1. Introduction
While the photooxidation of toluene in the gas phase has been studied extensively [
1,
2,
3], much less has been published on photooxidation in the liquid phase [
4,
5]. In either the gas phase or the liquid phase, catalyzed photooxidation of toluene has relied almost exclusively on titanium dioxide. Photoinduced holes in TiO
2 surfaces are very efficient at hydrogen abstraction and good results can be obtained with TiO
2 catalysis of hydrocarbon photooxidation, but TiO
2 is not well suited to applications intended to use sunlight. Its visible light absorptivity is virtually nil and most researchers use wavelengths reaching well down into the ultraviolet (UV) in order to get acceptable photoreaction rates or make extensive surface modifications by doping or thermal processing [
6,
7]. One of the objectives of the research presented in this paper was to demonstrate that the photooxidation of toluene could be effected by means of visible light. Secondarily, the potential to drive the photooxidation by sunlight alone is a topic that may have commercial implications in the future, should it become necessary to avoid the energy-consuming high temperatures and pressures currently necessary for the oxidation of hydrocarbons with molecular oxygen. A further disadvantage of titanium dioxide is that it is difficult to limit oxidation to the aldehyde or ketone. Longer irradiation, necessary to obtain a good yield, typically produces increasing amounts of the corresponding acid and also increasing mineralization rates [
4,
8].
We have used a variety of chlorometallates as catalysts for the oxidative photodecomposition of haloalkanes. Excited state metal complexes have been reported to act in several different ways to effect photocatalysis, all of which involve the creation of chlorine atoms. Among these are the reduction of the haloalkane [
9,
10,
11,
12,
13], oxidation of chloride counterions [
14], and photodissociation of chlorine atoms from the complex [
15,
16]. Chlorine atoms are able to abstract hydrogen from many C–H bonds, and have been used successfully to catalyze the photooxidation of ethanol [
17]. Their use in the catalyzed photooxidation of hydrocarbons is largely unexplored.
Photodissociation is probably the simplest source of chlorine atoms, and there are several materials that absorb light in the visible spectrum that undergo photodissociation and may be considered for heterogeneous photocatalysis. FeCl
3 supported on silica gel [
18] and FeCl
4ˉ immobilized on an ion exchange resin [
19] have been used to catalyze photooxidation. Polystyrene-based anion exchange resins in the chloride form have sometimes been found to be surprisingly effective [
20].
We investigated the photooxidation of toluene in the presence of each of these three materials, but we achieved better results using tetraethylammonium tetrachloroferrate, which is insoluble in toluene, the results from which are reported here. The absorption spectrum of the FeCl
4ˉ ion has a charge transfer band with a maximum at 360 nm, trailing well into the visible range, giving the material a yellow color. The chlorine atom produced upon photodissociation subsequently abstracts hydrogen from the substrate to produce a radical that can then react with molecular oxygen. Chlorine must eventually recoordinate to iron in order to restore the catalyst. Representing the solid Et
4NFeCl
4 matrix as Mx, the initial steps are assumed to take place thusly:
It has often been assumed because hydroperoxides accumulate during photooxidation reactions that they are formed through hydrogen abstraction by peroxyl radicals. However, ROOH bond energies are very low, usually less than 370 kJ/mol [
21,
22], so that hydrogen abstraction by peroxyl radicals is normally an endothermic process. The benzylperoxyl radicals formed in Equation (3) do not abstract hydrogen, but instead self-terminate with the elimination of O
2, through two channels with comparable rates [
23,
24]:
Channel (4b), the Russell mechanism [
23], yields benzyl alcohol and benzaldehyde in equal amounts. In the gas phase in the presence of oxygen, benzoxyl radicals, formed via Channel (4a), produce benzaldehyde and hydroperoxyl radicals to the exclusion of other reactions [
24], as represented in Equation (5).
