Photocatalytic Reduction of CO2 from Simulated Flue Gas with Colored Anatase
Abstract
:1. Introduction
2. Results and Discussion
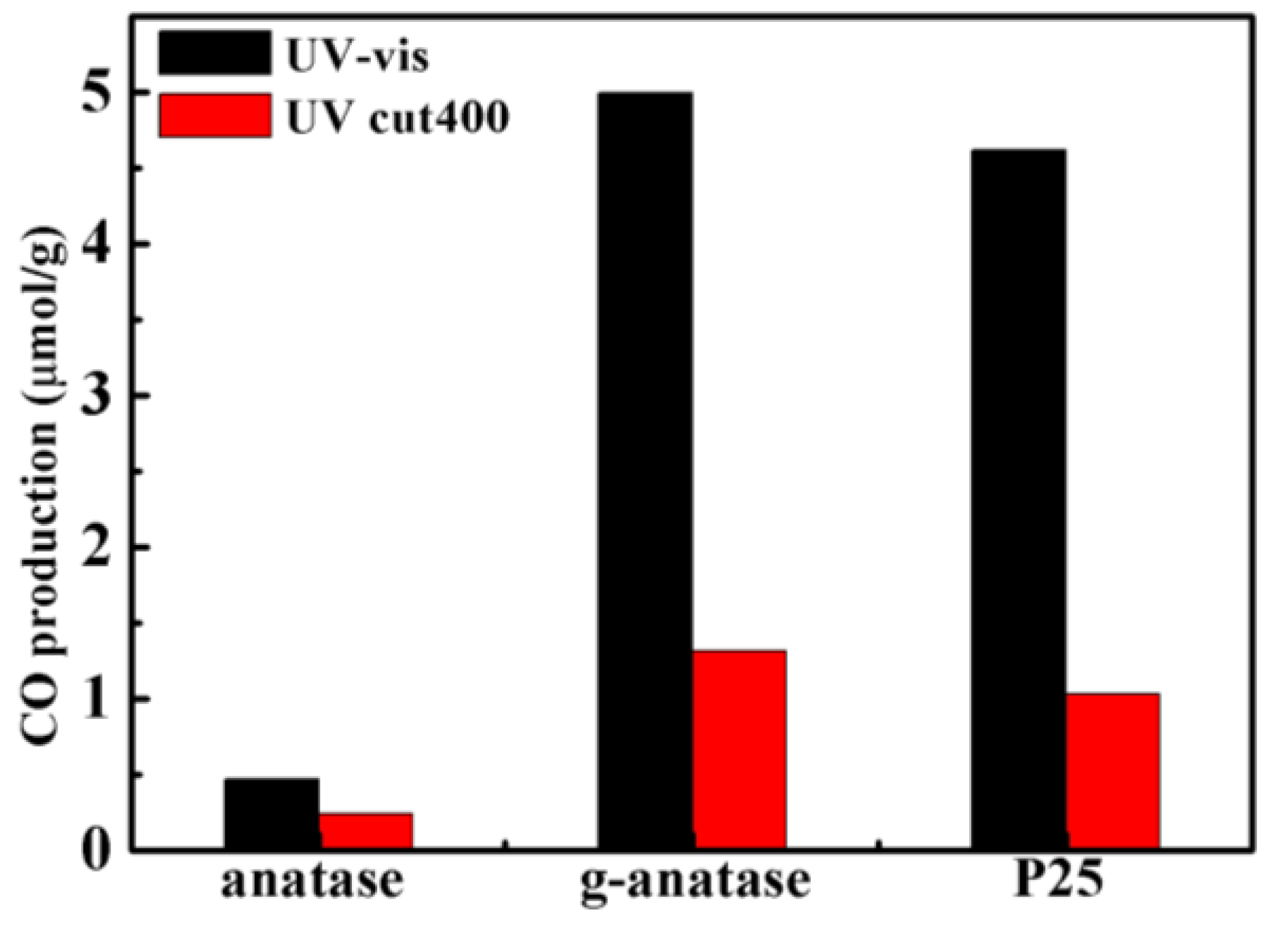
2.1. CO2 Photoreduction Performance
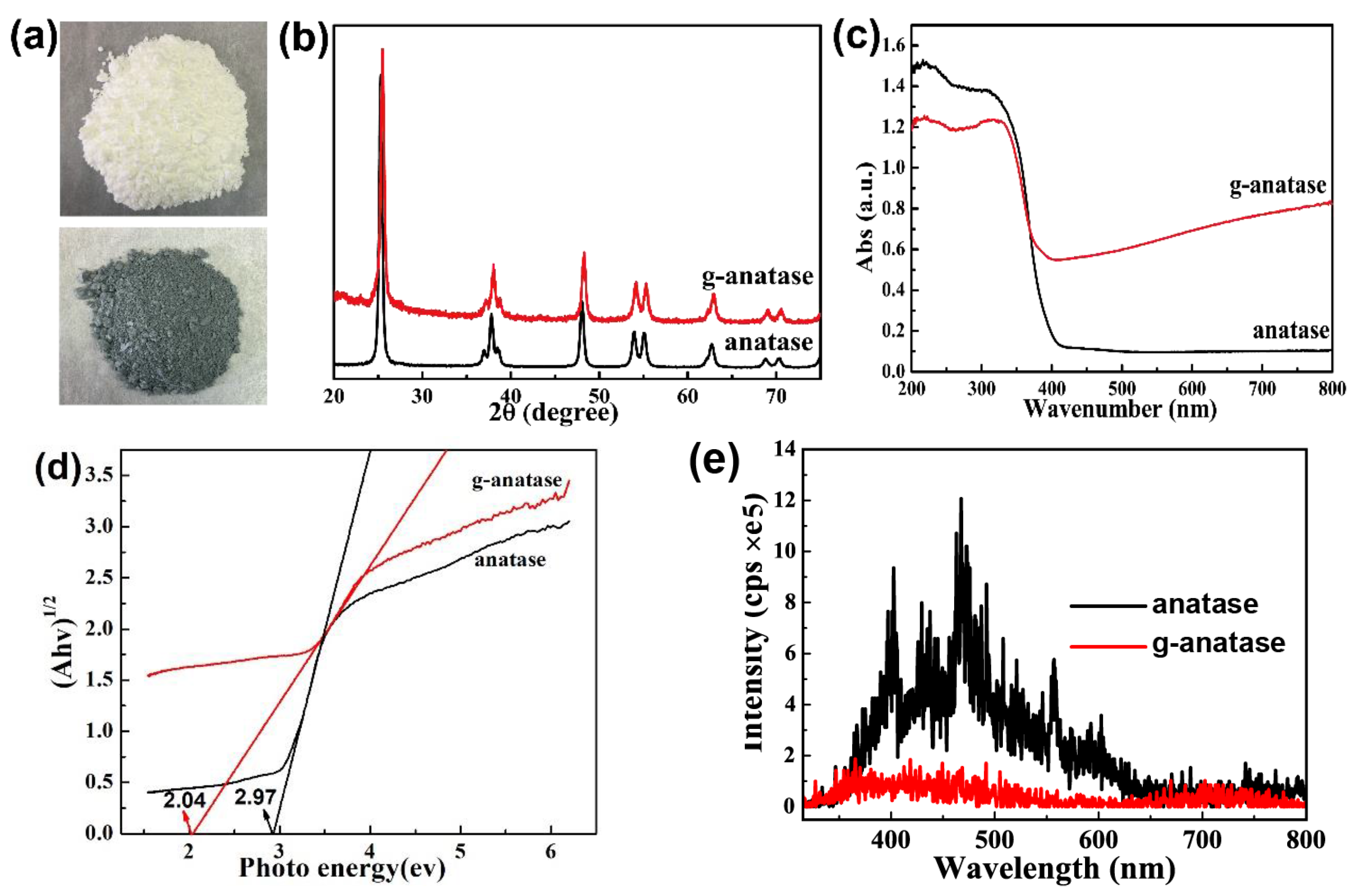
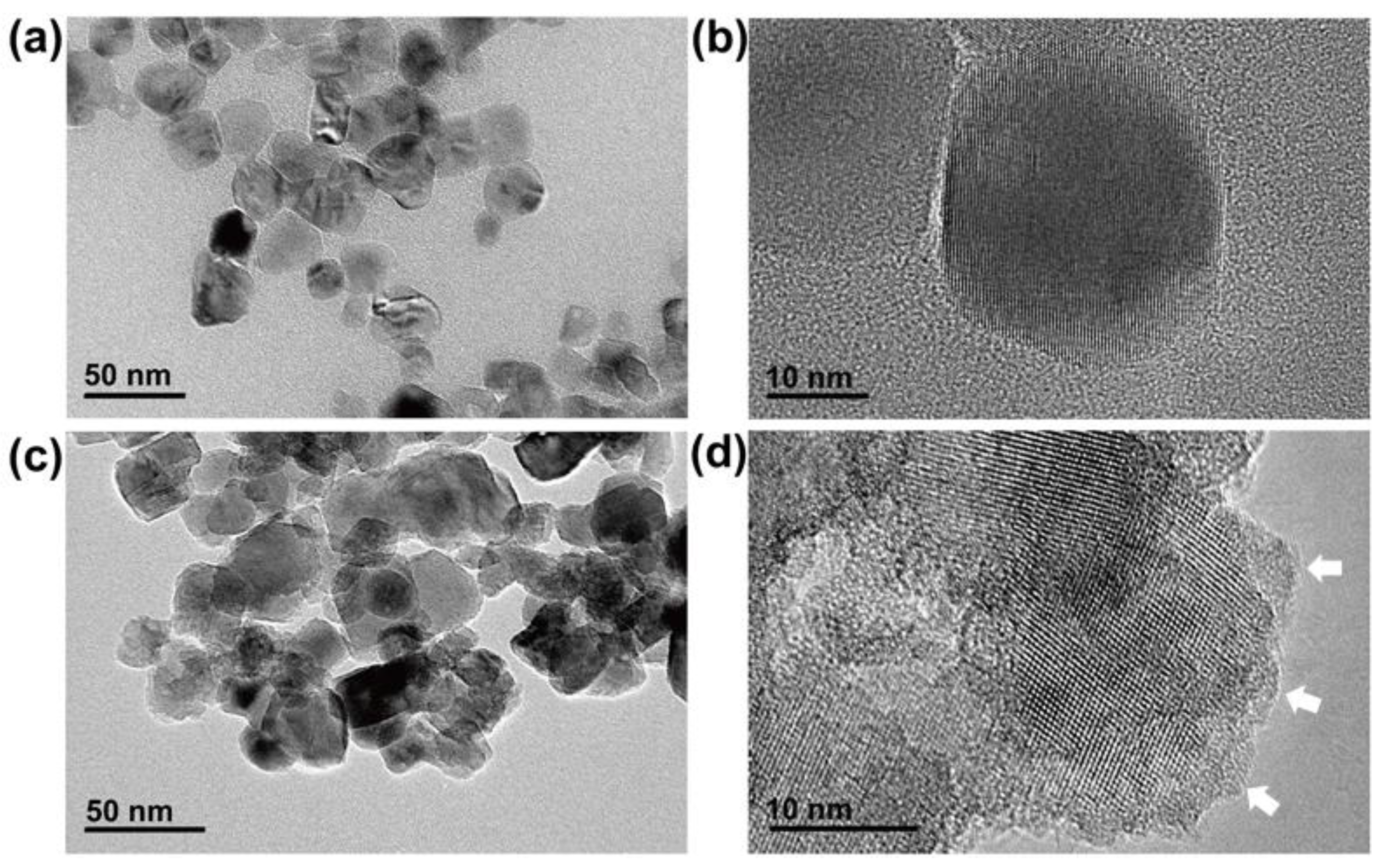
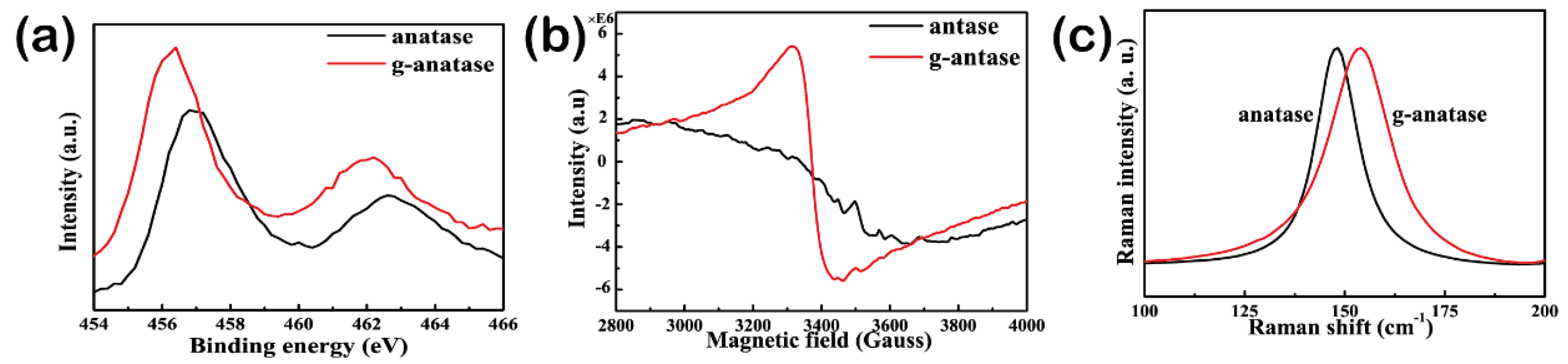
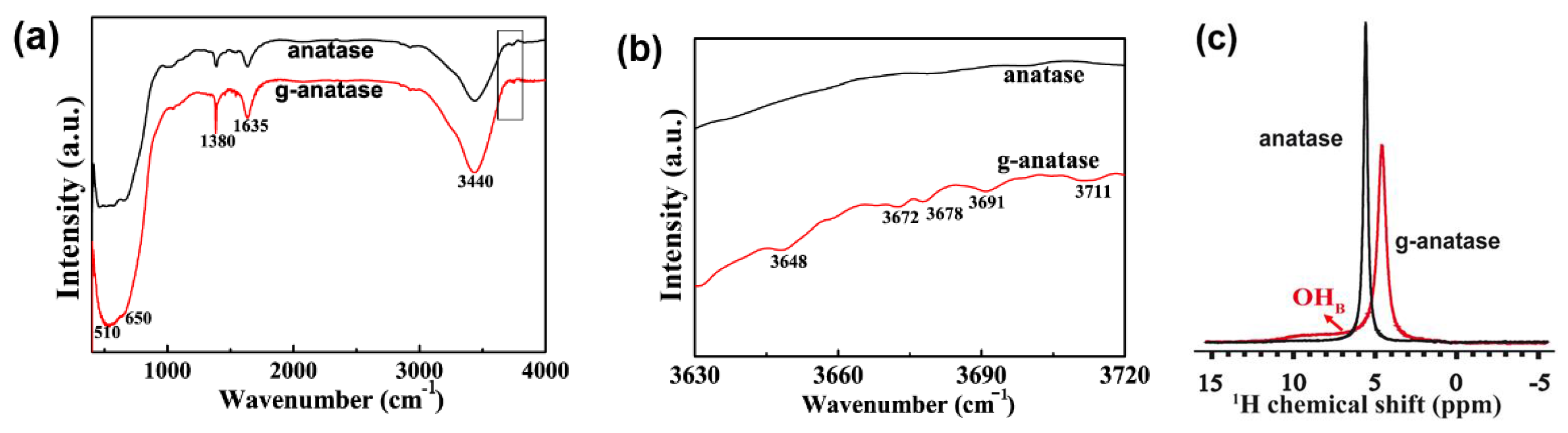
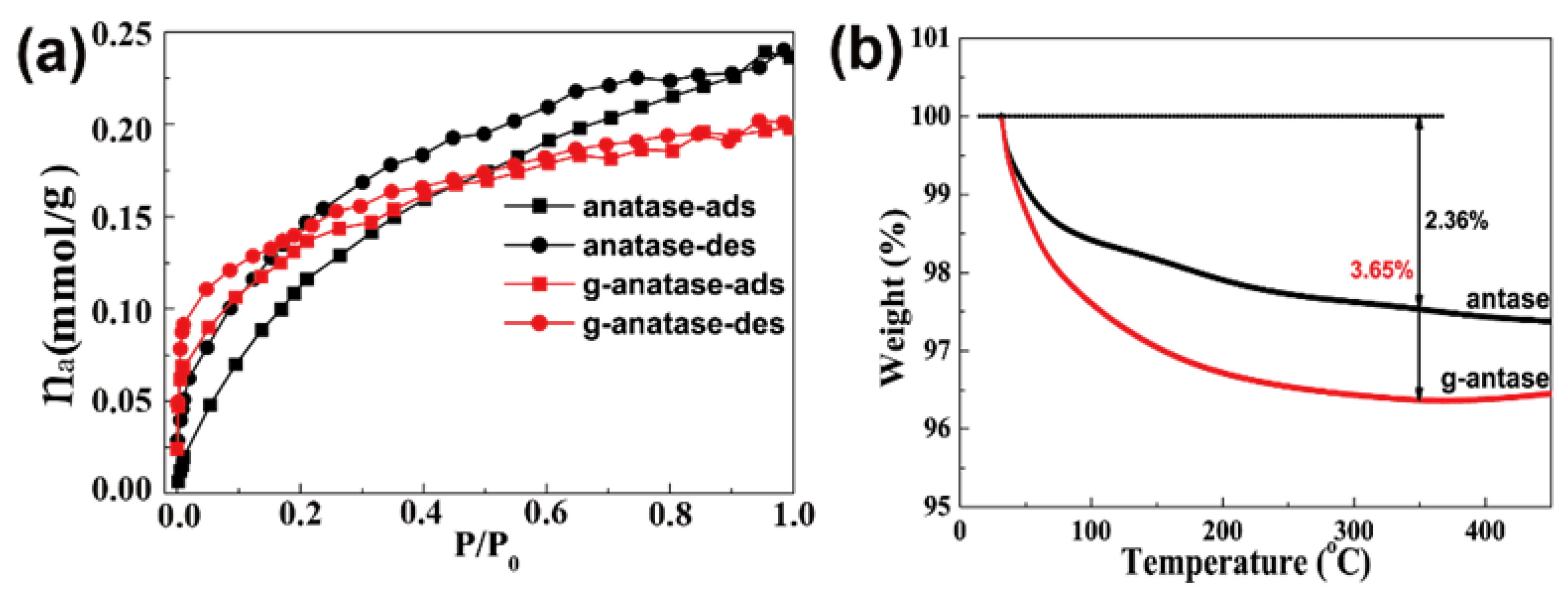
2.2. Characterization of Colored Anatase
3. Experimental Section
3.1. Surface Defective Anatase Preparation
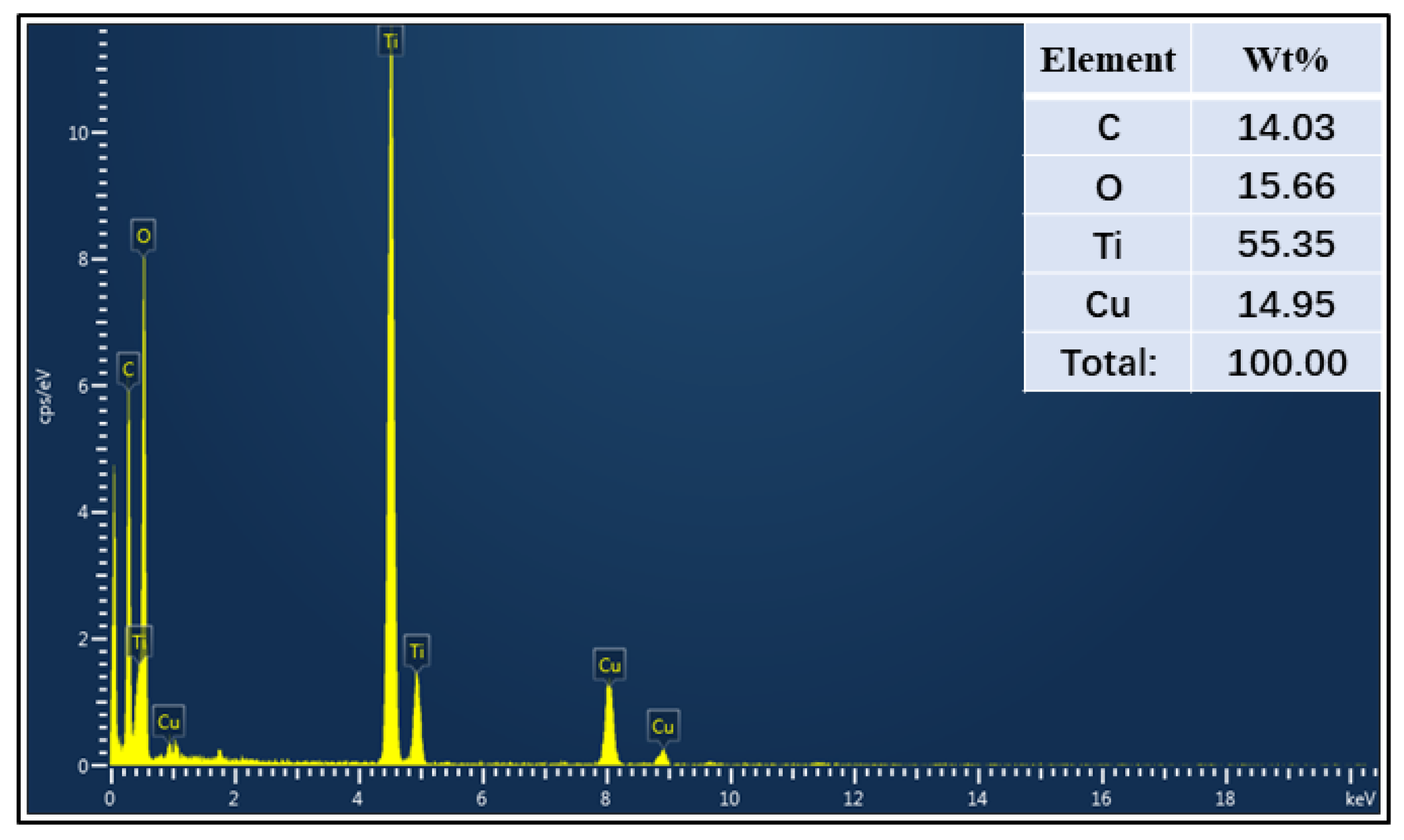
3.2. Sample Characterization
3.3. The Gas-Solid Reaction System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, J.G.; Low, J.X.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qin, J.; Jiang, M.; Ding, Z.; Hou, Y. Enhanced selective photocatalytic CO2 reduction into CO over Ag/CdS nanocomposites under visible light. Appl. Surf. Sci. 2017, 391, 572–579. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Zakaria, Z.Y. Photo-induced reduction of CO2 to CO with hydrogen over plasmonic Ag-NPs/TiO2 NWs core/shell hetero-junction under UV and visible light. J. CO2 Util. 2017, 18, 250–260. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Synergistic effect in plasmonic Au/Ag alloy NPs co-coated TiO2 NWs toward visible-light enhanced CO2 photoreduction to fuels. Appl. Catal. B Environ. 2017, 204, 548–560. [Google Scholar] [CrossRef]
