In order to create more open mesopores in the microporous zeolites, four different treatment methods were investigated. The alkaline post-processing adopted Na2CO3 (Treatment A), TMAOH/NaOH mixture (Treatment B), and NaOH (Treatment C) as desilication sources respectively, reacting at 65 °C for 1.5 h. A sequential mild acid treatment with HCl was performed after desilication of zeolites with NaOH (Treatment D), in order to remove Lewis acid sites generated after mesoporosity introduction.
2.1. Nitrogen Physisorption Characterization
Table 1 summarizes the texture properties of HZSM-5 zeolites obtained by different post-treatment methods. The parent HZSM-5 is characterized by typical microporous material. The mesoporous surface area is only 18 m
2/g, which is negligible compared to the microporous surface area of 395 m
2/g. For HZSM-5 after different alkaline treatments, the mesoporous surface area all approximately increase to about 34 m
2/g, while the corresponding microporous surface area decreases synchronously. A further mild HCl washing after desilication with NaOH makes little change to mesopores, but the microporous surface area was restored to 394 m
2/g for HZSM-5-D. The changes of mesoporous volume and microporous volume in each sample were in accordance with the corresponding trends of specific surface area.
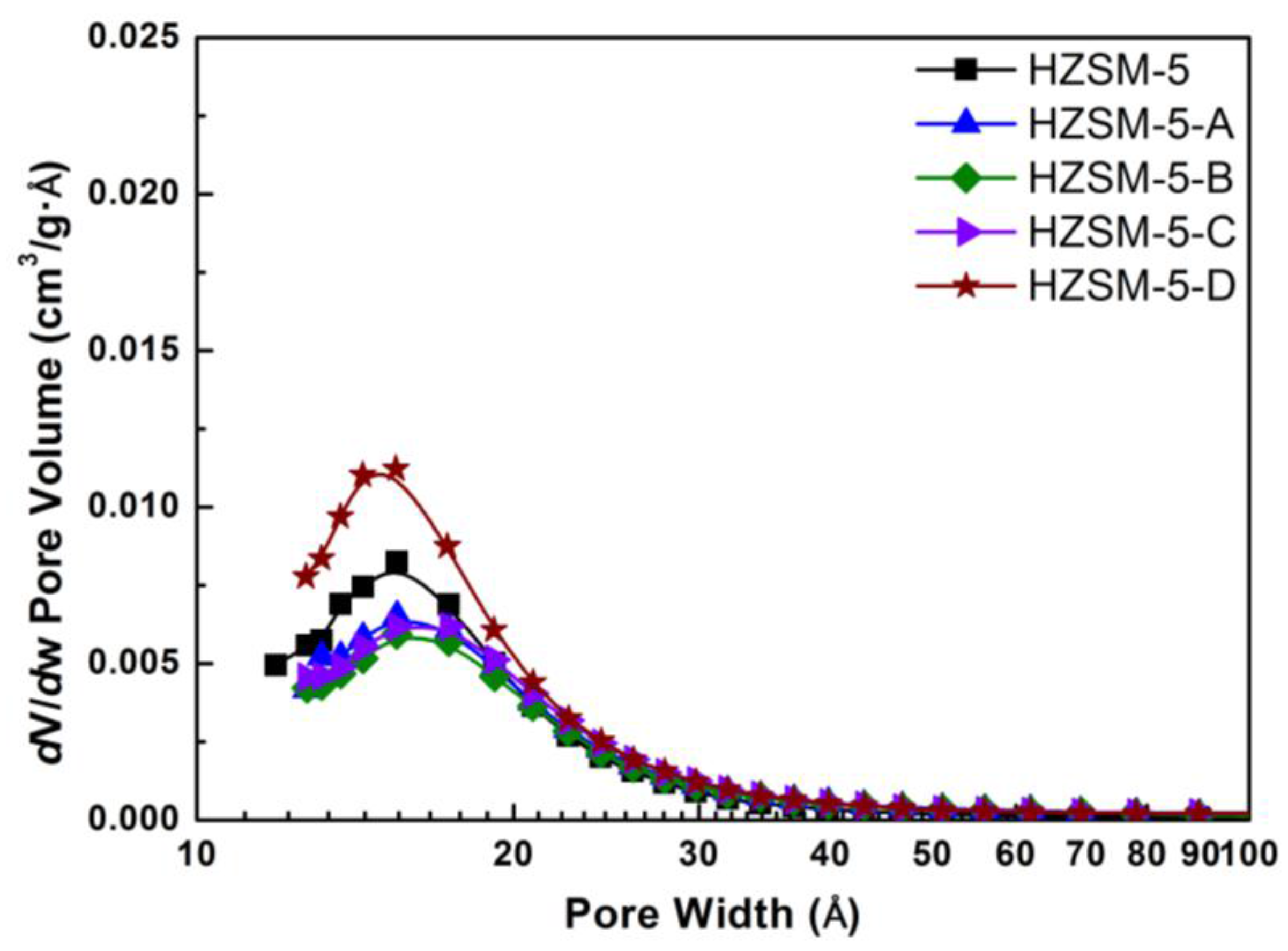
