Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources
Abstract
:1. Introduction
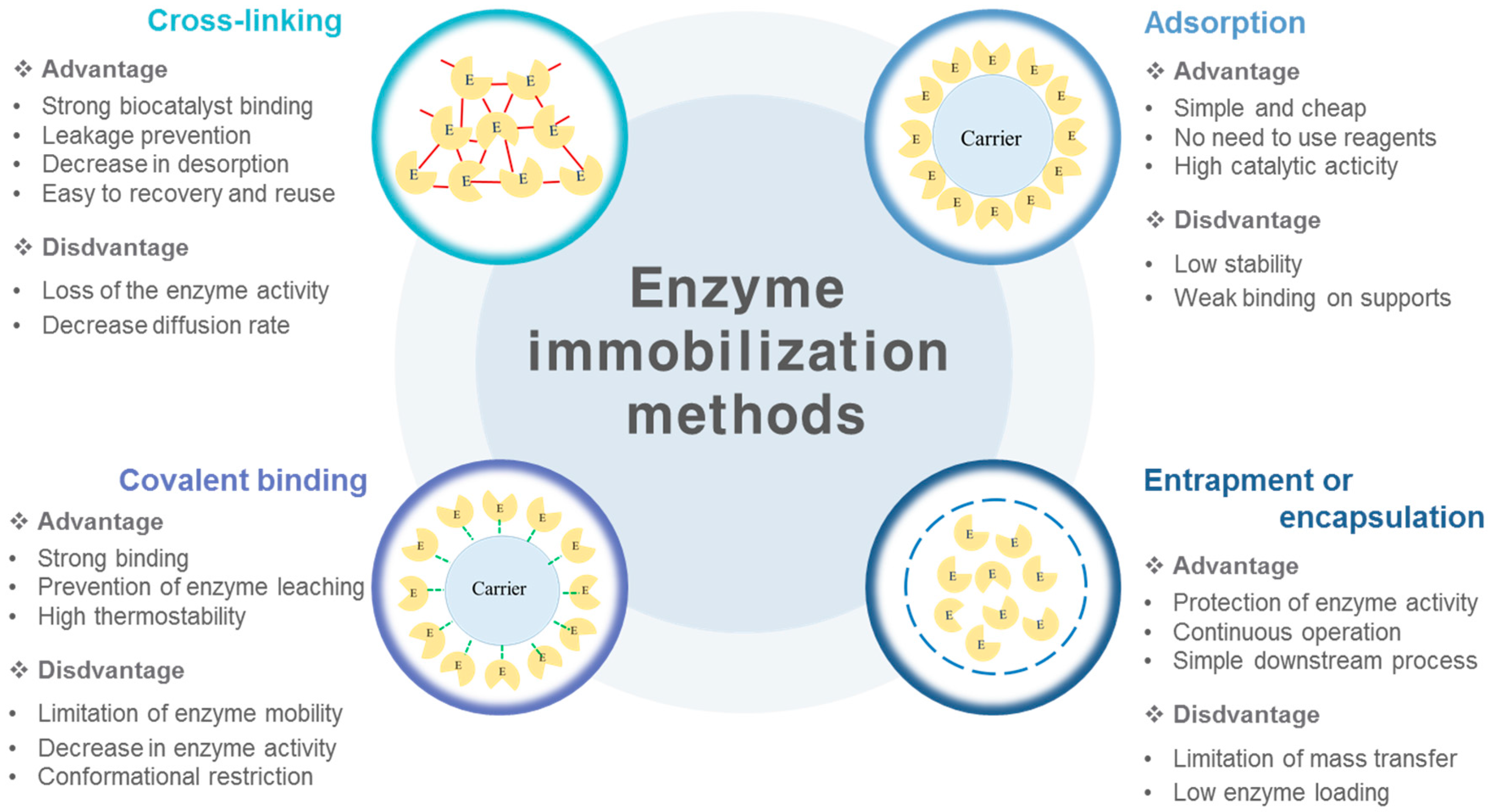
2. Enzyme Immobilization Techniques
2.1. Cross-Linking Immobilization
2.2. Adsorption Immobilization
2.3. Covalent Immobilization
2.4. Entrapment Immobilization
3. Development of Nano-Immobilized Lipase Biocatalyst
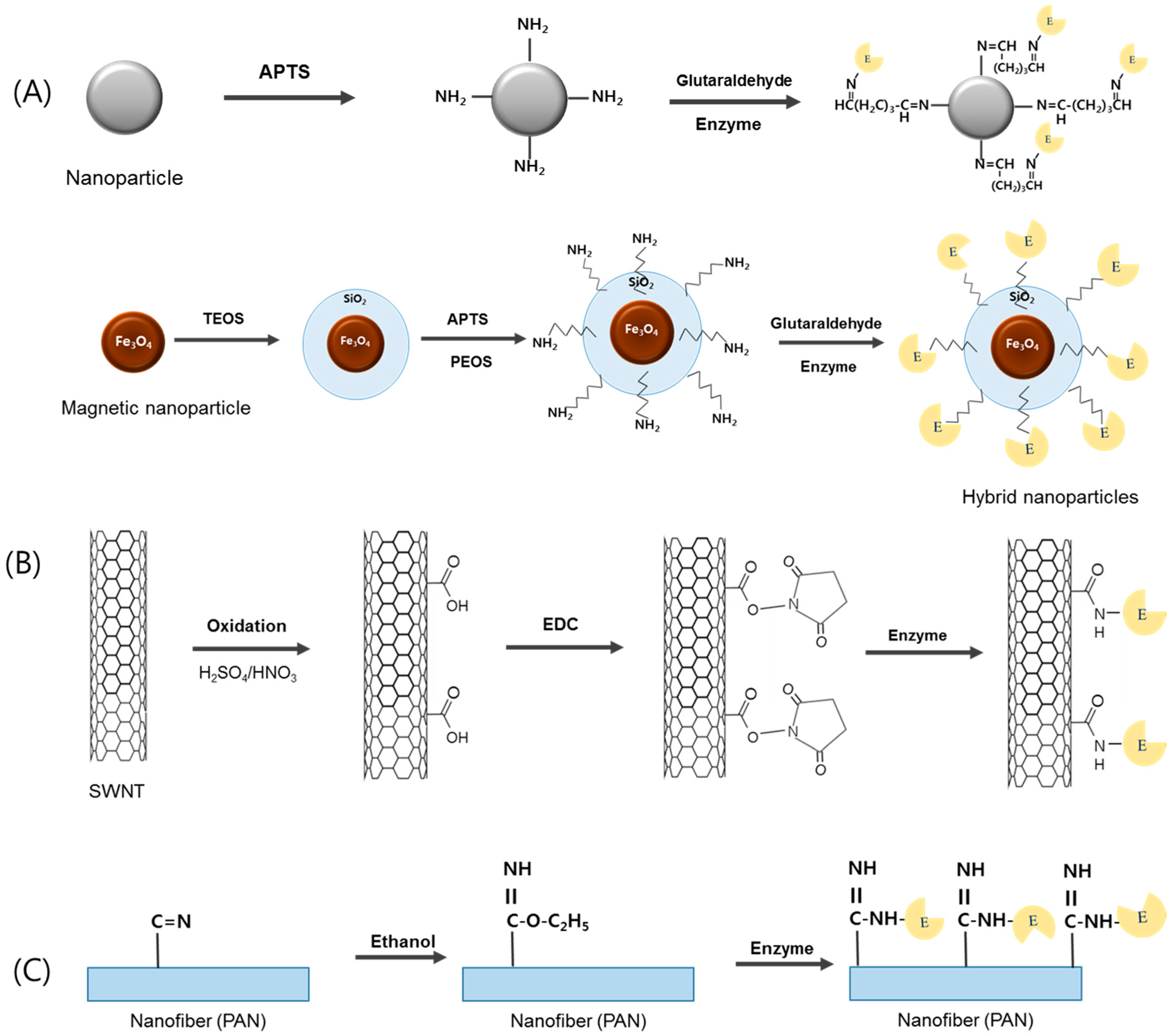
3.1. Nanoparticles-Based Lipase Immobilization
3.1.1. Non-Magnetic Nanoparticles
3.1.2. Magnetic Nanoparticles
3.2. Carbon Nanotubes-Based Lipase Immobilization
3.3. Electrospun Nanofibers-Based Lipase Immobilization
4. Biodiesel Production Using Nano-Immobilized Lipase
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentationfeedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar]
- Ho, S.H.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Perspectives on microalgal CO2-emissionmitigation systems: A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhair, S. Production of biodiesel: Possibilities and challenges. Biofuels Bioprod. Biorefin. 2007, 1, 57–66. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Berbel, J.; Verdugo-Escamilla, C. An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 1437–1452. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Gharat, N.; Rathod, V.K. Ultrasound assisted enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. Ultrason. Sonochem. 2013, 20, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Kondo, A. Enzymatic biodiesel production: An overview of potential feedstocks and process development. Bioresour. Technol. 2013, 135, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Azócar, L.; Heipieper, H.J.; Navia, R. Biotechnological processes for biodiesel production using alternative oils. Appl. Microbiol. Biotechnol. 2010, 88, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Yasin, N.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Lee, O.K.; Seong, D.H.; Lee, C.G.; Lee, E.Y. Sustainable production of liquid biofuels from renewable microalgae biomass. J. Ind. Eng. Chem. 2015, 29, 24–31. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization carrier. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, E.Y. Environmentally-benign dimethyl carbonate-mediated production of chemicals and biofuels from renewable bio-oil. Energies 2017, 10, 1790. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sardar, M. Enzyme immobilization: An overview on nanoparticles as immobilization matrix. Biochem. Anal. Biochem. 2015, 4. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.A.; Medintz, I.L.; Claussen, J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Gupta, M.N.; Kaloti, M.; Kapoor, M.; Solanki, K. Nanomaterials as matrices for enzyme immobilization. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011, 39, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEAs): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Serrano, P.; Cao, L.; Van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates with enhanced activity: Application to lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, J.; Yao, S.; Nie, L.; Deng, G.; Kuang, Y. Amperometric glucose biosensor based on adsorption of glucose oxidase at platinum nanoparticle-modified carbon nanotube electrode. Anal. Biochem. 2004, 331, 89–97. [Google Scholar] [CrossRef]
- Kim, H.; Lee, I.; Kwon, Y.; Kim, B.C.; Ha, S.; Lee, J.H.; Kim, J. Immobilization of glucose oxidase into polyanilinenanofiber matrix for biofuel cell applications. Biosens. Bioelectron. 2011, 26, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, I.; Choi, S.H.; Lee, O.K.; Kim, J.; Lee, E.Y. Nanoimmobilization of marine epoxide hydrolase of Mugil cephalus for repetitive enantioselective resolution of racemic styrene oxide in aqueous buffer. J. Nanosci. Nanotechnol. 2013, 13, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Gong, P.; Xu, D.; Dong, L.; Yao, S. Stabilization of α-chymotrypsin by covalent immobilization on amine-functionalized superparamagnetic nanogel. J. Biotechnol. 2007, 128, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Nair, S.; Kim, J.; Kwak, J.H.; Grate, J.W.; Kim, S.H.; Gu, M.B. Preparation of biocatalytic nanofibres with high activity and stability via enzyme aggregate coating on polymer nanofibres. Nanotechnology 2005, 16, S382–S388. [Google Scholar] [CrossRef] [PubMed]
- Fischback, M.B.; Youn, J.K.; Zhao, X.; Wang, P.; Park, H.G.; Chang, H.N.; Kim, J.; Ha, S. Miniature biofuel cells with improved stability under continuous operation. Electroanalysis 2006, 18, 2016–2022. [Google Scholar] [CrossRef]
- Liu, H.; Hu, N. Study on direct electrochemistry of glucose oxidase stabilized by cross-linking and immobilized in silica nanoparticle films. Electroanalysis 2007, 19, 884–892. [Google Scholar] [CrossRef]
- Kim, J.; Grate, J.W.; Wang, P. Nanobiocatalysis and its potential applications. Trends Biotechnol. 2008, 26, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Daubresse, C.; Grandfils, C.; Jerome, R.; Teyssie, P. Enzyme immobilization in nanoparticles produced by inverse microemulsion polymerization. J. Colloid Interface Sci. 1994, 168, 222–229. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Vijayakrishnan, V.; Schimossek, K. Entrapment of lipases in hydrophobic magnetite-containing sol-gel materials: Magnetic separation of heterogeneous biocatalysts. J. Mol. Catal. A Chem. 1998, 134, 251–258. [Google Scholar] [CrossRef]
- Yang, H.H.; Zhang, S.Q.; Chen, X.L.; Zhuang, Z.X.; Xu, J.G.; Wang, X.R. Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal. Chem. 2004, 76, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.I.N.G.; Li, M.; Patil, A.J.; Mann, S. Fabrication of protein/silica core–shell nanoparticles by microemulsion-based molecular wrapping. Adv. Mater. 2004, 16, 1838–1841. [Google Scholar] [CrossRef]