- House, R.L.; Iha, N.Y.M.; Coppo, R.L.; Alibabaei, L.; Sherman, B.D.; Kang, P.; Brennaman, M.K.; Hoertz, P.G.; Meyer, T.J. Artificial photosynthesis: Where are we now? Where can we go? J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 32–45. [Google Scholar] [CrossRef]
- Nakajima, T.; Tamaki, Y.; Ueno, K.; Kato, E.; Nishikawa, T.; Ohkubo, K.; Yamazaki, Y.; Morimoto, T.; Ishitani, O. Photocatalytic reduction of low concentration of CO2. J. Am. Chem. Soc. 2016, 138, 13818–13821. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramos, J.; DiMeglio, J.L.; Rosenthal, J. Efficient reduction of CO2 to CO with high current density using in situ or ex situ prepared Bi-Based materials. J. Am. Chem. Soc. 2014, 136, 8361–8367. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.Y.; Li, Z. Methanol synthesis in the catalytic reduction of CO2 under the visible light by BiFeO3 modified with carbon nanotubes. J. Chin. Ceram. Soc. 2009, 37, 1869–1872. [Google Scholar]
- Ponzoni, C.; Rosa, R.; Cannio, M.; Buscaglia, V.; Finocchio, E.; Nanni, P.; Leonelli, C. Electrophoretic deposition of multiferroic BiFeO3 submicrometric particles from stabilized suspensions. J. Eur. Ceram. Soc. 2012, 33, 1325–1333. [Google Scholar] [CrossRef]
- Liu, G.D.; Xie, S.J.; Zhang, Q.H.; Tian, Z.F.; Wang, Y. Carbon dioxide-enhanced photosynthesis of methane and hydrogen from carbon dioxide and water over Pt-promoted polyaniline–TiO2 nanocomposites. Chem. Commun. 2015, 51, 13654–13657. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Zhao, H.L.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.X.; Zhu, Y.; Wang, Y.; Wang, C. Enhanced CO2 photoreduction activity of black TiO2-coated Cu nanoparticles under visible light irradiation: Role of metallic Cu. Appl. Catal. A 2016, 510, 34–41. [Google Scholar] [CrossRef]
- Liu, L.J.; Jiang, Y.Q.; Zhao, H.L.; Chen, J.T.; Cheng, J.L.; Yang, K.S.; Li, Y. Engineering co-exposed {001} and {101} facets in oxygen-deficient TiO2 nanocrystals for enhanced CO2 photoreduction under visible light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Peng, C.; Reid, G.; Wang, H.F.; Hu, P. Perspective: Photocatalytic reduction of CO2 to solar fuels over semiconductors. J. Chem. Phys. 2017, 147, 030901. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Special Report on Carbon Dioxide Capture and Storage; Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005. [Google Scholar]
