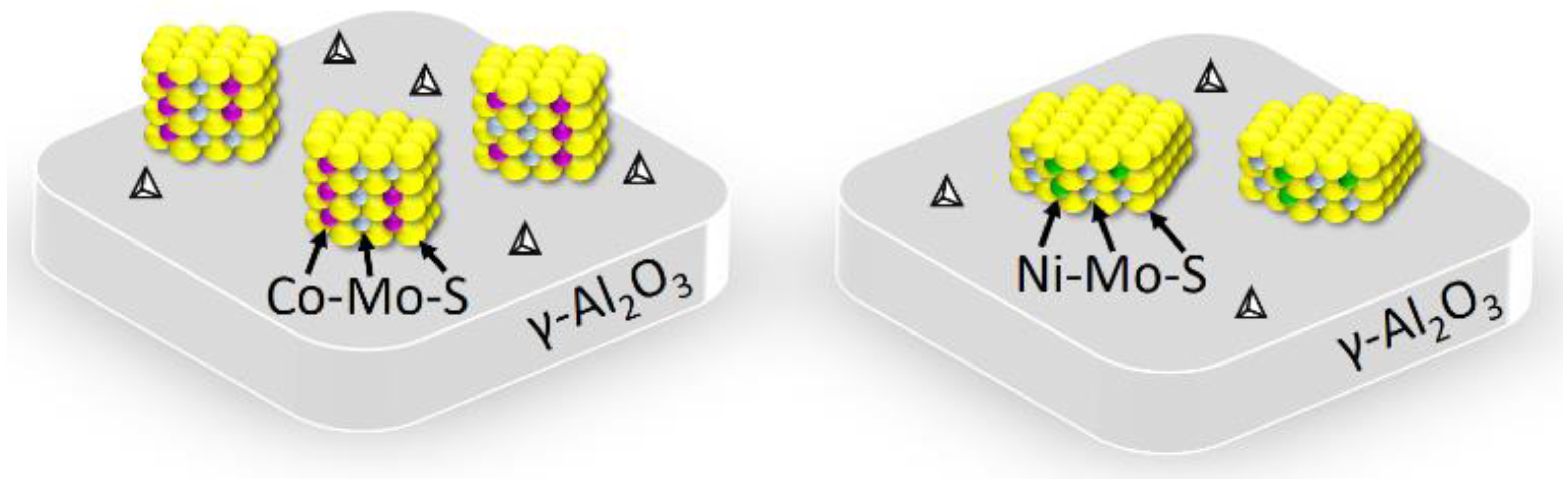
2.1. Characterizations of the Mesoporous Sulfide Catalysts
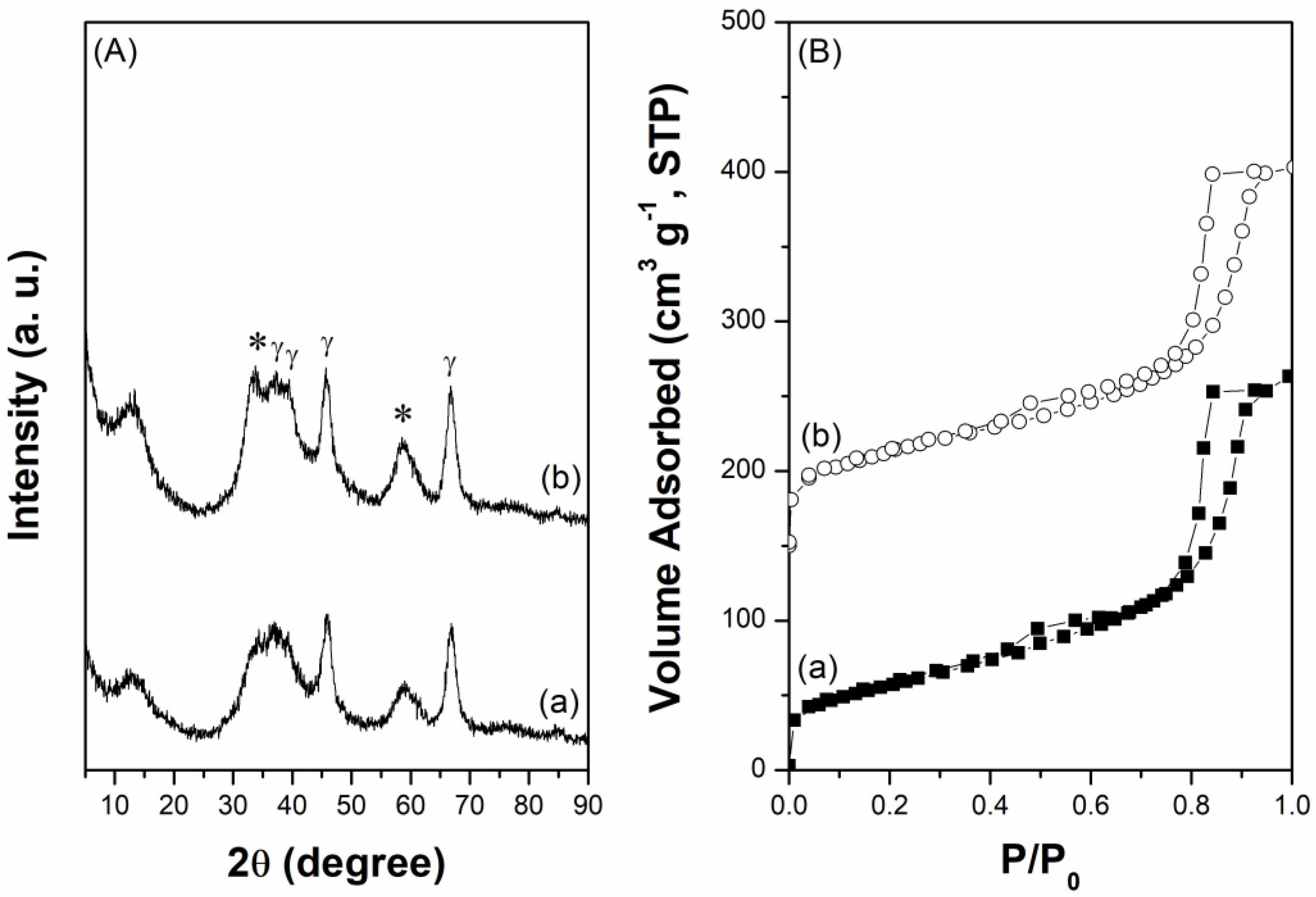
The wide-angle X-ray diffraction (XRD) patterns show that the crystallinity of the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst is slightly lower than that of the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst (
Figure 1A). The corresponding N
2 adsorption-desorption isotherms show that both catalysts possess a classical type-IV isotherm with an H
1 hysteresis loop in the relatively high P/P
0 region (
Figure 1B), suggesting the presence of a largely mesoporous framework.