The hydroperoxyl radical is thought to exchange hydrogens with a benzylperoxy radical, driven by the very low O‒H bond dissociation energy (247 kJ/mol) in the hydroperoxyl radical [
22], causing the buildup of benzyl hydroperoxide.
Benzylhydroperoxide can be expected to reoxidize iron(II) sites to iron(III), breaking the O–O bond and yielding another benzoxyl radical that may combine with oxygen to produce yet another aldehyde as in Equation (5).
Coordinated hydroxide can be neutralized by HCl, produced during the original hydrogen abstraction, Equation (2), restoring the catalyst to its original form.
One of the goals of this work was to find an alternative to TiO2 that would avoid oxidation products beyond benzaldehyde with yields as good as, or better than, those produced with TiO2. Secondarily, a catalyst that was selective for benzyl alcohol could become useful synthetically. A further goal was to be able to carry out the photooxidation of toluene at normal temperature and pressure with sunlight or simulated sunlight (λ > 320 nm) or, a fortiori, with visible light alone.
2. Results
2.1. Catalyst Selection
Several different materials were used as heterogeneous sources of photogenerated chlorine atoms: Dowex 1X-10, an anion exchange resin in the chloride form, FeCl
4ˉ on Dowex 1X-10, Et
4N[FeCl
4], and FeCl
3 on silica gel. The product yields were compared for 20-min photolyses of toluene (1 mL, with 1% acetic acid), using 20 mg of each material. The results are shown in
Table 1.
Because one of the goals of this work was to achieve a higher yield of benzyl alcohol, tetraethylammonium tetrachloroferrate was used for most of the experiments herein reported. We found that yield maximized between 10 and 20 mg of Et
4N[FeCl
4] per mL, falling off rapidly below that range and slowly above it, similar to what we have observed in other systems [
17].
2.2. Addition of a Polar Accelerant
Because the photooxidation mechanism proposed above involves a number of ionic and hydrogen bonding species, to which toluene could prove inhospitable, a comparison of the reaction rate in the presence of small additions of acetonitrile, acetone, or acetic acid was undertaken, with results as shown in
Table 2.
As expected, the addition of polar substances to toluene increased the rate of photooxidation. Acetic acid was used for all subsequent experiments because it promoted a higher oxidation rate with less unwanted benzyl chloride than with acetonitrile. Further experiments showed that the optimum fraction of acetic acid was approximately 1%.
2.3. Product Profile as a Function of Time
Several experiments were conducted in which small aliquots of the photolysate were removed during the course of the reaction. They were diluted with toluene and subjected to gas chromatography-mass spectrometric (GC-MS) analysis.
Figure 1 shows a characteristic result.
The ratio of acetaldehyde to benzyl alcohol remained constant at approximately 2.5 throughout the course of the reaction, indicating that the conversion of benzyl alcohol to benzaldehyde does not play a significant role.
2.4. Photolysis of Benzyl Alcohol
When irradiated in the presence of Et
4N[FeCl
4], benzyl alcohol was converted to benzaldehyde more rapidly than toluene was converted to benzyl alcohol and benzaldehyde. Data are shown in
Table 3.
In neither case was a significant amount of benzyl chloride produced. The higher rate of oxidation in the mixed solvent can be attributed to its lower viscosity.
Despite the relatively rapid reaction of benzyl alcohol, it was of negligible importance in the toluene photooxidation experiments reported here, because the benzyl alcohol fraction remained below 1%.