Figure 1 shows the pore size distribution of HZSM-5 zeolites obtained by different post-treatment methods, which was calculated according to the adsorption branch of isotherms using the BJH method. It is obvious that the microporous size distribution of parent HZSM-5 is very concentrated. Alkaline treatment has no effect on the location of the distribution maximum for micropore, but the intensity decreases. For hierarchical HZSM-5, there are a small amount of mesopores formed in a pore width of 2–6 nm. Consistent with the specific surface area, the HZSM-5-D possess slightly more micropores than parent HZSM-5.
Table 2 summarizes the texture properties of Hβ zeolites obtained by different post-treatment methods. The specific surface area of parent Hβ is larger than HZSM-5, and contains a certain amount of mesopores itself (86 m
2/g). Different alkaline treatments process had varying impacts on the formed mesopores. There is only a slight increase in the mesoporous surface area of Hβ-A and Hβ-B. The mesoporous surface area of Hβ-C is twice as large as parent Hβ, while the microporous surface area decreased correspondingly. The mesoporous surface area of Hβ-D further increased, while the microporous surface area remained at 421 m
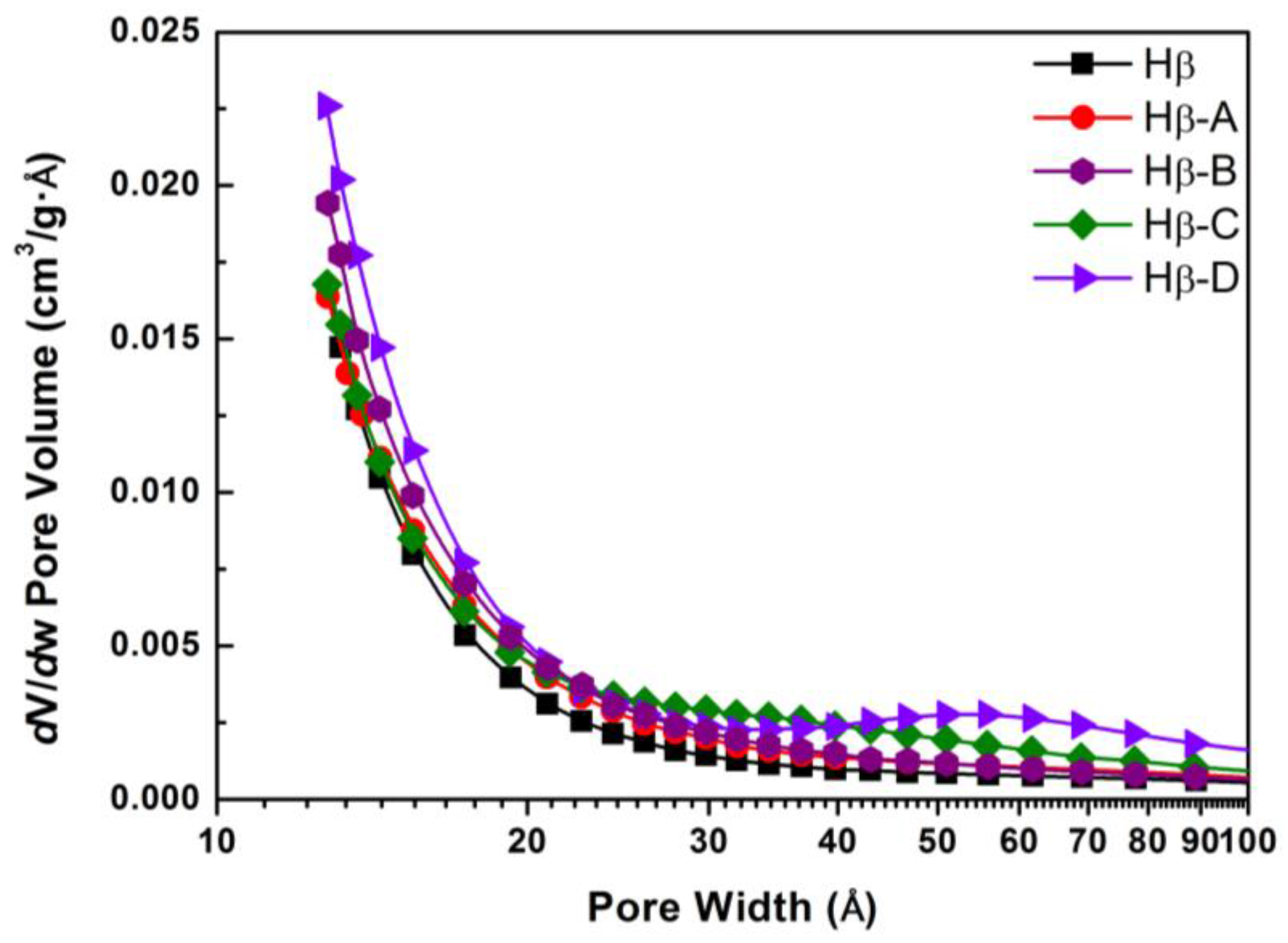
2/g. As can be seen from pore size distribution in
Figure 2, the hierarchical Hβ still retains a large amount of micropores, while the newly-formed mesopores are not very concentrated. For Hβ-A and Hβ-B, the additional mesopores are mainly distributed in the range of 2–4 nm, while for Hβ-C and Hβ-D, the pore size distribution of the additional mesopores is shifted to larger pore diameters (3–6 nm and 4–9 nm, respectively).