- Wang, T.; Meng, X.; Liu, G.G.; Chang, K.; Li, P.; Kang, Q.; Liu, L.Q.; Li, M.; Ouyang, S.X.; Ye, J.H. In situ synthesis of ordered mesoporous Co-doped TiO2 and its enhanced photocatalytic activity and selectivity for the reduction of CO2. J. Mater. Chem. A 2015, 3, 9491–9501. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Li, Y.; Ackerman, E.A.; Gajdardziska-Josifovska, M.; Li, H.L. Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels. Appl. Catal. A 2011, 400, 195–202. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; Mson, J.A.; Kong, X.Q.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2014, 519, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Q.; Zhao, Z.; Niu, M.; Mao, C.Y.; Cao, D.P.; Cheng, D.J.; Feng, P.Y.; Sun, Z.C. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Varley, J.B.; Rinke, P.; Umezawa, N.; Kresse, G.; van de Walle, C.G. Hybrid functional studies of the oxygen vacancy in TiO2. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 085212. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Infrared absorption of reduced rutile TiO2 single crystals. Phys. Rev. 1959, 113, 1222–1226. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, G.J.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Xiang, Q.; Lv, K.; Yu, J. Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2 nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air. Appl. Catal. B Environ. 2010, 96, 557–564. [Google Scholar] [CrossRef]
- Jiang, X.D.; Zhang, Y.P.; Jiang, J.; Rong, Y.S.; Wang, Y.C.; Wu, Y.C.; Pan, C.X. Characterization of oxygen vacancy associates within hydrogenated TiO2: A positron annihilation study. J. Phys. Chem. C 2012, 116, 22619–22624. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Peter, Y.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium b nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Bozhilov, K.; Dillon, R.J.; Wang, L.; Smith, P.; Zhao, X.; Bardeen, C.; Feng, P. Active facets on titanium(III)-doped TiO2: An effective strategy to improve the visible-light photocatalytic activity. Angew. Chem. Int. Ed. 2012, 51, 6223–6226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Zou, X.X.; Liu, J.K.; Su, J.; Zuo, F.; Chen, J.S.; Feng, P.Y. Facile synthesis of thermal-and photostable titania with paramagnetic oxygen vacancies for visible-light photocatalysis. Chem. Eur. J. 2013, 19, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbu, G.D.; Ramakrishnan, V.; Sasirekha, V.; Ramasamy, S.; Kumar, J. Influence of particle size on the phonon confinement of TiO2 nanoparticles. J. Exp. Nanosci. 