Table 1 tabulates the physicochemical property of the mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts. The N
2 physisorption data reveal that the specific surface area (
SBET), total pore volume (V
total), and pore size (Ф
p) of the mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts are independent of Ni and Co. (denoted as the promoters), whereas the NH
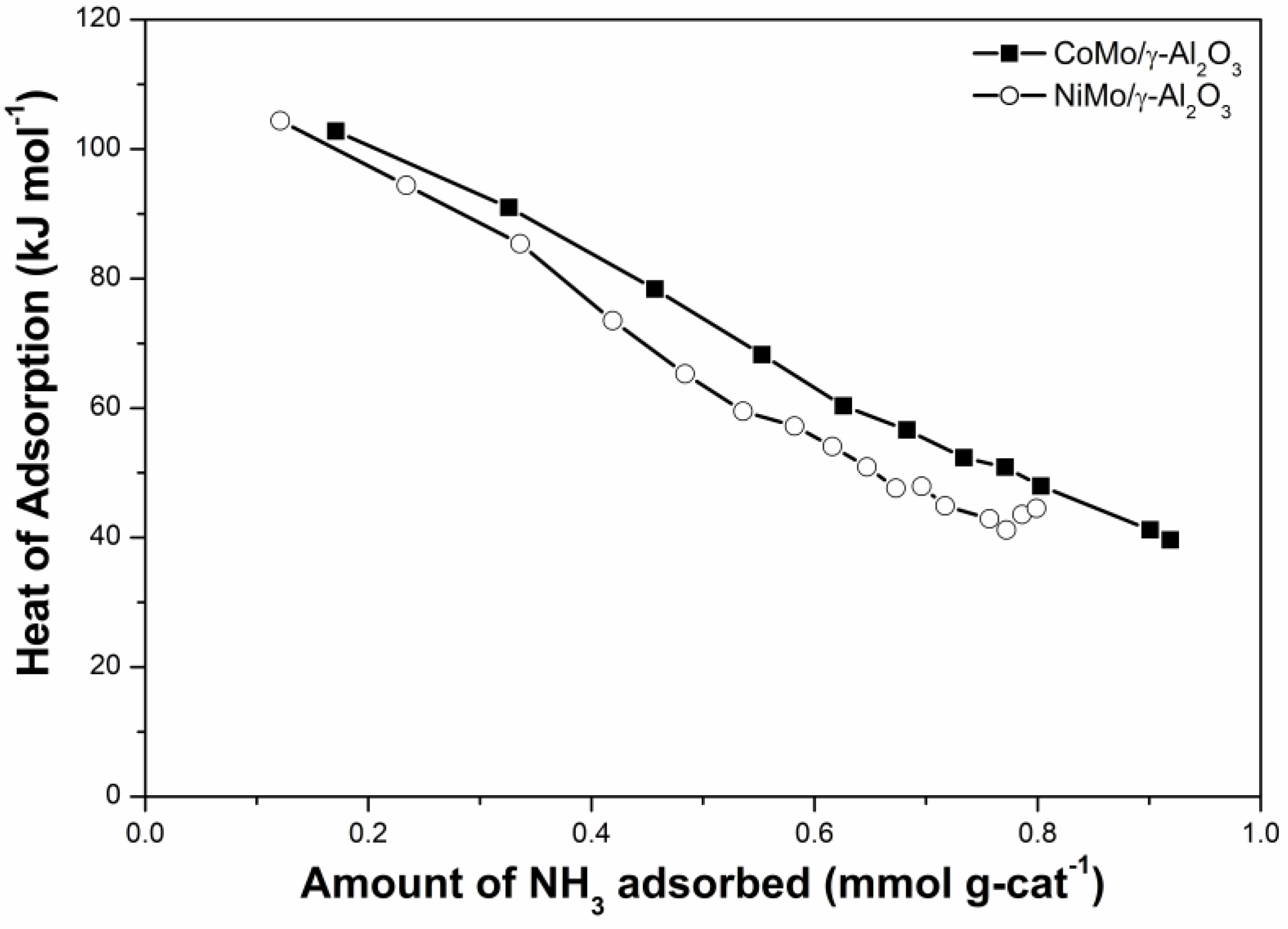
3 and NO uptakes vary slightly. The differential heat of NH
3 adsorption study further indicates that the acidic strength and capacity of mesoporous sulfide catalysts can be enhanced if Ni is replaced by Co (
Figure 2). This result implies that the surface fine structures of the mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts are slightly different from each other, such as the acidic sites, coordinatively unsaturated sites (CUS), and brim sites. A previous study has shown that the activity and selectivity of mesoporous sulfide catalysts in the hydrotreating of crude oils are highly related to their surface fine structures, which, in turn, influences the composition and fuel properties of the hydrotreated oils [
17].