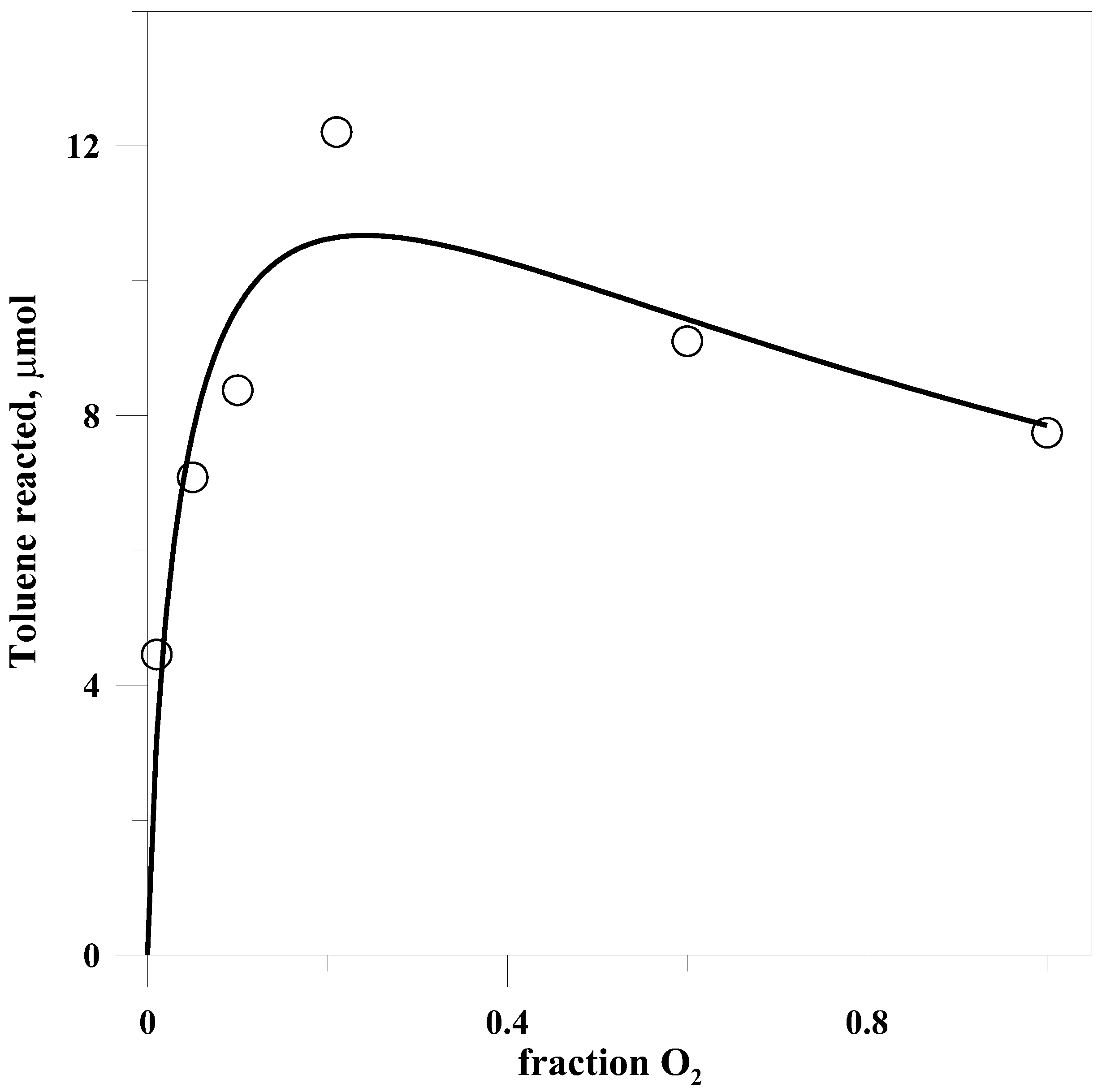
2.5. Dependence of Yield on Oxygen
The amount of toluene undergoing photooxidation varied in a complex fashion with the fraction of O
2 above it, as is seen in
Figure 2. In addition, the fraction of benzyl chloride in the products varied in an inverse manner with the partial pressure of oxygen. This is as would be expected from the competition between Cl and O
2 for the benzyl radical.
