New Trendy Magnetic C-Scorpionate Iron Catalyst and Its Performance towards Cyclohexane Oxidation
Abstract
:1. Introduction
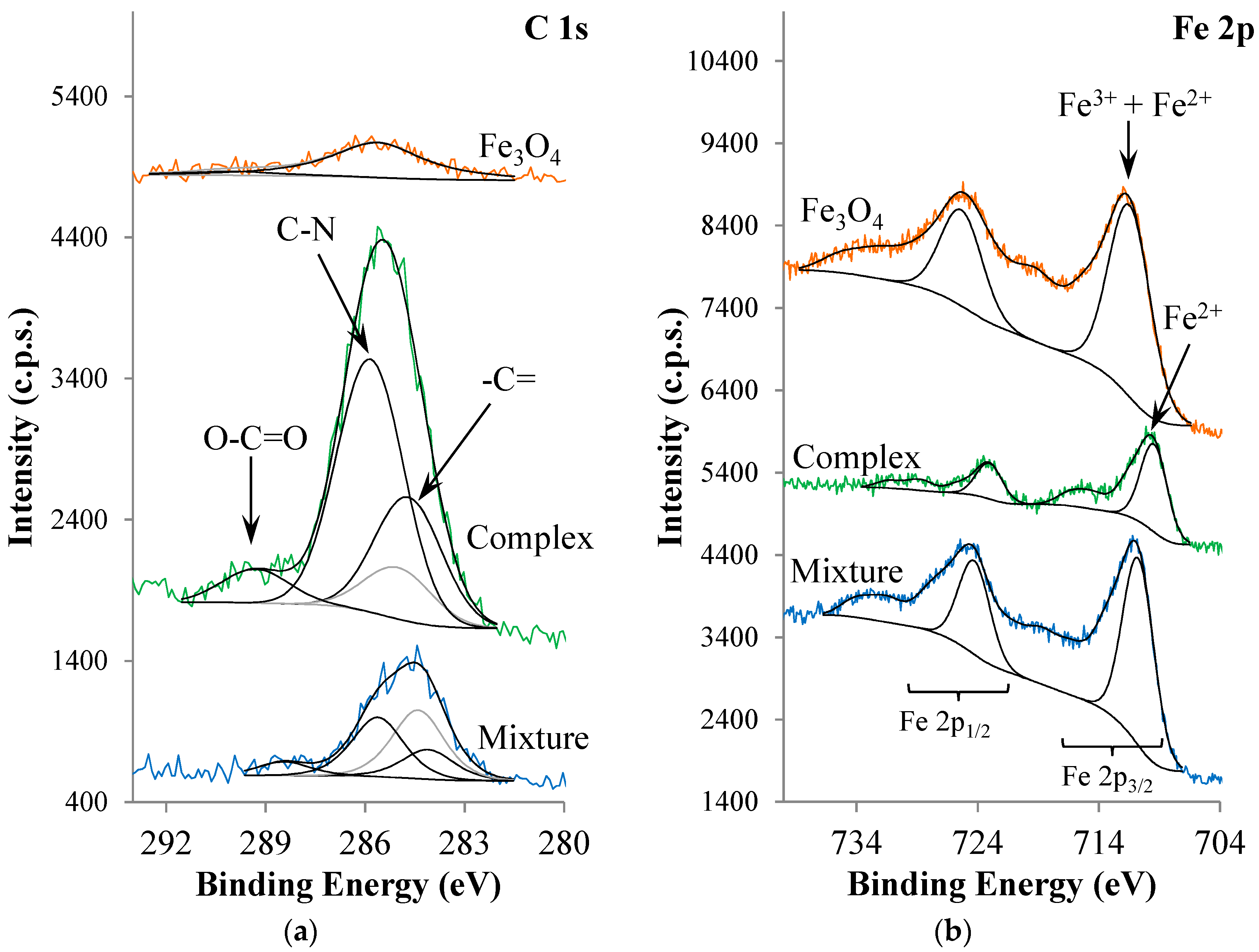
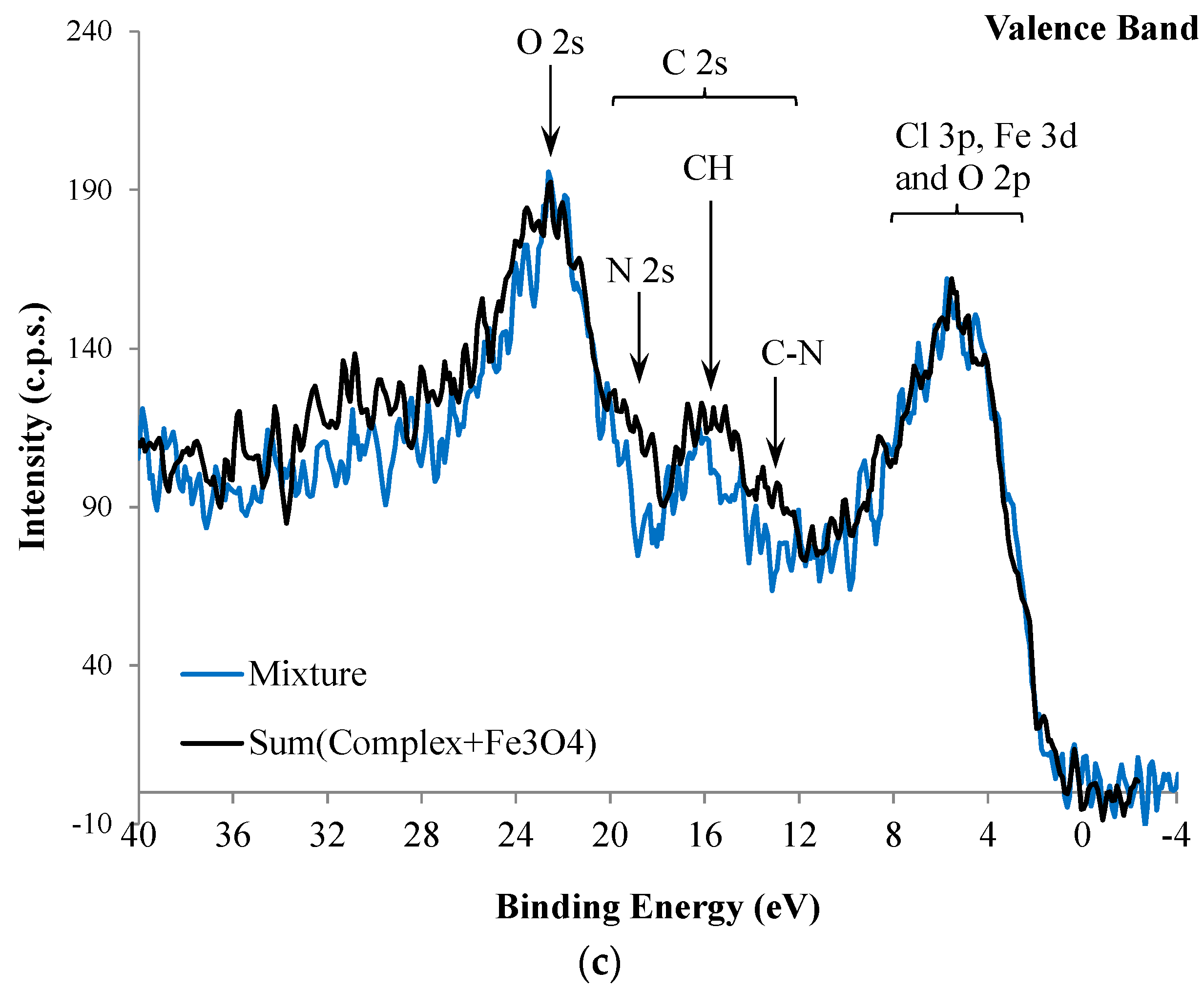
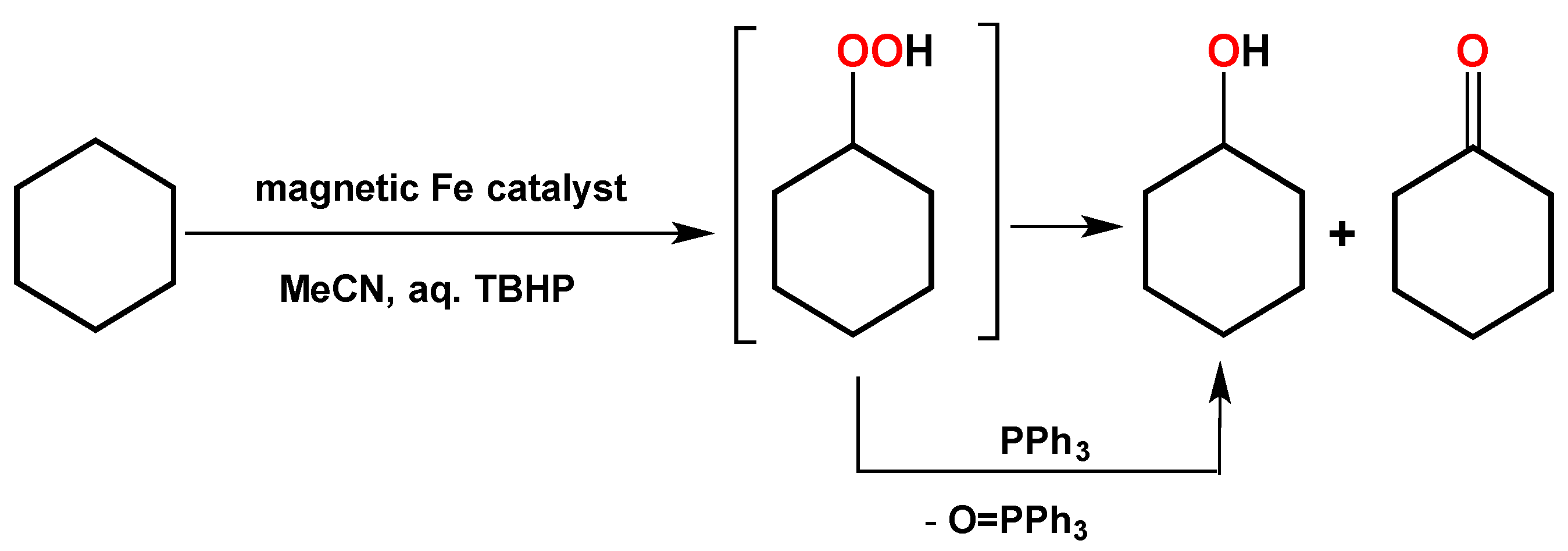
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Section
3.2. Preparation of the Dispersed Catalyst
3.3. Procedure for the Catalytic Oxidations
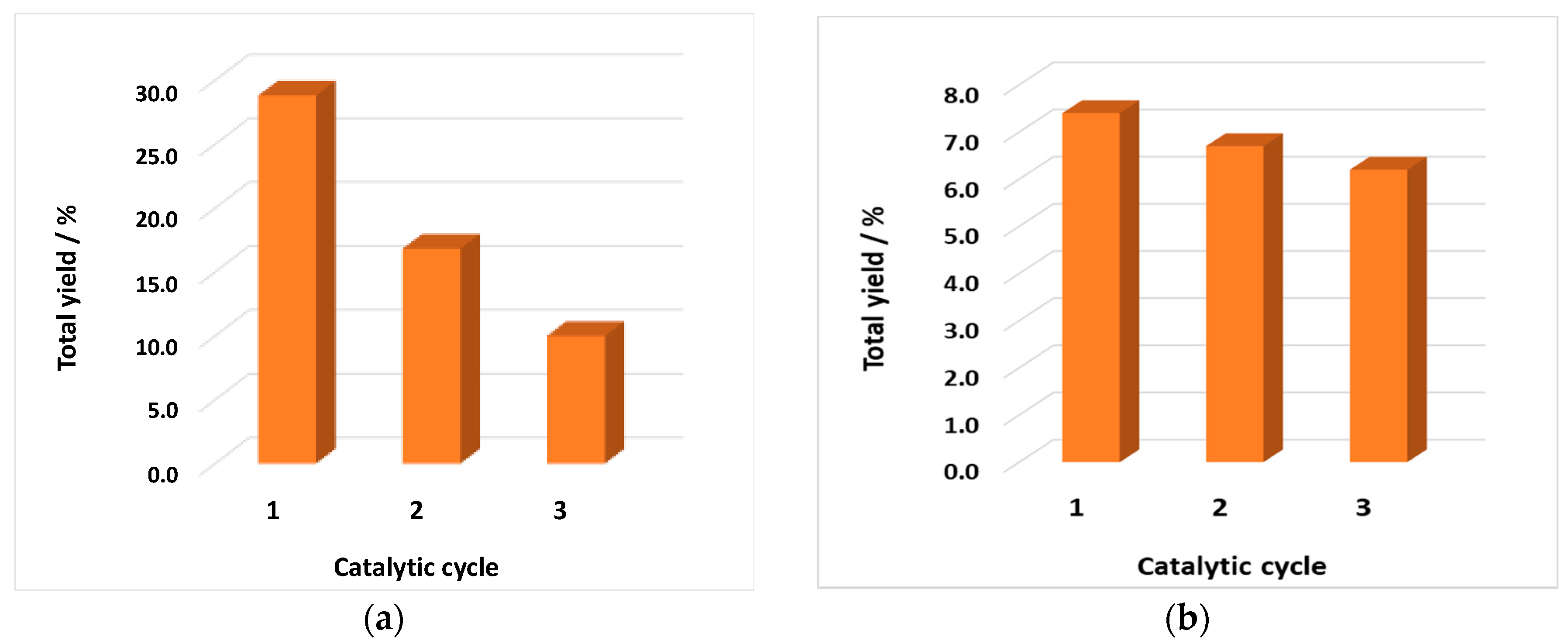
3.4. Catalyst Recycling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically Separable Nanocatalysts: Bridges between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Stubbs, L.P.; Ho, F.; Liu, R.; Ship, C.P.; Maguire, J.A.; Hosmane, N.S. Magnetic Nanomixtures: A New Perspective in Catalysis. ChemCatChem 2010, 2, 365–374. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.-M. Magnetically Recoverable Nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. Fast-Growing Field of Magnetically Recyclable Nanocatalysts. Chem. Rev. 2014, 114, 6949–6985. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin, Germany, 2008. [Google Scholar]
- Stolle, A.; Ranu, B. (Eds.) Ball Milling towards Green Synthesis Applications, Projects, Challenges; Royal Society of Chemistry (RCS): London, UK, 2015. [Google Scholar]
- Ribeiro, A.P.C.; Fontolan, E.; Alegria, E.C.B.A.; Kopylovich, M.N.; Bertani, R.; Pombeiro, A.J.L. The influence of multiwalled carbon nanotubes and graphene oxide additives on the catalytic activity of 3d metal catalysts towards 1-phenylethanol oxidation. J. Mol. Catal. A Chem. 2017, 426, 557–563. [Google Scholar] [CrossRef]