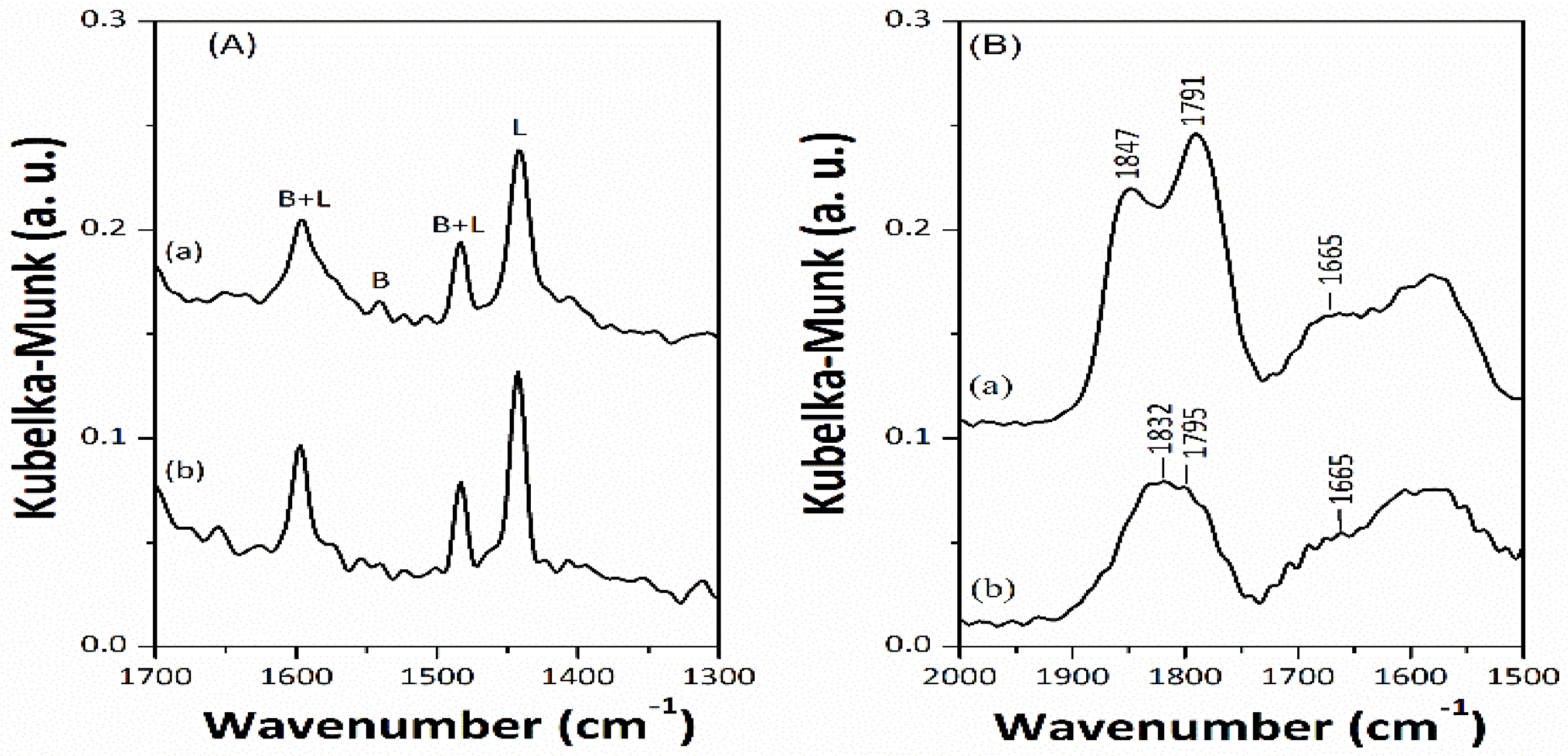
To understand the surface fine structures better, mesoporous sulfide catalysts were investigated by in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy using pyridine and NO as probe molecules. High-resolution transmission electron microscopy (HRTEM) images were also obtained. In the in situ DRIFT spectra with pyridine adsorption (
Figure 3A), the characteristic DRIFT signals centered at 1442 and 1540 cm
−1 are assigned to pyridine adsorbed on Lewis and Bronsted acid sites, respectively [
18,
19,
20]. The DRIFT signal centered at 1442 cm
−1 is stronger than others, indicating that mesoporous sulfide catalysts are mostly related to Lewis solid acids. For the in situ DRIFT spectra with NO adsorption (
Figure 3B), the characteristic DRIFT signals centered at 1665 and around 1832–1847 cm
−1 are assigned to NO adsorbed on the CUS regions nearby the MoS
x and CoS
x (or NiS
x) species, respectively [
21]. It is worth noting that NO preferentially adsorbs on the CUS regions at the edges or corners of the Co- and Ni-incorporated MoS
2-like slabs but it does not adsorb on the molybdenum(VI) cations or the mesoporous alumina framework [
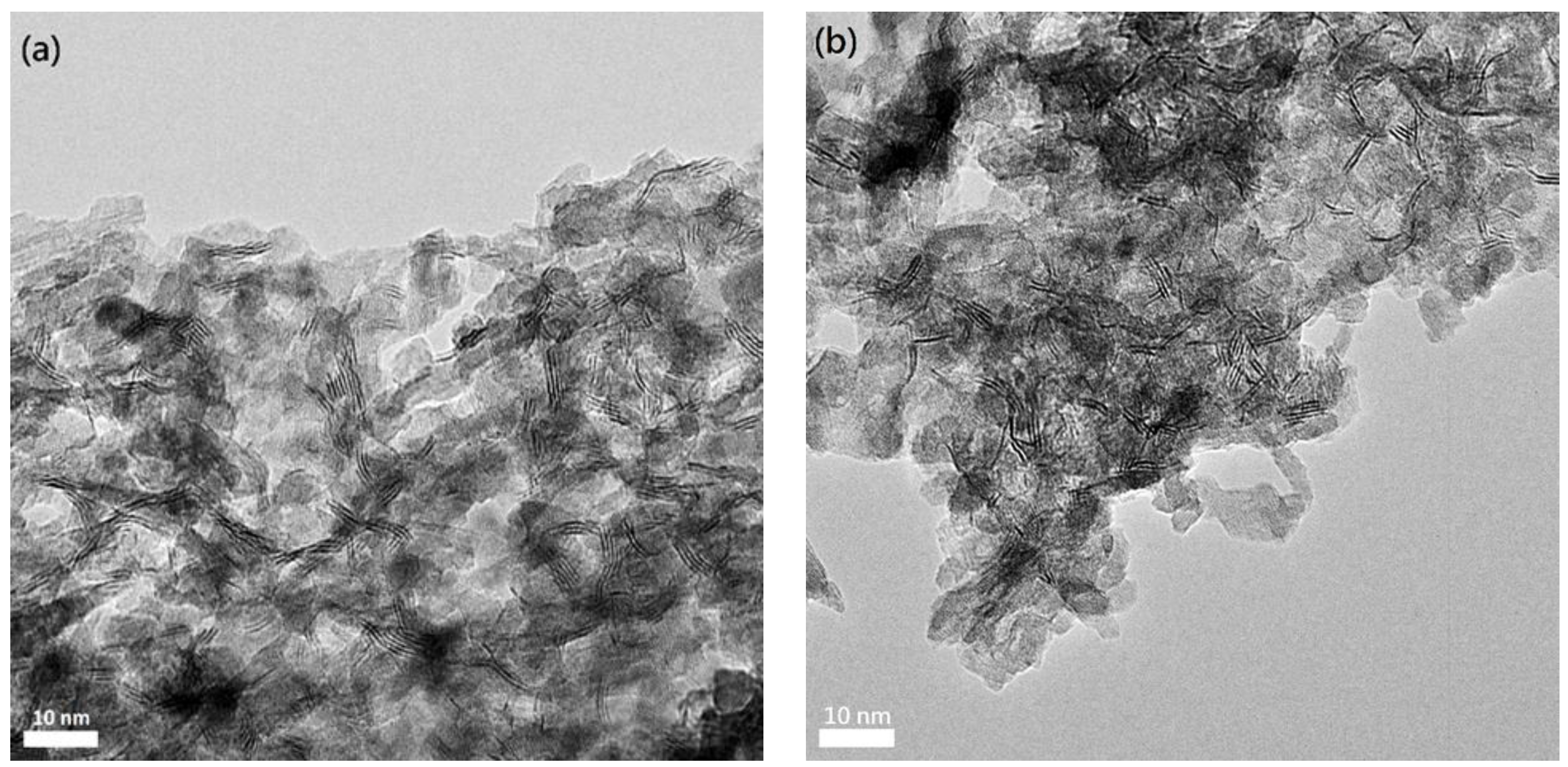
22]. The surface fine structures of the mesoporous sulfide catalysts were directly observed by the HRTEM technique (
Figure 4). The HRTEM images show that the mesoporous sulfide catalysts contain well-dispersed MoS
2-like slabs in which the stacking number, length, and height are varied slightly with the incorporation of Co. and Ni. The average values of Co- or Ni-incorporated MoS
2-like slabs obtained by statistical methods are tabulated in
Table 1. The Co-incorporated MoS
2-like slabs (stacking number ≈ 3.8, width ≈ 4.5 nm, height ≈ 2.0 nm) are obviously smaller but higher than the Ni-incorporated counterparts (stacking number ≈ 2.6, width ≈ 5.9 nm, height ≈ 1.5 nm).
The chemical environments of the mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts prepared by similar procedures have been previously examined by extended X-ray absorption fine structure (EXAFS) measurements [
22,
23,
24]. The unsupported MoS
2 crystal was used as a reference material, where the coordination numbers of molybdenum and sulfur (denoted N(Mo) and N(S), respectively) were close to 6. The EXAFS calculations revealed that mesoporous sulfide catalysts possessed very small N(Mo) values of ca. 2–3 and moderate N(S) values of ca. 4.5–5.5, indicating the presence of highly-dispersed MoS