Despite the complex relationship between yield and the fraction of O
2, the data are consistent with a simple kinetic model based on the quenching of the FeCl
4ˉ* excited state by O
2 and the competition for the benzyl radical just described. Again using Mx to represent the solid matrix, we can write
The rate at which the excited state is created can be represented at a single wavelength by
I0f/V, where
I0 is the incident intensity,
f the fraction of light absorbed at that wavelength, and
V the sample volume. The rate over all wavelengths is the integral of this function, but may be taken as a constant,
h, for this analysis. The rate constants for radiative plus non-radiative deactivation (
ka), oxygen quenching (
kb), and dissociation (
kc), are referred to the number of accessible FeCl
4ˉ sites,
nFe, rather than to concentrations, since the FeCl
4ˉ ions are not mobile. By using the steady-state approximation for the number of excited state tetrachloroferrate ions, the rate of dissociation may be represented by the following equation, typical for photochemical processes with quenching [
25],
The fate of the chlorine atom created by photodissociation can be summed up by the equations
The steady-state approximation may be applied to the concentrations of chlorine atoms and benzyl radicals, leading to the result below, with coefficients
a = kb/kch,
b = (ka+kc)/kch, and
c = k3/k1k2.
This equation was fit to the experimental data, yielding the solid line in
Figure 2. From the fit one can derive only a limited amount of information about the rate constants, the main item of interest being that the ratio of
ka + kc (rate constants for deactivation and dissociation) to
kb (rate constant for oxygen quenching) is approximately 6, whereby
kb is to be understood as the pseudo-first order quenching rate constant with the partial pressure of O
2 equal to 1.0 atm.
When nitrogen was bubbled through a toluene-Et4NCl mixture before exposure to light, benzyl chloride was the sole product, as would be expected from the foregoing. The reaction is, of course, stoichiometric rather than catalytic.
2.6. Effect of Added Salts
Photochemical reactions initiated by the photodissociation of chlorine atoms are typically accelerated in the presence of dissolved chloride or bromide ions [
20,
26,
27], while other types of photoreactions involving metal complex excited states are sometimes retarded, or even quenched entirely [
28,
29]. The acceleration observed with reactions initiated by the photodissociation of chlorine atoms can be attributed to the formation of Cl
2ˉ ions, the equilibrium constant for which is approximately 2 × 10
5 in aqueous solution [
30], and certainly larger in nonpolar solvents. The formation of Cl
2ˉ retards the recombination of Cl atoms with FeCl
3ˉ (on the solid surface) in the solvent cage [
31,
32,
33,
34].
Adding 2 mg of Hx
4NCl more than doubled the amount of toluene undergoing oxidation, as can be seen in
Table 4. More salt did not increase the yield, presumably because 2 mg yielded enough chloride ion to react almost completely with chlorine atoms as they were produced.
Bromide ion proved equally effective. In accordance with expectations, when Bu
4NBr was added to 1.00 mL of toluene (with 1% acetic acid), the yields of benzaldehyde and benzyl alcohol were increased, as can be seen in
Table 5. The amount of toluene undergoing photooxidation approximately doubled, with the yield of benzyl alcohol increasing substantially more than the yield of benzaldehyde. As was the case with Hx
4NCl, adding more than approximately 1 mg of salt per mL of toluene did not increase the yield further.
Less expected was the change in the dependence of yield on the partial pressure of O
2 in the presence of either salt. In the absence of salt, as shown in
Figure 2, the yield passed through a maximum at approximately 0.2 atm O
2, but with either salt present the yield increased continuously with O
2 partial pressure, see
Table 5.
This behavior is consistent with the mechanism outlined in Equations (9) through (16). The equilibrium constant for the formation of BrClˉ from Brˉ and Cl∙ is even larger than that for the formation of Cl
2ˉ, approximately 7 × 10
12 in water [
30]. Thus, both Brˉ and Clˉ stabilize the chlorine atom, effectively increasing the rate constant,
kc, for photodissociation, leading to proportionately smaller values of
a and
b in Equation (17). A fit of Equation (17) to a series of experiments in which the O
2 partial pressure was varied while the other experimental variables, including the amount of Bu
4NBr, were held constant, yielded good agreement between calculated and experimental yields. The ratio of
kb to
ka +
kc was 0.2, compared to the value of 6 obtained without added salt, which would indicate a large increase in
kc, the rate constant for dissociation of excited state FeCl
4ˉ.