Under alkaline treatment conditions, larger mesopores are created by amorphization and skeleton atom leaching. Compared with the strong alkaline nature of NaOH solution, the treatment process using Na
2CO
3 and TMAOH/NaOH solution is milder in a weak alkaline environment. Zhao studied the effect of different alkaline treatment conditions on hierarchical HBEA zeolites [
16]. In Na
2CO
3 solution, the formation of intracrystalline mesopores was not selective. For TPAOH/NaOH treatment, the pore formation process showed a moderate desilication in the intra-mesopores, because TPA
+ ions bound to the zeolite surface in alkaline environment act as suitable pore-directing agents. Pure NaOH treatment provides no affinity to the zeolite surface, resulting in the unselective dissolution of the crystals on the unprotected surface. We noticed that there was an approximate mesoporous surface area for the hierarchical HZSM-5 zeolites under different alkaline treatment conditions. However, the surface area of mesopores formed in hierarchical Hβ zeolites was directly related to the basicity of alkaline solution. More mesopores are produced in the higher alkaline NaOH solution, while the micropores portion is well preserved. The main reason may be due to the different pore structure of the two zeolites. HZSM-5 contains 10-membered ring channels (0.51 nm × 0.55 nm and 0.53 nm × 0.56 nm), whereas Hβ contains 12-membered ring channels (0.66 nm × 0.67 nm and 0.56 nm × 0.56 nm). It is obvious that Hβ zeolite is more sensitive to the alkaline post-treatment.