2-like slabs. Moreover, the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst with smaller N(Mo) and N(S) values should have smaller MoS
2-like slabs. This speculation is supported by our characterization results. Combining the wide-angle XRD, gas adsorption, in situ DRIFT, and HRTEM studies with previous EXAFS data, the differences in surface fine structures between the mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts are proposed in
Scheme 1. The mesoporous CoMo/γ-Al
2O
3 sulfide catalyst has a relatively large number of acidic sites and CUS, which is presumably due to the presence of the small Co-incorporated MoS
2-like slabs with high stacking numbers on the mesoporous alumina framework. These small Co-incorporated MoS
2-like slabs should have more active sites at the edges or corners. On the other hand, the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst has a relatively small number of acidic sites and CUS, probably related to the large Ni-incorporated MoS
2-like slabs with lower stacking numbers impregnated on the mesoporous alumina framework. These large Ni-incorporated MoS
2-like slabs may contribute to the larger number of active sites at the brims.
2.2. Hydrotreating Model Diesel Oils
The ability of the mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
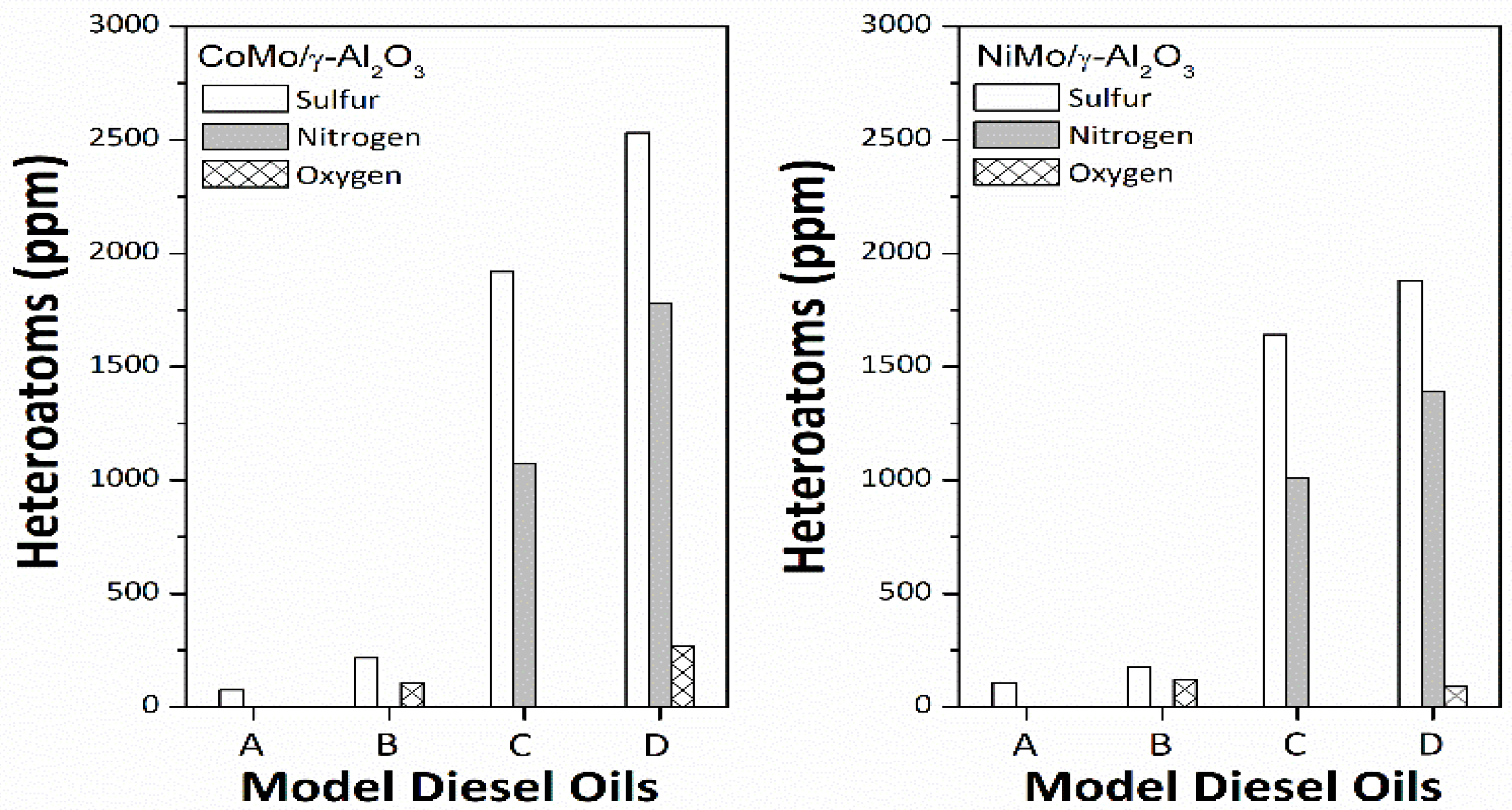
3 sulfide catalysts to simultaneously remove heteroatoms (sulfur, oxygen, and nitrogen) from model diesel oils A–D (
Table S1) was examined using a high-pressure stainless-steel batch-type reactor at 330 °C and 5 MPa of H
2 for 1 h (
Figure 5). When model diesel oil A was used as an oil feedstock, the sulfur contents of hydrotreated oils over mesoporous sulfide catalysts were remarkably reduced to 74–107 ppm. Apparently, the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst is superior to mesoporous NiMo/γ-Al
2O
3 sulfide catalyst in the removal of sulfur from model diesel oil A. It is reasonable to surmise that the Co-incorporated MoS