2.7. Photonic Efficiency
Light from a 100-W mercury lamp was passed through a 365-nm interference filter to irradiate 1.0 mL of toluene (1% acetic acid, with 1 mg of Hx4NCl) with 20 mg of Et4N[FeCl4] for one hour. The intensity of light hitting the sample was 32.2 mW, equivalent to 1.0 × 10‒7 einstein/s. A total of 15.7 μmol of toluene was oxidized, which amounts to a photonic efficiency of 0.042 mol/einstein at 365 nm.
2.8. Comparison with Published Results Using Other Catalysts
We cannot readily compare the results presented above using Et
4N[FeCl
4] to literature studies using TiO
2 to catalyze the photooxidation of toluene, because of a lack of TiO
2 experiments on neat toluene with focused irradiation from an external light source. A comparison is possible with the catalyst FeCl
4ˉ on Amberlite [
19], data for which are reproduced in
Table 6. Despite the uncertainties in comparing neat toluene with toluene diluted in another solvent,
Table 6 includes results from two studies on the photooxidation of toluene in acetonitrile solutions, one catalyzed by TiO
2 [
8] and the other by alumina-supported V
2O
5 [
5].
One of the difficulties in comparing experiments in neat toluene with those performed on toluene in acetonitrile is that toluene has a viscosity about twice as great as that of acetonitrile. Lower viscosities in solution will increase reaction rates in general, compensating in part for the expected decrease in the rates of biomolecular steps in the photooxidation as the toluene concentration is lowered. We were unable to make a direct comparison of Et4N[FeCl4]-catalyzed photooxidation rates in neat toluene with those in acetonitrile solutions of toluene, because Et4N[FeCl4] dissolves in acetonitrile and cannot function as a heterogeneous catalyst.
Perhaps even more important than the higher yields observed with Et
4N[FeCl
4] is the much higher fraction of benzyl alcohol in the product mix. In addition, no benzoic acid was observed with Et
4N[FeCl
4], which was also the case with alumina-supported vanadium (V) oxide as the catalyst [
5]. By contrast, the TiO
2-catalyzed experiment shown in
Table 6 generated a variety of additional oxidation products, especially
p-cresol, the yield of which was more than half that of benzaldehyde [
8]. It should be noted that with Et
4N[FeCl
4] as the catalyst, some benzyl chloride was also formed. The fraction of benzyl chloride in the product mix was as high as 25% in shorter experiments with a relatively high concentration of a tetraalkylammonium salt, but was less than 1% in longer experiments with no salt and a filter cutting off irradiation wavelengths below about 340 nm
2.9. Comparison with Published Results Using TiO2 in an Immersion Reactor
As noted in the previous section, although TiO2 has been used extensively for the photooxidation of toluene, it is difficult to find a usable comparison to the experiments reported here, because a large fraction of the literature on toluene photooxidation deals with toluene in the gas phase or at low concentration in a solvent.
It is clear from these studies that with TiO
2 as the catalyst oxidation proceeds beyond benzaldehyde and yields products from oxidation of the benzene ring. One study, for example, examined only benzoic acid as the oxidation product [
35], while in another the amount of
p-cresol formed exceeded the amount of benzaldehyde during the entire experiment [
36].
One detailed study of product development from the TiO
2-catalyzed photooxidation of toluene was done by Navio et al. using an immersion lamp [
4]. In order to have as direct a comparison as possible, we carried out a similar experiment with Et
4N[FeCl
4] as a catalyst. The results are shown in
Table 7.