2.3. NH3-TPD Characterization
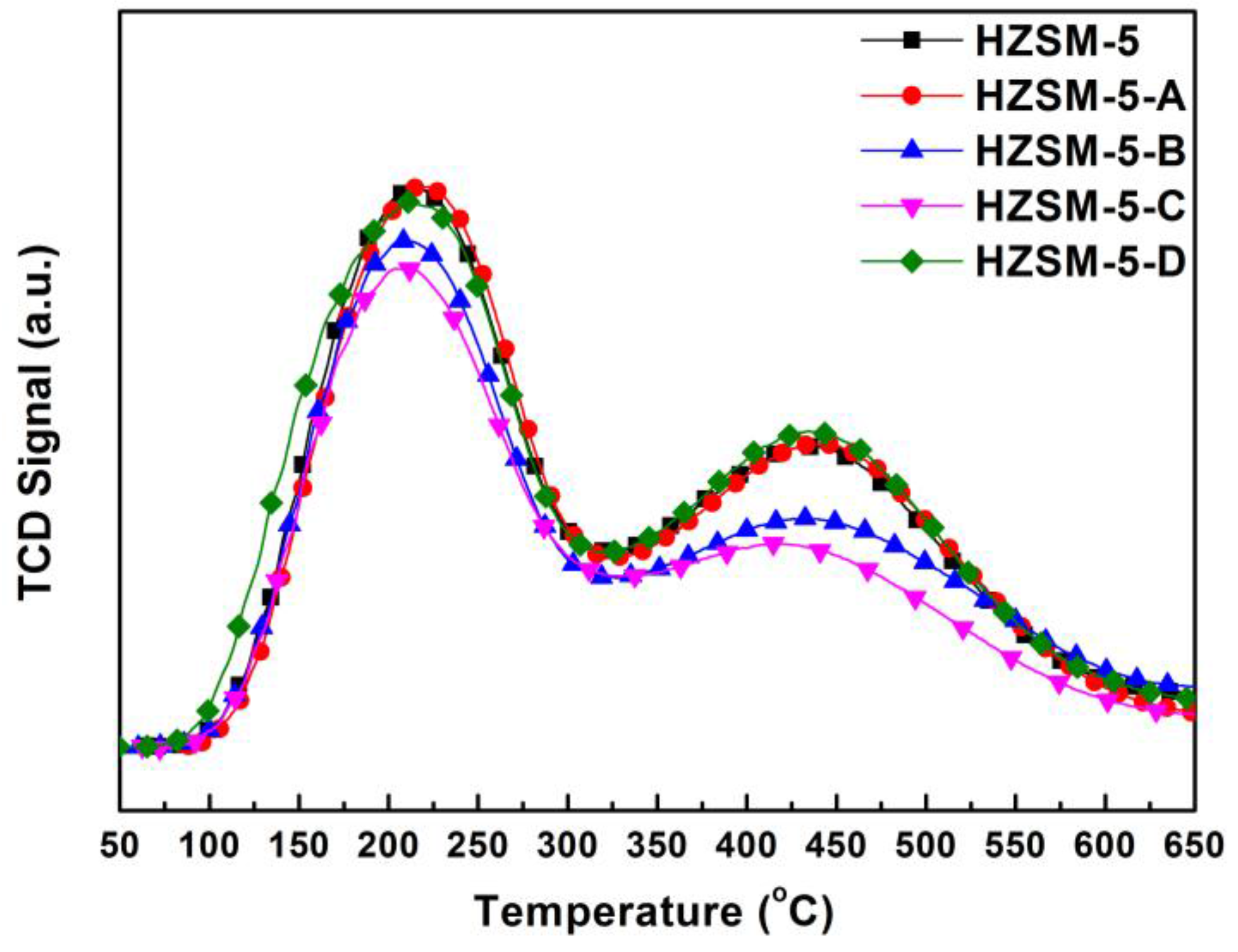
Figure 5 shows the NH
3-TPD patterns of parent HZSM-5 and the hierarchical HZSM-5 obtained by different post-treatment methods.