- Estes, L.L.; Schweizer, M. Fibers, 4 Polyamide Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2011; Volume 11, pp. 41–49. [Google Scholar]
- Ahmed, J.; Ahuja, S.; Ali, I.; Anderson, C.; Anderson, M.J.; Arai, Y.; Braglia, M.; Babirad, S.; Bag, D.; Bagchi, A.; et al. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Clerici, M.G.; Ricci, M.; Strukul, G. Metal-Catalysis in Industrial Organic Processes; Chiusoli, G.P., Maitlis, P.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2006. [Google Scholar]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 15–16, 2236–2252. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-homoscorpionate oxidation catalysts—Electrochemical and catalytic activity. Catalysts 2017, 7, 12. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Mendes, M.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Liquid phase oxidation of xylenes catalyzed by the tripodal C-scorpionate iron(II) complex [FeCl2{κ3-HC(pz)3}]. Polyhedron 2017, 125, 151–155. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate vanadium, iron and copper complexes as selective catalysts for the peroxidative oxidation of cyclohexane under mild conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A Gen. 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Peixoto de Almeida, M.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-scorpionate Fe(II) complex in carbon materials for cyclohexane oxidation with hydrogen peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Naumkin, A.; Kraut-Vass, V.A.; Gaarenstroom, S.W.; Powell, C.J. (Eds.) NIST X-ray Photoelectron Spectroscopy Database; Standard Reference Database 20, Measurement Services Division of the National Institute of Standards and Technology (NIST); Material Measurement Laboratory (MML): Gaithersburg, MD, USA, 2012.
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning Cyclohexane Oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organometallics 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Silva, M.F.C.G.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Silva, T.F.S.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Org. Chem. 2011, 24, 764–773. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Matias, I.A.S.; Duarte, T.A.G.; Pombeiro, A.J.L. Catalytic performance of Fe(II)-scorpionate complexes towards cyclohexane oxidation in organic, ionic liquid and/or supercritical CO2 media: A comparative study. Catalysts 2017, 7, 230. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Pombeiro, A.J.L. Highly selective cyclohexane oxidation catalyzed by ferrocene in ionic liquid medium. Lett. Org. Chem. 2017, 14, 571–574. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. C. R. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Kefini, K.K.; Msagati, T.A.M.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterization and applications in electronic devices. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Alegria, E.C.B.A.; Fontolan, E.; Ribeiro, A.P.C.; Kopylovich, M.N.; Domingos, C.; Ferraria, A.M.; Bertani, R.; Botelho do Rego, A.M.; Pombeiro, A.J.L. Simple solvent-free preparation of dispersed composites and their application as catalysts in oxidation and hydrocarboxylation of cyclohexane. Mater. Today Chem. 2017, 5, 52–62. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar] [CrossRef]

| Atomic Ratios | Expected | Experimental | |
|---|---|---|---|
| [FeCl2{κ3-HC(pz)3}] | [FeCl2{κ3-HC(pz)3}] | [FeCl2{κ3-HC(pz)3}] + Fe3O4 | |
| Fe/N | 0.17 | 0.18 | 2.4 |
| N/Cl | 3.0 | 4.0 | 1.7 |
| C/N | 1.7 | 2.2 | 2.7 |
| Fe3O4 | |||
| O/Fe | 1.3 | - | 1.2 |
| Entry | Time/h | Additive | Yield/% b | A/K c | Total TON d | Total TOF/h−1 e | ||
|---|---|---|---|---|---|---|---|---|
| CyO (K) | CyOH (A) | Total | ||||||
| 1 | 1 | Hpca | 7.3 | 11.3 | 18.6 | 1.5 | 93 | 93 |
| 2 | 3 | Hpca | 9.9 | 18.9 | 28.8 | 1.9 | 144 | 48 |
| 3 | 6 | Hpca | 10.3 | 19.2 | 29.5 | 1.9 | 147 | 25 |
| 4 | 3 | no additive | 3.3 | 1.5 | 4.8 | 0.5 | 24 | 8 |
| 5 | 1 | HNO3 | 1.2 | 2.3 | 3.5 | 1.9 | 17.5 | 18 |
| 6 | 1 | H2SO4 | 1.8 | 1.9 | 3.7 | 1.1 | 18.5 | 19 |
| 7 | 1 | TEMPO | 1.2 | 1.7 | 2.9 | 1.4 | 14.5 | 15 |
| 8 f | 3 | Hpca | 14.7 | 12.3 | 27 | 0.8 | 135 | 45 |
| 9 g | 3 | Hpca | 2.9 | 2 | 4.9 | 0.7 | 24.5 | 8 |
| 10 h | 1 | Hpca | 5.3 | 6.2 | 11.5 | 1.2 | 57.5 | 58 |
| 11 i | 1 | Hpca | 10.3 | 16.3 | 26.6 | 1.6 | 133 | 133 |
| 12 j | 1 | Hpca | 4.1 | 8.3 | 12.4 | 2.0 | 62 | 62 |
| 13 k | 6 | Hpca | 12.4 | 20.6 | 33.0 | 0.6 | 165 | 28 |
| 14 l | 6 | Hpca | 1.6 | 0.9 | 2.5 | 0.6 | 12.5 | 2 |