Given the differences in lamp intensity and irradiation time, Et
4N[FeCl
4] performed as well as, or better than, TiO
2. With Et
4N[FeCl
4], however, a considerable amount of benzyl alcohol was produced, nearly 40% of the total, while none was found with TiO
2. Navio et al. sampled the photolysate over a 12-h period, finding that the yield of benzaldehyde actually began decreasing after seven hours’ irradiation, when the rate of further oxidation to benzoic acid and carbon dioxide eventually exceeded the rate of formation of benzaldehyde [
4]. The maximum yield is what is reported in
Table 8. The Et
4N[FeCl
4] experiment also generated 1.0 mmol of benzyl chloride as an unwanted side product.
2.10. Direct Comparison with of Et4N[FeCl4] with TiO2
To obtain the best comparison possible with titanium dioxide, experiments were undertaken under identical conditions, using 1 mL of toluene (1% acetic acid) and 20 mg of catalyst, either TiO
2 (anatase) or Et
4N[FeCl
4]. Results are shown in
Table 8.
The results show that catalysis by Et4N[FeCl4] consistently produced more benzaldehyde and much more benzyl alcohol than catalysis by TiO2, the advantage being higher with visible light. Despite the very low absorptivity of TiO2 above 380 nm, some photooxidation did take place with a 390 nm cutoff filter (from Newport), whereas none was detected with a 385 nm cutoff filter (Melles Griot) that despite its lower cutoff wavelength (50% T) had lower transmission overall between 380 and 400 nm.
2.11. Sunlight Experiments
Several irradiations were carried out in sunlight, in some cases with light focused on a stirred mixture through a 3″ glass
f/0.5 lens, and generally with an attached balloon containing air or oxygen. The best results, however, were obtained on unstirred samples in Petri dishes, with the catalyst simply lying on the bottom of the dish.
Table 9 compiles results from three experiments in Petri dishes, together with a similar experiment employing FeCl
3 on silica gel as the catalyst.
FeCl3 on SiO2 was photocatalytically active in sunlight, but the yield was lower than with Et4N[FeCl4], and only a small amount of benzyl alcohol was produced.
The photonic efficiency for the solar-induced oxidation of toluene may be estimated very roughly by making some approximations about the wavelength range active in inducing the reaction. The total solar insolation (i.e., the solar irradiance reaching the earth) during the experiment may be estimated, given the latitude (37.3° N) and the time of day [
37]. Values during the summer were typically near 1000 W/m
2. If we assume that all wavelengths up to 450 nm are photoactive—and equally active—the fraction of solar insolation that is photochemically active would be 9.2% [
38]. Thus the photochemically active radiation incident on a Petri dish, without allowing for the fraction reflected, was approximately 90 mW/cm
2. Taking the average wavelength of this radiation as 380 nm, this corresponds to 0.2 einsteins incident on the Petri dish in six hours. Using the best result reported in
Table 7, this yields a photonic efficiency of about 0.7%. While considerably smaller than the 4.2% found at 365 nm in the laboratory, the lack of stirring and the reflection from the glass that was not accounted for in the sunlight experiments renders the results broadly comparable.
2.12. Selectivity
The TiO
2-catalyzed photooxidation of toluene in the liquid phase yields very little benzyl alcohol [
4,
8,
36], which is, of course, desirable if benzaldehyde is the synthetic goal. Unfortunately, the absolute yield of benzaldehyde under these conditions reaches a maximum early in the process, after which the further oxidation of benzaldehyde is faster than its formation [
4,
8,
36]. The oxidation of benzaldehyde to benzoic acid does not occur with alumina-supported V
2O
5 as the photocatalyst [
5], and some benzyl alcohol (~5%) is formed along with benzaldehyde.
Et4N[FeCl4] not only affords a higher yield of primary products than does TiO2, it also avoids the problem of further oxidation. But perhaps most important, if benzyl alcohol is the synthetic goal, it consistently yields an alcohol to aldehyde ratio that approaches 1:2.