- Naik, R.R.; Tomczak, M.M.; Luckarift, H.R.; Spain, J.C.; Stone, M.O. Entrapment of enzymes and nanoparticles using biomimetically synthesized silica. Chem. Commun. 2004, 15, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoo, Y.J. Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnol. Bioprocess Eng. 2014, 19, 553–567. [Google Scholar] [CrossRef]
- Kim, J.; Grate, J.W. Single-enzyme nanoparticles armored by a nanometer-scale organic/inorganic network. Nano Lett. 2003, 3, 1219–1222. [Google Scholar] [CrossRef]
- Yan, M.; Ge, J.; Liu, Z.; Ouyang, P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J. Am. Chem. Soc. 2006, 128, 11008–11009. [Google Scholar] [CrossRef] [PubMed]
- Antczak, M.S.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis–key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Aarthy, M.; Saravanan, P.; Gowthaman, M.K.; Rose, C.; Kamini, N.R. Enzymatic transesterification for production of biodiesel using yeast lipases: An overview. Chem. Eng. Res. Des. 2014, 92, 1591–1601. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Netto, C.G.; Andrade, L.H.; Toma, H.E. Enantioselective transesterification catalysis by Candida antarctica lipase immobilized on superparamagnetic nanoparticles. Tetrahedron Asymmetry 2009, 20, 2299–2304. [Google Scholar] [CrossRef]
- Ji, P.; Tan, H.S.; Xu, X.; Feng, W. Lipase covalently attached to multi walled carbon nanotubes as an efficient catalyst in organic solvent. AIChE. J. 2010, 56, 3005–3011. [Google Scholar] [CrossRef]
- Li, S.F.; Chen, J.P.; Wu, W.T. Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. J. Mol. Catal. B Enzym. 2007, 47, 117–124. [Google Scholar] [CrossRef]
- Verma, M.L.; Puri, M.; Barrow, C.J. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit. Rev. Biotechnol. 2016, 36, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2013, 97, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Ching, C.B.; Xu, R. Lipase immobilization on modified zirconia nanoparticles: Studies on the effects of modifiers. Process Biochem. 2009, 44, 1245–1251. [Google Scholar] [CrossRef]
- Kim, M.I.; Ham, H.O.; Oh, S.D.; Park, H.G.; Chang, H.N.; Choi, S.H. Immobilization of Mucor javanicus lipase on effectively functionalized silica nanoparticles. J. Mol. Catal. B Enzym. 2006, 39, 62–68. [Google Scholar] [CrossRef]