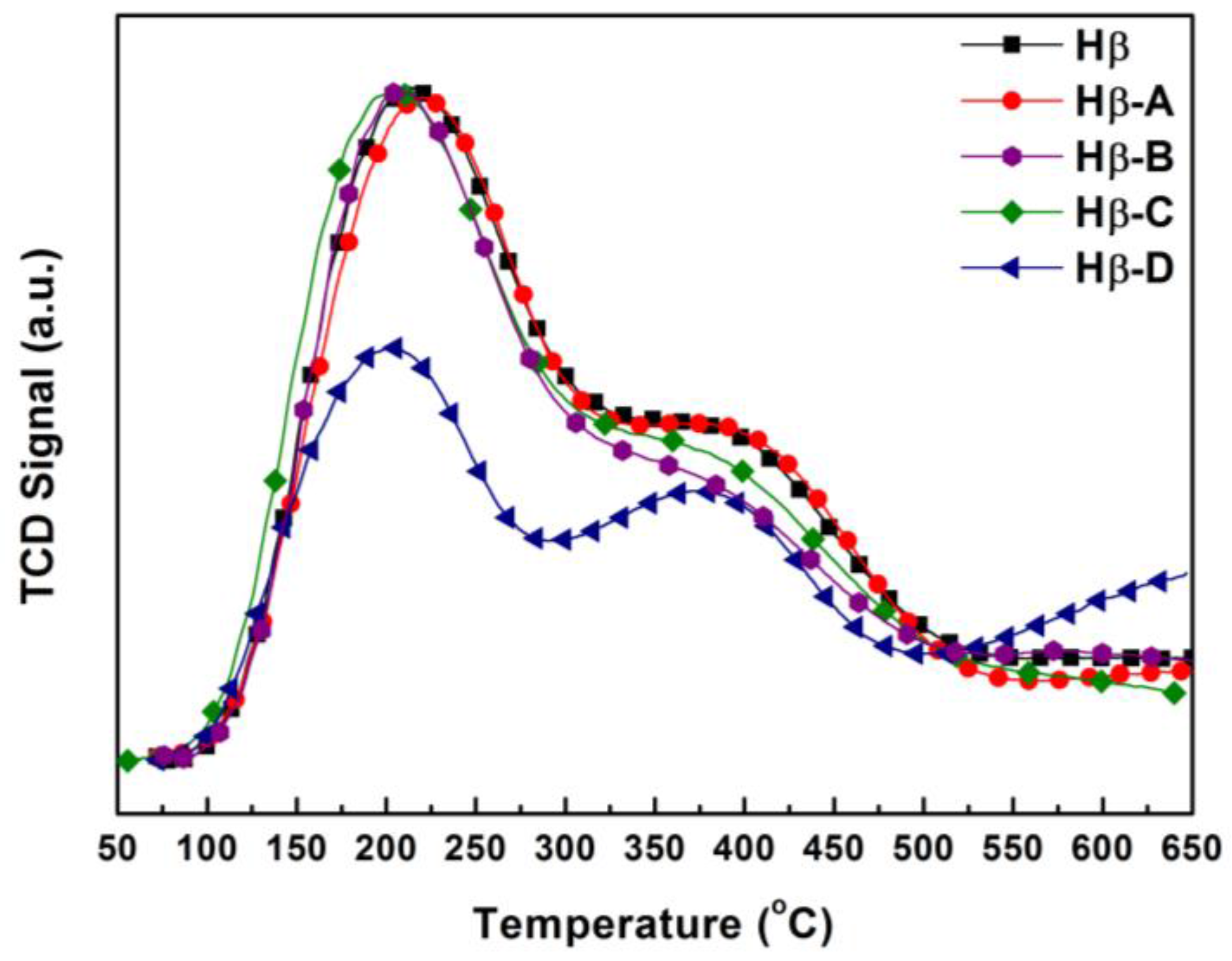
Figure 6 shows the NH
3-TPD patterns of parent Hβ and the hierarchical Hβ obtained by different post-treatment methods. Two ammonia desorption peaks appear in all zeolites. The low temperature peak is derived from the weakly adsorbed ammonia molecules via hydrogen bonding, instead of the acid sites [
19]. The high temperature peaks can be ascribed to strong Brønsted acid sites. It had been previously reported that Brønsted acid sites mainly exist over hierarchical zeolites formed in the partial desilication [
18]. Na
+ ions existed in the cationic sites of zeolite during alkaline treatment process, which could be removed by following ion exchanging with NH
4+; thus, the Brønsted acidity could be recovered.
Different alkaline treatments processes did not change the low temperature peak of the hierarchical HZSM-5. The high temperature peak remained essentially in the original position, indicating that alkaline treatment has tiny effect on the strength of the Brønsted acid sites. The peak area slightly decreased for HZSM-5-B and HZSM-5-C, indicating partial desilication reduces the number of Brønsted acid sites. The NH3-TPD patterns of the hierarchical Hβ exhibit similar desorption peaks to the parent one, indicating the alkaline treatment of Hβ Zeolites may not significantly decrease the acidity. For the hierarchical zeolites, we also noted that the loss of relative XRD crystallinity did not correlate well with the amount of acid sites, especially for HZSM-5-C and Hβ-A. The characteristic tetrahedrally coordinated aluminosilicate framework of zeolites results in Brønsted acid sites. In the harsh treatment condition of strong alkaline environment, larger mesopores are created by a combination of amorphization and framework atom leaching, which both affect the amount of acid sites. The relationship between the amount of acid sites determined from NH3-TPD and relative crystallinity is more complicated.