2014, 9, 661–668. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Zhang, M.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Kumar, P.M.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: Correlation to presence of surface states. Thin Solid Films 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Szczepankiewicz, S.H.; Colussi, A.J.; Hoffmann, M.R. Infrared spectra of photoinduced species on hydroxylated titania surfaces. J. Phys. Chem. B 2000, 104, 9842–9850. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Brennecke, J.F.; Kamat, P.V. Origin of catalytic effect in the reduction of CO2 at nanostructured TiO2 films. ACS Catal. 2014, 4, 3249–3254. [Google Scholar] [CrossRef]
- Lindan, P.; Harrison, N.; Gillan, M. Mixed dissociative and molecular adsorption of water on the rutile (110) surface. Phys. Rev. Lett. 1998, 80, 762–765. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Lapkin, A.A.; Plucinski, P.K.; Friedrich, J.M.; Walsh, F.C. Reversible Storage of Molecular Hydrogen by Sorption into Multilayered TiO2 Nanotubes. J. Phys. Chem. B 2005, 109, 19422–19427. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, A.Y.; Fujiwara, T.; Yagi, H.; Akutsu, H.; Nosaka, Y. Photocatalytic Reaction Sites at the TiO2 Surface as Studied by Solid-State 1H NMR Spectroscopy. Langmuir 2003, 19, 1935–1937. [Google Scholar] [CrossRef]
- Murrell, J.N.; Sorbie, K.S. New analytic form for the potential energy curves of stable diatomic states. J. Chem. Soc. Faraday Trans. 1996, 92, 2791–2798. [Google Scholar] [CrossRef]
- Morcombe, C.R.; Zilm, K.W. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 2003, 162, 479–486. [Google Scholar] [CrossRef]
- Hayashi, S.; Hayamizu, K. Chemical Shift Standards in High-Resolution Solid-State NMR (1) 13C, 29Si, and 1H Nuclei. Bull. Chem. Soc. Jpn. 1991, 64, 685–687. [Google Scholar] [CrossRef]

| Catalysts | Light Source | CO2 Concentration | CO Production (µmol/h) | Reference |
|---|---|---|---|---|
| TiO2−x/exposed {001}/{101} facets (through NaBH4 reduction) | UV: 100-W mercury vapor lamp; visible: 450-W Xe lamp (λ > 400 nm) | 4 mL/min CO2 + H2O continuous flow | UV:CO = 0.44, Vis:CO = 0.208 | [13] |
| Brookite TiO2-x (thermal treatment in He) | 150-W solar simulator | 2 mL/min, CO2 + H2O continuous flow | CO + CH4 = 0.315 | [11] |