- Miletić, N.; Abetz, V.; Ebert, K.; Loos, K. Immobilization of Candida antarctica lipase B on polystyrene nanoparticles. Macromol. Rapid Commun. 2010, 31, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Luo, G.; Dai, Y. Effect of solvents and precipitanton the properties of chitosan nanoparticles in a water-in-oil microemulsion and its lipase immobilisation performance. Bioresour. Technol. 2010, 101, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Kamel, G.; Sparago, C.; Bordi, F.; Lupi, S.; Diociaiuti, M.; Palocci, C. Structure-activity relationships of Candida rugosa lipase immobilised on polylactic acid nanoparticles. Soft Matter 2011, 7, 2653–2662. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Yang, C.T.; Ching, C.B.; Xu, R. Immobilization of lipases on hydrophobilized zirconia nanoparticles: Highly enantioselective and reusable biocatalysts. Langmuir 2008, 24, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Dyal, A.; Loos, K.; Noto, M.; Chang, S.W.; Spagnoli, C.; Shafi, K.V.; Ulman, A.; Cowman, M.; Gross, R.A. Activity of Candida rugosa lipase immobilized on γ-Fe2O3 magnetic nanoparticles. J. Am. Chem. Soc. 2003, 125, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Ponvel, K.M.; Kim, M.; Hwang, S.; Ahn, I.S.; Lee, C.H. Immobilization of lipase on hydrophobic nano-sized magnetite particles. J. Mol. Catal. B Enzym. 2009, 57, 62–66. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, J.K.; Kim, M.J.; Lee, C.J. Immobilization of lipase on single walled carbon nanotubes in ionic liquid. Bull. Korean Chem. Soc. 2010, 31, 650–652. [Google Scholar] [CrossRef]
- Lee, S.H.; Doan, T.T.N.; Won, K.; Ha, S.H.; Koo, Y.M. Immobilization of lipase within carbon nanotube–silica composites for non-aqueous reaction systems. J. Mol. Catal. B Enzym. 2010, 62, 169–172. [Google Scholar] [CrossRef]
- Shah, S.; Solanki, K.; Gupta, M.N. Enhancement of lipase activityin non-aqueous media upon immobilisation on multi-walled carbon nanotubes. Chem. Cent. J. 2007, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Buang, N.A.; Mahat, N.A.; Jamalis, J.; Huyop, F.; Aboul-Enein, H.Y.; Wahab, R.A. Simple adsorption of Candida rugosa lipase onto multi-walled carbon nanotubes for sustainable production of the flavor ester geranyl propionate. J. Ind. Eng. Chem. 2015, 32, 99–108. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized multi-wall carbon nanotubes for lipase immobilisation. Adv. Eng. Mater. 2010, 12, B179–B183. [Google Scholar] [CrossRef]
- Ye, P.; Xu, Z.K.; Wu, J.; Innocent, C.; Seta, P. Nanofibrous membranes containing reactive groups: Electrospinning from poly(acrylonitrile-co-maleic acid) for lipase immobilization. Macromolecules 2009, 39, 1041–1045. [Google Scholar] [CrossRef]
- Huang, X.J.; Chen, P.C.; Huang, F.; Ou, Y.; Chen, M.R.; Xu, Z.K. Immobilization of Candida rugosa lipase on electrospun cellulose nanofiber membrane. J. Mol. Catal. B Enzym. 2011, 70, 95–100. [Google Scholar] [CrossRef]
- Song, J.; Kahveci, D.; Chen, M.; Guo, Z.; Xie, E.; Xu, X.; Besenbacher, F.; Dong, M. Enhanced catalytic activity of lipase encapsulated in PCL nanofibers. Langmuir 2012, 28, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Nakane, K.; Hotta, T.; Ogihara, T.; Ogata, N.; Yamaguchi, S.J. Synthesis of (Z)-3-Hexen-1-yl acetate by lipase immobilised in polyvinylalcohol nanofibers. J. Appl. Polym. Sci. 2007, 106, 863–867. [Google Scholar] [CrossRef]