2.4. Catalytic Fast Pyrolysis of Kraft Lignin
Lignin is very chemically inert, due to its complex three-dimensional network structure formed by different non-phenolic phenylpropanoid units, which were linked with a variety of C–O and C–C bonds [
2,
4,
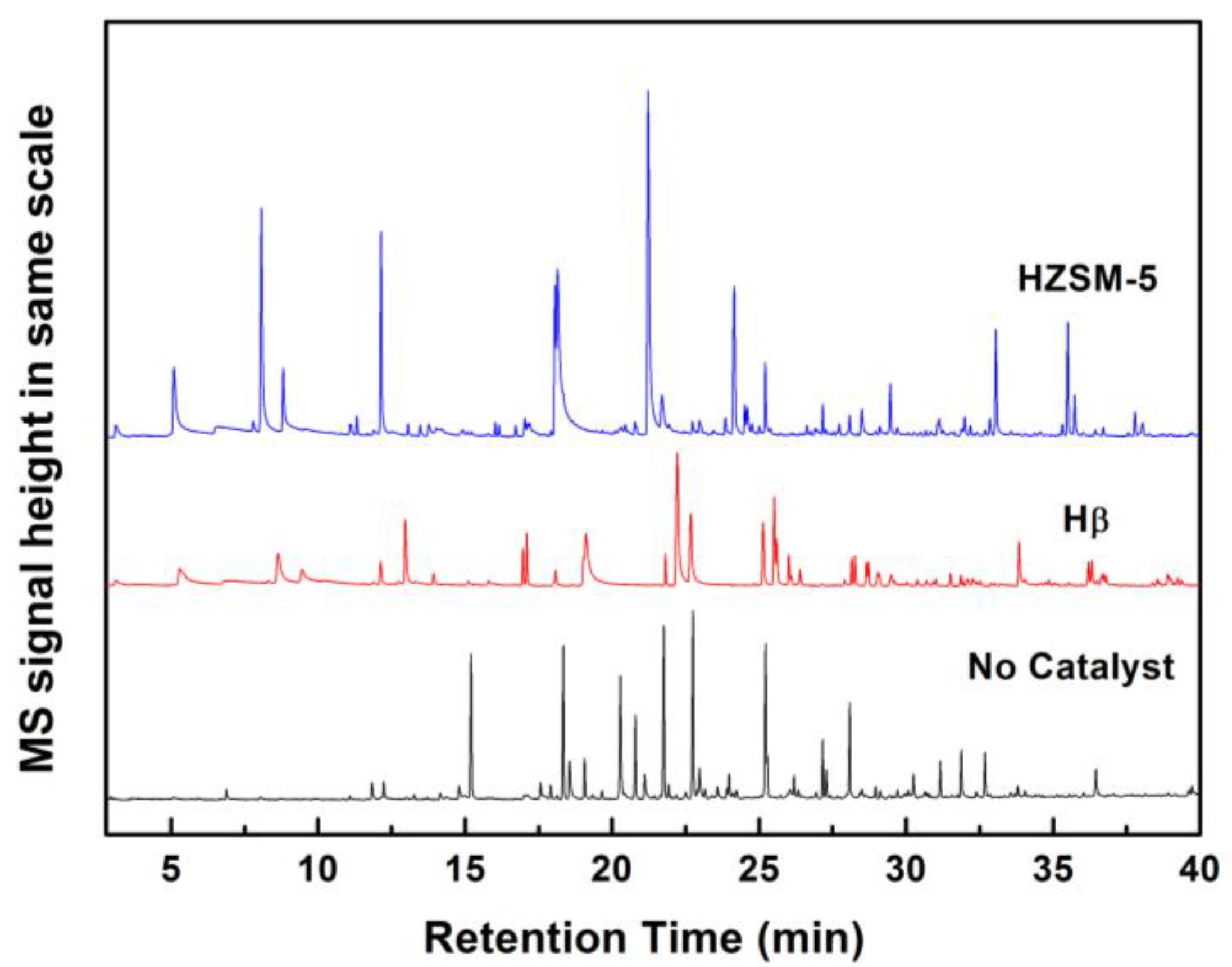
5]. Pyrolysis can destroy these phenyl-propane units in the polymer lattice. As shown in
Figure 7, the pyrograms of lignin over parent HZSM-5 and Hβ Zeolites were compared with those of non-catalytic fast pyrolysis. The yield of the condensable volatiles during Py-GC/MS experiments is semi-quantitatively evaluated by the sum of the absolute peak area of the total ion chromatogram peak (calibrated with the precise lignin sample weight), while the selectivity of a certain category of compound is represented by the percentage of peak area measured in total area. The light gases derived from the deoxygenation of oxygenates during CFP of lignin, mainly CO
2, CO, and other non-condensable gas, are not included in the sum of the absolute peak areas of condensable liquid products. As reported in previous studies by Bokhoven [
15], the yield of light gases was about 5% when the lignin was pyrolyzed at 650 °C on different zeolites catalysts. Because the lignin has a high resistance to pyrolysis, the polymer structure can only be depolymerized into aromatic monomers at the pyrolysis temperature of 600 °C in this work; thus, the C–C bond will not be further broken to produce smaller molecules of light gases. It has also been reported previously that the yield of light gases produced by lignin pyrolysis is much lower than that of cellulose and hemicellulose [
20]. Therefore, in the pyrolysis conditions of this work, pyrolysis products are mainly the condensable volatiles and coke correlating with each other. The products from lignin pyrolysis are very complex. It becomes very difficult to measure individual component. The retention time and the characteristic ion fragments of each compound in the total ion current chromatogram are shown in
Table S1. Non-CFP of lignin mainly produces oxygen-containing species with very few aromatic hydrocarbons. During the process of lignin pyrolysis, Bokhoven verified that depolymerized lignin products undergo successive reactions to form phenol alkoxy ketones, phenol alkoxy, phenols, aromatic alkoxy, and, finally, aromatic hydrocarbon [
14,
15]. The presence of zeolites greatly changed the composition of pyrolysis products, leading to improved deoxygenation of the high oxygen-containing compounds into aromatics. The GC-detected condensable pyrolysis products were divided into 4 categories: oxygenates (predominantly phenols, guaiacols, and syringols), monocyclic aromatics (BTX), naphthalene, and polycyclic aromatic hydrocarbons (PAH). The retention time and the characteristic ion fragments of each compound in the total ion current chromatogram are shown in