2-like slabs result in higher activity in the HDS of dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (DMDBT) than the Ni-incorporated counterparts. When model diesel oils B–D containing stearic acid or quinoline were used as oil feedstocks, the sulfur- and nitrogen-containing compounds mostly remained in the hydrotreated oils treated over the mesoporous sulfide catalysts, whereas the oxygen-containing compounds were mostly removed (>98%). With mesoporous sulfide catalysts, it is easier to achieve the HDO of stearic acid than the HDS of DBT and DMDBT and the hydrodenitrogenation (HDN) of quinoline. In addition, the heteroatom concentrations of the hydrotreated oils over the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst are obviously lower than those over the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst. The influences of oxygen- and nitrogen-containing compounds on the hydrotreating performance of mesoporous sulfide catalysts in the hydrotreating of model diesel oils B–D may be related to the interactions of model compounds adsorbed on the catalytically active sites and other surface fine structures.
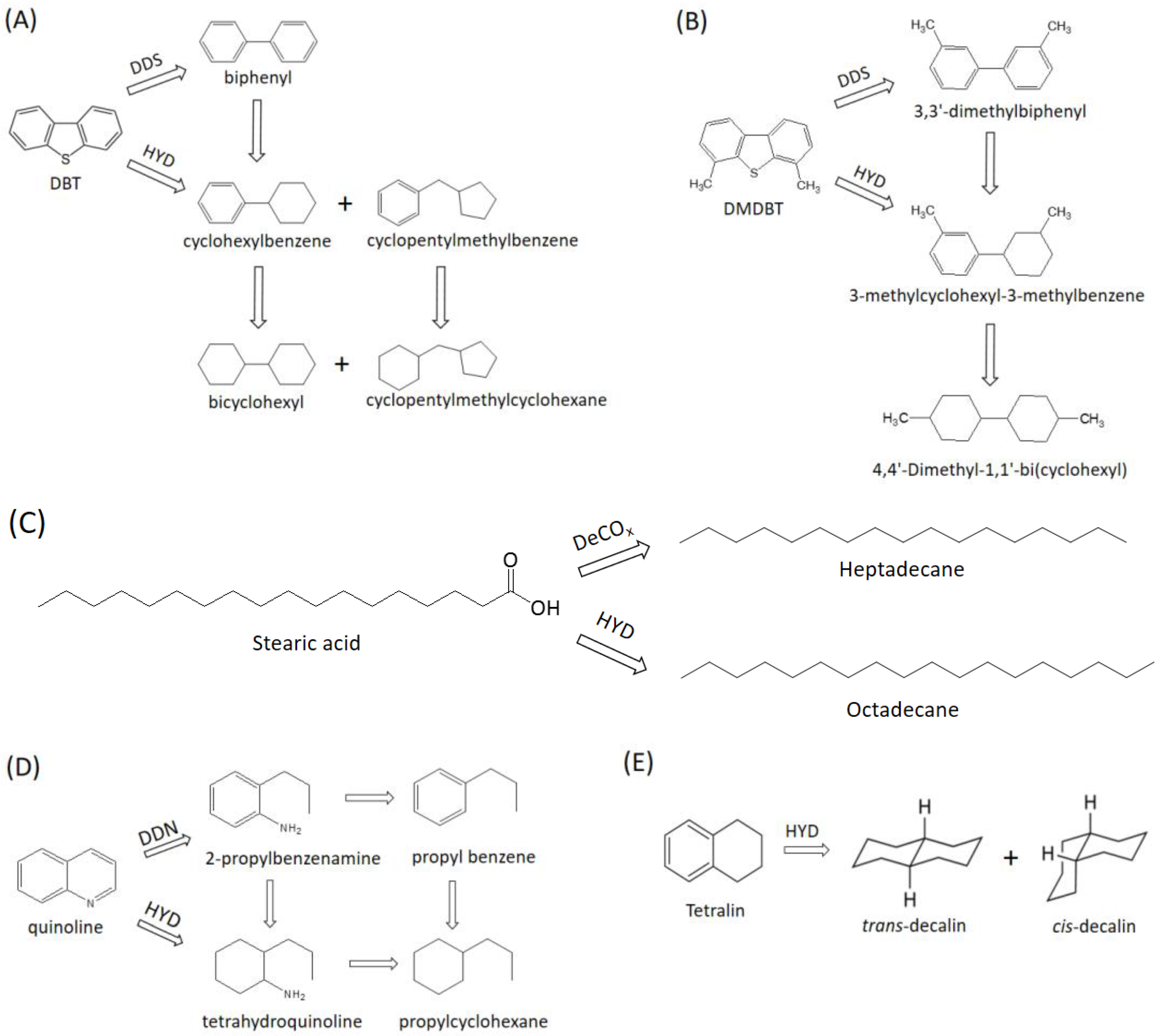
To understand the catalytic behavior of the mesoporous sulfide catalysts in the hydrotreating of model diesel oils A–D, the possible reaction pathways for HDS, HYD, HDO, and HDN are illustrated in
Scheme 2 [
25,
26,
27]. In the HDS of DBT, biphenyl is formed by the direct desulfurization (DDS) pathway, whereas cyclohexylbenzene and cyclopentylmethylbenzene are formed by the HYD and subsequent isomerization/desulfurization pathway. Bicyclohexyl and cyclopentylmethyl cyclohexane are obtained if the DDS and HYD products are further hydrogenated. Similarly, the HDS of DMDBT can be catalyzed by the DDS and HYD pathways. In the HDO of stearic acid, heptadecane is formed by the decarboxylation/decarbonylation (DeCO
x) pathway, whereas octadecane is formed by the HYD and subsequent deoxygenation pathways. In the HDN of quinoline, propylbenzene is formed by the direct denitrogenation (DDN) pathway, whereas propylcyclohexane is formed by HYD and subsequent denitrogenation pathway. In the HYD of tetralin,
cis- and
trans-decalin are formed by the isomerization and HYD of octalin as hydrogenated intermediates. Based on the proposed reaction mechanisms, the catalytic behavior of the mesoporous sulfide catalysts in these model reactions can be thoroughly discussed, including the hydrotreating activity, the conversion of model compounds, and the selectivity for products (
Figure 6 and
Figure 7). The detailed data are tabulated in
Tables S2–S3 (ESI). The hydrotreating activity was calculated by dividing the molar faction of oil desulfurized by the catalyst after the first hour of reaction.