- Lei, L.; Liu, X.; Li, Y.; Cui, Y.; Yang, Y.; Qin, G. Study on synthesis of poly (GMA)-grafted Fe3O4/SiOx magnetic nanoparticles using atom transfer radical polymerization and their application for lipase immobilization. Mater. Chem. Phys. 2011, 125, 866–871. [Google Scholar] [CrossRef]
- Huang, S.H.; Liao, M.H.; Chen, D.H. Direct binding and characterizationof lipase onto magnetic nanoparticles. Biotechnol. Prog. 2003, 19, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Effect of silica coating on Fe3O4 magnetic nanoparticles for lipase immobilization and their application for biodiesel production. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zhao, C.; Ding, Y.; Xu, P. Biodiesel production in packed-bed reactors using lipase–nanoparticle biocomposite. Bioresour. Technol. 2011, 102, 6352–6355. [Google Scholar] [CrossRef] [PubMed]
- Marta, Z.; Dorota, C.; Tomasz, S.; Adam, S.; Katarzyna, W.; Joanna, S.; Halina, K.; Marszałł, M.P. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization-New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Sakai, S.; Liu, Y.; Yamaguchi, T.; Watanabe, R.; Kawabe, M.; Kawakami, K. Production of butyl-biodiesel using lipase physically-adsorbed onto electrospun polyacrylonitrile fibers. Bioresour. Technol. 2010, 101, 7344–7349. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Fan, Y.H.; Hu, R.F.; Wu, W.T. Pseudomonas cepacia lipase immobilized onto the electrospun PAN nanofibrous membranes for biodiesel production from soybean oil. J. Mol. Catal. B Enzym. 2011, 72, 40–45. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Raita, M.; Arnthong, J.; Champreda, V.; Laosiripojana, N. Modification of magnetic nanoparticle lipase designs for biodiesel production from palm oil. Fuel Process. Technol. 2015, 134, 189–197. [Google Scholar] [CrossRef]
- Karimi, M. Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Effect of solvents and oil content on direct transesterification of wet oil-bearing microalgal biomass of Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized lipase as the biocatalyst. Bioresour. Technol. 2013, 135, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: Effect of water, t-butanol and blue silica gel contents. Renew. Energy 2016, 91, 196–206. [Google Scholar] [CrossRef]
| Nanomaterials | Strain | Carrier | Type of Binding | Special Feature | Refs. |
|---|---|---|---|---|---|
| Nanoparticles | Pseudomonas cepacia | Zironia | Covalent | Increased activity and enantioselectivity | [47] |
| Mucor japonicus | Silica | Covalent | Enhanced enzyme loading and enzyme stability | [48] | |
| Candida antarctica | Polystyrene | Adsortion | High hydrolytic activity | [49] | |