| Entry | Time/h | Additive | Yield/% b | Total TON c | Total TOF/h−1 d | ||
|---|---|---|---|---|---|---|---|
| CyO (K) | CyOH (A) | Total | |||||
| 1 a | 1 | Hpca | 0 | 2.3 | 2.3 | 11.5 | 12 |
| 2 a | 3 | Hpca | 0 | 3.3 | 3.3 | 16.5 | 6 |
| 3 a | 6 | Hpca | 0.6 | 6.8 | 7.4 | 37 | 6 |
| 4 a | 3 | no additive | 0 | 5.7 | 5.7 | 28.5 | 10 |
| 5 a,e | 6 | Hpca | 0 | 2 | 2 | 10 | 2 |
| 6 a,f | 3 | Hpca | 0 | 1.1 | 1.1 | 5.5 | 2 |
| 7 g | 6 | Hpca | 2.3 | 5 | 7.3 | 36.5 | 6 |
| 8 h | 6 | Hpca | 2.1 | 4.9 | 7.0 | 35.0 | 6 |
| 9 i | 6 | Hpca | 3.7 | 3.2 | 6.9 | 34.5 | 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.P.C.; Matias, I.A.S.; Alegria, E.C.B.A.; Ferraria, A.M.; Botelho do Rego, A.M.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. New Trendy Magnetic C-Scorpionate Iron Catalyst and Its Performance towards Cyclohexane Oxidation. Catalysts 2018, 8, 69. https://doi.org/10.3390/catal8020069
Ribeiro APC, Matias IAS, Alegria ECBA, Ferraria AM, Botelho do Rego AM, Pombeiro AJL, Martins LMDRS. New Trendy Magnetic C-Scorpionate Iron Catalyst and Its Performance towards Cyclohexane Oxidation. Catalysts. 2018; 8(2):69. https://doi.org/10.3390/catal8020069
Chicago/Turabian StyleRibeiro, Ana P. C., Inês A. S. Matias, Elisabete C. B. A. Alegria, Ana M. Ferraria, Ana M. Botelho do Rego, Armando J. L. Pombeiro, and Luísa M. D. R. S. Martins. 2018. "New Trendy Magnetic C-Scorpionate Iron Catalyst and Its Performance towards Cyclohexane Oxidation" Catalysts 8, no. 2: 69. https://doi.org/10.3390/catal8020069