Figure 6 shows that the decreases in hydrotreating activity and conversions of DBT, DMDBT, and tetralin follow the order: model diesel oils A > B >> C > D. The HDS of DMDBT and the HYD of tetralin over the mesoporous sulfide catalysts are particularly inhibited by quinoline in model diesel oils C and D. However, stearic acid and quinoline were almost consumed in a short reaction period although they seem to compete with each other for the catalytically active sites. These observations suggest, again, that the catalytically active sites for the HDS of DBT and DMDBT, the HDO of stearic acid, the HDN of quinoline, and the HYD of tetralin over the mesoporous sulfide catalysts are the same or are close to each other. As estimated from the catalytic data, the competition among these reactions follows the order: HDN of quinoline ≈ HDO of stearic acid > HDS of DBT >> HDS of DMDBT ≈ HYD of tetralin, which should be directly related to the sequence of competitive adsorption of various model compounds on the catalytically active sites. Therefore, it can be said that the catalytically active sites of mesoporous sulfide catalysts give high priority to the HDN of quinoline and the HDO of stearic acid; thus, the HDS of DBT and DMDBT, and the HYD of tetralin are strongly inhibited at the same time, particularly for the latter two model compounds.
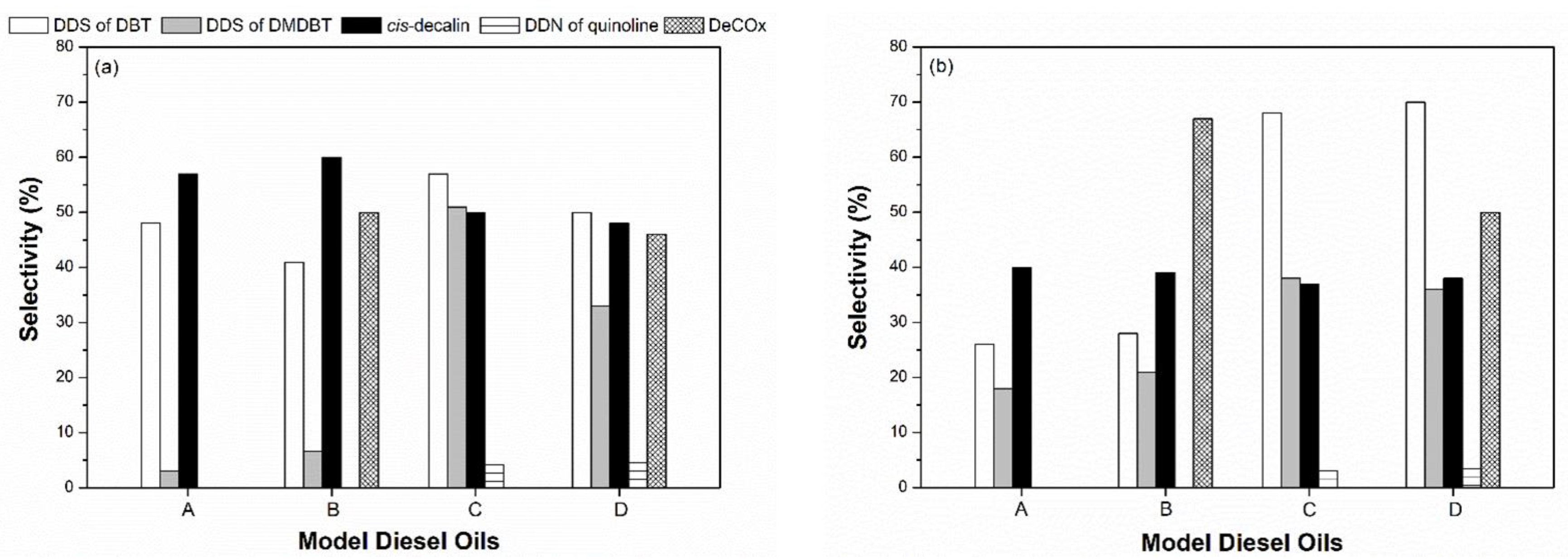
The selectivity of mesoporous sulfide catalysts in these model reactions is also influenced by the competitive adsorption of various model compounds on the catalytically active sites (
Figure 7). For the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst, the selectivity for the DDS of DBT was decreased by stearic acid, increased by quinoline, and remained nearly unchanged if both model compounds are present. The reverse is true for the selectivity for the HYD of tetralin to
cis-decalin. On the other hand, the selectivity for the DDS of DMDBT was increased by stearic acid and, in particular, quinoline. The selectivity for the DDN of quinoline and the DeCOx of stearic acid was subject to slight variation. For the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst, the selectivity for the DDS of DBT and DMDBT was positively influenced by stearic acid and, in particular, quinoline, while the selectivity for the DeCO
x of stearic acid was decreased by quinoline. The latter observation is similar to those of a recent study reported by Deliy et al. [
27]. They showed that the DeCO
x products were mainly formed during the HDO of methyl palmate over sulfided NiMo/γ-Al
2O
3 catalyst, preferably in the presence of aromatic molecules. The selectivity for the HYD of tetralin to
cis-decalin and the DDN of quinoline is subject to slight variation. With these results in mind, we can presume that the nitrogen atom in quinoline has a greater influence on the selectivity of mesoporous sulfide catalysts in the hydrotreating of model diesel oils than other heteroatoms present in the model compounds. In addition, these model compounds influence the reaction pathways over mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts differently, presumably associated with the differences in binding strength and spatial location of reactant molecules on the catalytically active sites.