Before addressing the question of why this is so, it is useful to examine the alcohol to aldehyde ratio in the direct, uncatalyzed, photooxidation of toluene under UV irradiation. In both the liquid [
39] and the gas phase [
24] benzaldehyde is the predominant product, with an alcohol to aldehyde ratio of about 1:3. Another study has shown that the ratio in liquid toluene is highly dependent on temperature [
40]. In both liquid and gas phase the alcohol to aldehyde ratio was found to be constant over the entire irradiation time [
24,
40], demonstrating that the aldehyde is a not a secondary product.
The uncatalyzed photolysis results are consistent with the two reaction channels that have been proposed [
24], one yielding benzaldehyde exclusively (4a) and the other yielding benzyl alcohol and benzaldehyde in equal amounts (4b). The 1:3 ratio of alcohol to aldehyde suggests that the benzoxyl radical produced in Channel (4a) may abstract hydrogen at a rate competitive with its reaction with oxygen via Equation (5).
The benzyl alcohol to benzaldehyde ratio in the Et4N[FeCl4]-catalyzed photooxidation of toluene is quite similar to the ratio in the uncatalyzed photolysis. Therefore, a better way to state the question of selectivity is to ask why TiO2 suppresses the formation of benzyl alcohol.
This has been explored experimentally for titanium dioxide with cyclohexane as the substrate [
41,
42]. Two studies concluded that a high proportion of the alcohol produced occurs while the alkane is adsorbed on the TiO
2 surface. The alcohol is even more strongly adsorbed, leading to a large fraction converted to the aldehyde before a product molecule is released into solution [
41,
42]. The conversion of benzyl alcohol to benzaldehyde should be still more efficient, as recent studies by Higashimoto et al. have shown that adsorption of benzyl alcohol to TiO
2 gives rise to visible light absorption by a TiO
2-benzylic alcohol complex [
43,
44], enhancing the conversion to benzaldehyde even further.
This is completely consistent with observations, since a smaller fraction of benzyl alcohol is found in the TiO
2-catalyzed photooxidation of toluene than the fraction of cyclohexanol in the TiO
2-catalyzed photooxidation of cyclohexane [
41]. The adsorption is evidently weaker on alumina-supported V
2O
5 [
5] leading to higher alcohol to aldehyde ratios, and still weaker (or nonexistent) on Et
4N[FeCl
4], so that a substantial amount of benzyl alcohol is formed in solution, or released into solution from the Et
4N[FeCl
4] solvent cage.
From a synthetic point of view, the alcohol to aldehyde ratio is still low in the Et
4N[FeCl
4]-catalyzed photooxidation of toluene. Nevertheless, it is vastly better than that achieved with TiO
2, FeCl
3 on silica gel, V
2O
5 on alumina, or FeCl
4ˉ on an Amberlite anion exchange resin. Only two of the factors varied during the experiments reported here seemed to have an influence on the alcohol to aldehyde ratio. The addition of a bromide salt appears to increase the alcohol fraction in the product, but with the penalty that, except when cutting off almost all the UV (which causes the overall yield to decline), the amount of benzyl chloride is increased. Additionally, the alcohol/aldehyde ratio is controlled to some extent by the fraction of dioxygen above the irradiated suspension.
Table 10 shows the relevant data.
While a higher fraction of alcohol seems to be produced at lower partial pressures of O
2, the overall yield decreases significantly as the O
2 fraction is reduced below 20%, as can be seen in
Figure 2.
3. Materials and Methods
Tetraethylammonium tetrachloroferrate(III) was obtained from Sigma-Aldrich (Milwaukee, WI, USA). Toluene (GCMS grade) and titanium dioxide (anatase) were obtained from VWR Scientific (Radnor, PA, USA). Dowex 1-X10 (200–400 mesh) was from J. T. Baker (Phillipsburg, NJ, USA). Iron(III) chloride on silica gel (5% by mass), tetrahexylammonium chloride, benzyl alcohol, benzyl chloride, and benzaldehyde were obtained from Sigma-Aldrich. Tetrabutylammonium bromide was from Alfa Aesar (Haverhill, MA, USA).