| 2.5 at% Co-TiO2 (sol-gel method) | Visible light of 300-W Xe arc lamp with an L-42 glass filter | 3 mL DI H2O and 80 kPa CO2 gas | CO = 0.194, CH4 = 0.009 | [16] |
| 4% Cu@TiO2 (annealing at 450 °C for 2 h in high vacuum) | Visible light of 500-W Xe lamp (λ > 400 nm) | CO2 generated from NaHCO3/H2SO4 reaction | CO = 0.162, CH4 = 0.027 | [12] |
| 10% I-TiO2 hydrolysis of titanium isopropoxide (TTIP) in iodic acid solution followed by hydrothermal treatment | Visible light of 150-W solar simulator | CO2 + H2O mix gas | CO = 0.48 | [17] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Xia, M.; Marchetti, A.; Wang, X.; Cao, W.; Guan, H.; Kong, X. Photocatalytic Reduction of CO2 from Simulated Flue Gas with Colored Anatase. Catalysts 2018, 8, 78. https://doi.org/10.3390/catal8020078
Guan Y, Xia M, Marchetti A, Wang X, Cao W, Guan H, Kong X. Photocatalytic Reduction of CO2 from Simulated Flue Gas with Colored Anatase. Catalysts. 2018; 8(2):78. https://doi.org/10.3390/catal8020078
Chicago/Turabian StyleGuan, Yebin, Ming Xia, Alessandro Marchetti, Xiaohong Wang, Weicheng Cao, Hanxi Guan, and Xueqian Kong. 2018. "Photocatalytic Reduction of CO2 from Simulated Flue Gas with Colored Anatase" Catalysts 8, no. 2: 78. https://doi.org/10.3390/catal8020078