Recent studies have shown that the catalytic activity and selectivity of mesoporous CoMo/γ-Al
2O
3 and NiMo/γ-Al
2O
3 sulfide catalysts in the hydrotreating of petroleum- and coal-derived oils are not only associated with surface fine structures of Co- and Ni-incorporated MoS
2-like slabs as the catalytically active sites, but also strongly influenced by the spatial location of heteroatom-containing compounds, such as H
2S or amines, competitively adsorbed on the catalytically active sites [
25,
26,
28,
29,
30,
31,
32,
33]. In the HDS of DBT and DMDBT, it is generally agreed that the catalytically active sites for the DDS pathway are associated with the CUS regions at the edges or corners of Co- and Ni-incorporated MoS
2-like slabs, so-called the Co(Ni)-Mo-S structures, while those for the HYD pathway are related to the brim sites, which exhibit metal-like character [
25,
26]. In the upgrading of model diesel oil A over the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst, there is no doubt that small Co-incorporated MoS
2-like slabs with high stacking numbers are highly efficient in the HDS of DBT toward the DDS product, which is catalyzed by the Co-Mo-S structures at the edges or corners. In contrast, the main HDS route of DMDBT is the HYD pathway, which is catalyzed by the brim sites because of steric hindrance [
31]. The hydrotreating activity of the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst in the hydrotreating of model diesel oil A is poor by comparison, suggesting that large Ni-incorporated MoS
2-like slabs with lower stacking numbers contain relatively low numbers of catalytically active sites, i.e., the Ni-Mo-S structures, and show relatively low activity for HDS reactions. However, the HYD of tetralin to
trans-decalin over the brim sites is significantly enhanced, presumably because of the high electron density of the brim sites, which facilitate hydrogen dissociation corresponding to the high HYD ability of the large Ni-incorporated MoS
2-like slabs [
25]. The variation in selectivity for the HDS of DBT and DMDBT over the mesoporous sulfide catalysts could be related to their competitive adsorption on the catalytically active sites.
In the hydrotreating of model diesel oils B–D, the hydrotreating activity and selectivity of mesoporous sulfide catalysts in the HDS of DBT and DMDBT and the HYD of tetralin varied intensely. In the case of the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst, we speculate that a considerable number of the Co-Mo-S structures are occupied or hindered by the adsorption of stearic acid and, in particular, quinoline. The DDS pathway in the HDS of DBT was slightly inhibited, whereas the reverse is true for the DDS pathway in HDS of DMDBT. The selectivity of
cis-decalin was slightly enhanced by stearic acid although the HYD of tetralin was inhibited. Clearly, stearic acid occupies the Co-Mo-S structures and is quickly converted to the hydrotreated products (
n-heptadecane and
n-octadecane) under the severe reaction conditions. As a result, the HDS of DBT to the DDS product was negatively affected by the presence of stearic acid and its hydrotreated products. The HDS of DMDBT was slightly enhanced, presumably because more DMDBT is accessible to the catalytically active sites. This might be because the solvent environment near the catalytically active sites, such as the hydrophobic–hydrophilic properties, was affected by the hydrotreated products derived from the HDO of stearic acid. By comparison, quinoline promoted the HDS of DMDBT toward the DDS product remarkably, although the conversion was extremely low. In addition, the influence of quinoline on the HDS of DBT toward the DDS product was positive with a slight decrease in conversion. The results cannot be simply explained in the same way. According to recent studies reported by Prins et al., Lauritsen et al., and other pioneers, the DDS pathway occurs by the σ-adsorption of DMDBT on the Co-Mo-S structures via the sulfur atom, while the HYD pathway presumably proceeds by π-adsorption of DMDBT on the brim sites via the aromatic system [
25,
26,
27,
28,
29,
30,
31,
32]. In this study, presumably, the quinoline molecules can be adsorbed either on the Co-Mo-S structures or the brim sites of the Co-incorporated MoS
2-like slabs [
33]. The π-adsorption of bulky DMDBT molecules with two methyl groups in the 4 and 6 positions on the residual sites of the Co-incorporated MoS
2-like slabs is the first step of the HYD pathway and is significantly inhibited by steric hindrance (
Scheme S1). This means that more DMDBT is available for the DDS pathway through the σ-adsorption of DMDBT via the sulfur atoms because this results in less steric hindrance. In the case of the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst, the HDS of DBT and DMDBT was not negatively influenced by quinoline, and the selectivity toward the DDS product was also facilitated. Moreover, the influence of stearic acid on selectivity for the HDS of DBT and the HYD of tetralin was nearly negligible. This might be associated with the special surface properties of the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst; that is, (i) the number of the Ni-Mo-S structures for the DDS pathway is originally low, (ii) the brim sites with enhanced metallic properties have high HYD activity, and (iii) the adsorption strengths of stearic acid and quinoline on surface sites with relatively low CUS and weak acidity are supposedly low. Similarly, the desorption of the HDO and HDN products should be facilitated. Because of these special surface properties, the poisoning of the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst by stearic acid and quinoline becomes less intense.