The photolysis of toluene was generally carried out on 1.0 mL samples (unless otherwise noted) in glass spectrophotometer cells (Starna 1-SOG-10, % T attenuated to 50% at 315 nm). The irradiation source was a 100-W Osram HBO 100W/X3 mercury lamp in an Oriel Q housing, passed through a lowpass filter to remove desired portions of the UV, and focused on the sample. Two separate beams, at right angles, were used for irradiation. The two beams had different intensities as a result of somewhat different collection optics. Data within a single table was derived from the same beam, but data from one table in the text should not be compared with data from another. In many experiments a balloon containing air, O2, N2, or a mixture was affixed to the opening of the cuvette by means of a plastic pipette tip, which made a firm seal. A fan was used to maintain the temperature of the cuvette at 22 ± 2 °C. Incident light intensities were measured with a Thorlabs PM400 power meter (Thorlabs, Newton, NJ, USA) with an S314C thermal sensor. Most sunlight experiments were carried out in small Petri dishes (6-cm diameter), placed horizontally with no stirring and no focusing lens.
UV-visible spectra were recorded with a Cary 50 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). GC-MS measurements were carried out with a Shimadzu QP-2100 instrument (Shimadzu Scientific Instruments, Kyoto, Japan), with a Shimadzu 221-75954-30 column containing a p-bis(dimethylsiloxy)phenylene/dimethylsiloxane crosspolymer as stationary phase. The oven start temperature was 40 °C and a 30 °C linear temperature gradient was applied to a final temperature of 280 °C. A 20:1 split ratio was applied to sample injections. Species were identified from their mass spectra and by comparison with authentic samples. Total ion count peak areas were measured for known concentrations of benzaldehyde, benzyl alcohol, and benzyl chloride. A quadratic relationships was obtained in each case, with R2 > 0.999, which was used to determine concentrations.
4. Conclusions
Photocatalysis of the photooxidation of toluene by Et4N[FeCl4] operates through the formation of benzylperoxyl radicals following the dissociation of, and subsequent hydrogen abstraction by, chlorine atoms. This leads to significant differences in outcome compared to TiO2. Oxidation in the presence of TiO2 takes place mostly on the surface, while to a substantial degree the chlorine atoms that dissociate from Et4N[FeCl4] escape the solvent cage and react in free solution. In comparison to TiO2, Et4N[FeCl4] leads to a higher yield of benzaldehyde and a much higher yield of benzyl alcohol under near-UV excitation, the alcohol to aldehyde ratio approaching 1:2 with Et4N[FeCl4], compared to about 1:10 with TiO2. Not only is the overall yield of benzyl alcohol and benzaldehyde higher than with TiO2, the further oxidation to benzoic acid, cresol, and other products, including total mineralization, is avoided. The side product, benzyl chloride, is a potential problem, but only minimal amounts are produced with long irradiation times and irradiation wavelengths above 345 nm. As a potential green synthetic method for benzyl alcohol it leaves something to be desired, but the fraction of benzyl alcohol obtained, as much as 40%, is at least far superior to what can be obtained with TiO2.
Given the effectiveness of Et4N[FeCl4], the question that arises is why FeCl3 on silica gel and FeCl4ˉ on an Amberlite anion exchange resin, which also function by chlorine atom photodissociation, are so much less efficient. With respect to the overall yield, the answer probably lies in the higher concentration of photoactive FeClx centers in Et4N[FeCl4], which lacks the scaffolding in the other materials. That does not explain the difference in selectivity between Et4N[FeCl4] and FeCl3 on SiO2, where it is likely that silica gel adsorbs benzyl alcohol at least as strongly as does TiO2.