| Candida rugosa | Chitosan | Covalent | High enzyme loading and activity retention | [50] | |
| Candida rugosa | Polylactic acid | Adsorption | Enhanced activity and stability | [51] | |
| Candida rugosa | γ-Fe2O3 | Covalent | Enhanced stability | [53] | |
| Porcine pancreas | Magnetic | Adsorption | Good reusability | [54] | |
| Carbon nanotube | Pseudomonas cepacia | SWNT | Adsorption, covalent | Increased retention of enzyme activity | [55] |
| Rhizopus arrhizus | MWNT | Covalent | Enhanced resolution efficiency | [43] | |
| Candida rugosa, C. antarctica B, Thermomyces lanuginosus | MWNT | Adsorption | Enhanced stability | [56] | |
| Candida rugosa | MWNT | Adsorption | High enzyme activity | [57] | |
| Candida rugosa | MWNT | Adsorption | Enhanced activity and thermal stability | [58] | |
| Candida antarctica | MWNT | Adsorption | Enhanced activity and stability | [59] | |
| Nanofibers | Candida antarctica | Polyacrylnitrate | Covalent | High enzyme stability | [44] |
| Candida rugosa | Poly-(acrylonitrile-comaleic acid) | Covalent | High activity and enzyme loading | [60] | |
| Candida rugosa | Cellulose acetate | Covalent | Enhanced thermal stability | [61] | |
| Burkholderia cepacia | Polycaprolactane | Covalent | Enhanced catalytic activity and reusability | [62] | |
| Candida rugosa | Polyvinyl alcohol (PVA) | Covalent | Equivalent esterification activity to that of Novozyme 435 | [63] |
| Strain | Carrier | Substrate | Biodiesel Coversion (%) | Reusability (Days or Cycles) | Refs. |
|---|---|---|---|---|---|
| Pseudomonas cepacia | Fe3O4 | Soybean oil | 88 | 10 days | [67] |
| PAN-nanofiber | Rapeseed oil | 94 | 20 days | [73] | |
| Soybean oil | 90 | 10 cycles | [74] | ||
| Thermomyces lanuginosa | Amino-Fe3O4 | Soybean oil | 90 | 4 cycles | [75] |
| Palm oil | 97 | 5 cycles | [76] | ||
| Epoxy-silica | Canola oil | 99 | 20 cycles | [82] | |
| Burkholderia sp. | Amino-Fe3O4-SiO2 | Waste cooking oil | 91 | 3 cycles | [77] |
| Alkyl-Fe3O4-SiO2 | Olive oil | 90 | 10 cycles | [80] | |
| Chlorella vulgaris | 90 | 2 cycles | [81] | ||
| Rhizomucor miehei | PAMAM-mMWCNT | Waste cooking oil | 94 | 10 cycles | [78] |
| Epoxy-silica | Canola oil | 95 | 7 cycles | [82] | |
| Candida antarctica | Epoxy-Fe3O4-SiO2 | Waste cooking oil | 100 | 6 cycles | [79] |
| Epoxy-silica | Canola oil | 59 | 15 cycles | [82] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources. Catalysts 2018, 8, 68. https://doi.org/10.3390/catal8020068
Kim KH, Lee OK, Lee EY. Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources. Catalysts. 2018; 8(2):68. https://doi.org/10.3390/catal8020068
Chicago/Turabian StyleKim, Keon Hee, Ok Kyung Lee, and Eun Yeol Lee. 2018. "Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources" Catalysts 8, no. 2: 68. https://doi.org/10.3390/catal8020068