2.3. Co-Processing of Jatropha Bio-Oil with Model Diesel Oil or LGO
The catalytic performance of sulfided catalysts in the co-processing of Jatropha bio-oil mixed with model diesel oil A’ or LGO, denoted as oil feedstocks E and F, respectively, was further examined using a stainless-steel batch-type reactor at 330–350 °C and 5–7 MPa of H
2 for 3 h. The catalyst weight was varied in range of 10–20 wt % based on the oil feedstocks. The oil feedstocks made by mixing model diesel oil A’ (or LGO) with 10 wt % Jatropha bio-oil have high acid values in the range of 5.8–7.3 mg KOH g
−1. Jatropha bio-oil mainly consists of fatty acids and derivatives, in addition to small amounts of hydrocarbons and oxygen- and nitrogen-containing aromatic compounds, based on the gas chromatography–flame ionization detector (GC-FID) and gas chromatography–mass spectrometry (GC-MS) analyses (
Figures S1–S2, ESI). In
Table 2, the results show again that the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst is superior to the mesoporous CoMo/γ-Al
2O
3 sulfide catalyst in the simultaneous removals of sulfur, nitrogen, and oxygen from Jatropha bio-oil co-fed with model diesel oil A’ or LGO. The hydrotreating activity of mesoporous sulfide catalysts was decreased by replacing model diesel oil A’ with LGO. It appears likely that the aromatic sulfur compounds present in LGO are complex, making sulfur removal a difficult task. On the other hand, in addition to decreasing the hydrogen partial pressure during the reaction period, the byproducts formed during the HDS, HDO, and HDN processes, such as H
2S, H
2O, and NH
3, may occupy the catalytically active sites of mesoporous sulfide catalysts in a batch-type reactor (a closed system), and presumably cause catalyst deactivation. Hydrotreated oils with moderate sulfur contents (46–116 ppm) were only obtained under severe reaction conditions. To overcome these technical problems, the co-processing of Jatropha bio-oil with LGO over the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst was further performed in an up-flow fixed-bed reactor at 350 °C and 5–7 MPa of H
2 flow (150 Ncc min
−1) using a weight hourly space velocity (WHSV) value of 1.5 h
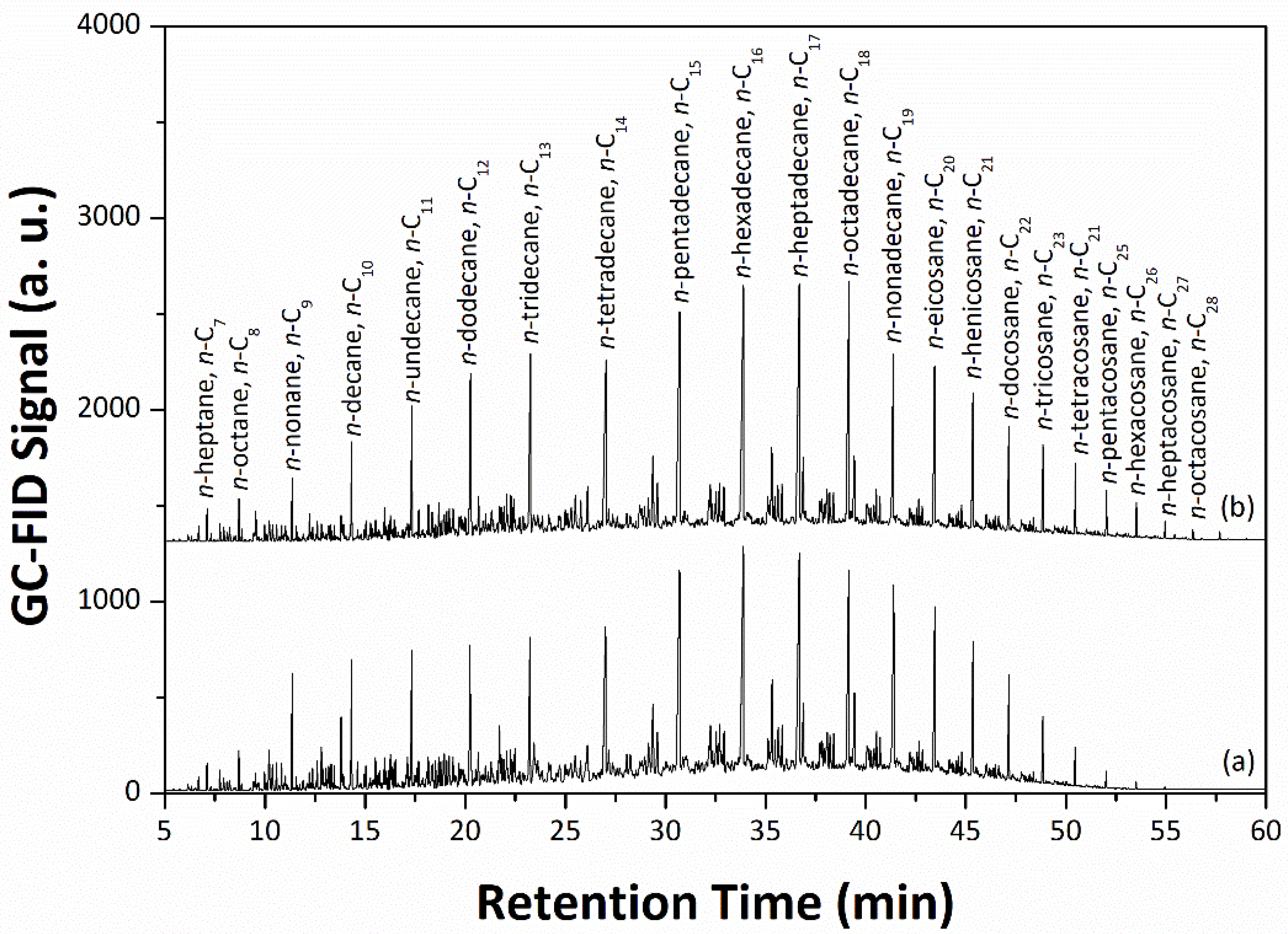
−1. After a time-on-stream (TOS) period of 96 h, the hydrotreated oils with low sulfur contents and almost no oxygen and nitrogen were obtained, particularly when the hydrogen pressure was increased to 7 MPa. The GC profiles show that the hydrotreated oil derived from the co-processing of Jatropha bio-oil with LGO over the mesoporous NiMo/γ-Al
2O
3 sulfide catalyst at 350 °C and 7 MPa of H
2 flow is similar to petrodiesel fuel (
Figure 8). In other words, the hydrotreated oil with reduced carbon footprint is a potential source of drop-in diesel-like fuel and is expected to be a greener fuel for the transport sector with low environmental impact.