Table S2.
The acid sites of zeolites can promote deoxygenation, decarboxylation, and decarbonylation, as well as cleavage, oligomerization, alkylation, isomerization, cyclization, and aromatization, to further convert the depolymerization products of lignin. Over the parent HZSM-5 and Hβ Zeolites, there are significant differences in the selectivity of condensable volatiles. Using HZSM-5 catalyst, 27.2% of oxygenates still exist in the condensable volatiles, mainly in trimethoxybenzene. On Hβ zeolite, oxygenates almost disappeared completely, only 1%. The deoxygenation effect of Hβ is much higher than that of HZSM-5. Considering the difference in pore structure between the two zeolites, more lignin-derived oxygenates can diffuse into the larger pores of Hβ, even larger molecules trimethoxybenzene; thereby, most of oxygenates can be effectively converted. It has also been reported previously that HZSM-5 is invalid for the conversion of bulkier lignin monomers due to size exclusion or pore plugging, while Hβ is very effective for deoxygenation of lignin-derived oxygenates [
13]. Zeolite strong acid sites are not the unique factor to achieve high conversion and selectivity to aromatics. The two most essential parameters are diffusion and the active site.
The yield of the condensable volatiles for the CFP over parent HZSM-5zeolite is much higher than that of non-CFP of lignin. The reason is tentatively speculated that the microporous zeolites help to stabilize reactive intermediates derived during lignin pyrolysis, which otherwise readily recombine to form char. On the other hand, Hβis not as effective as HZSM-5 in improving the yield of the condensable volatiles. The yields of condensable volatiles on parent Hβ zeolite are even lower than those of non-CFP. It is tentatively speculated that the reason for this is that less coke is present on the surface of HZSM-5, which is mainly derived from large size PAH molecule. It has been reported previously that the amount of coke derived from CFP of lignin is related to the pore size, and the larger pore size of the Hβ zeolites is favorable for the formation of coke occurring from polymerization reactions [
13]. For HZSM-5 zeolite, the formation of large condensed molecules from side reactions was inhibited due to the smaller pore size. For Hβ zeolite, the concentration of PAH in the condensable volatiles is twice as much as HZSM-5. There is a considerable amount of 3 and 4 ring aromatics produced over Hβ zeolite, indicating that these large PAH are not hindered by the steric hindrance.
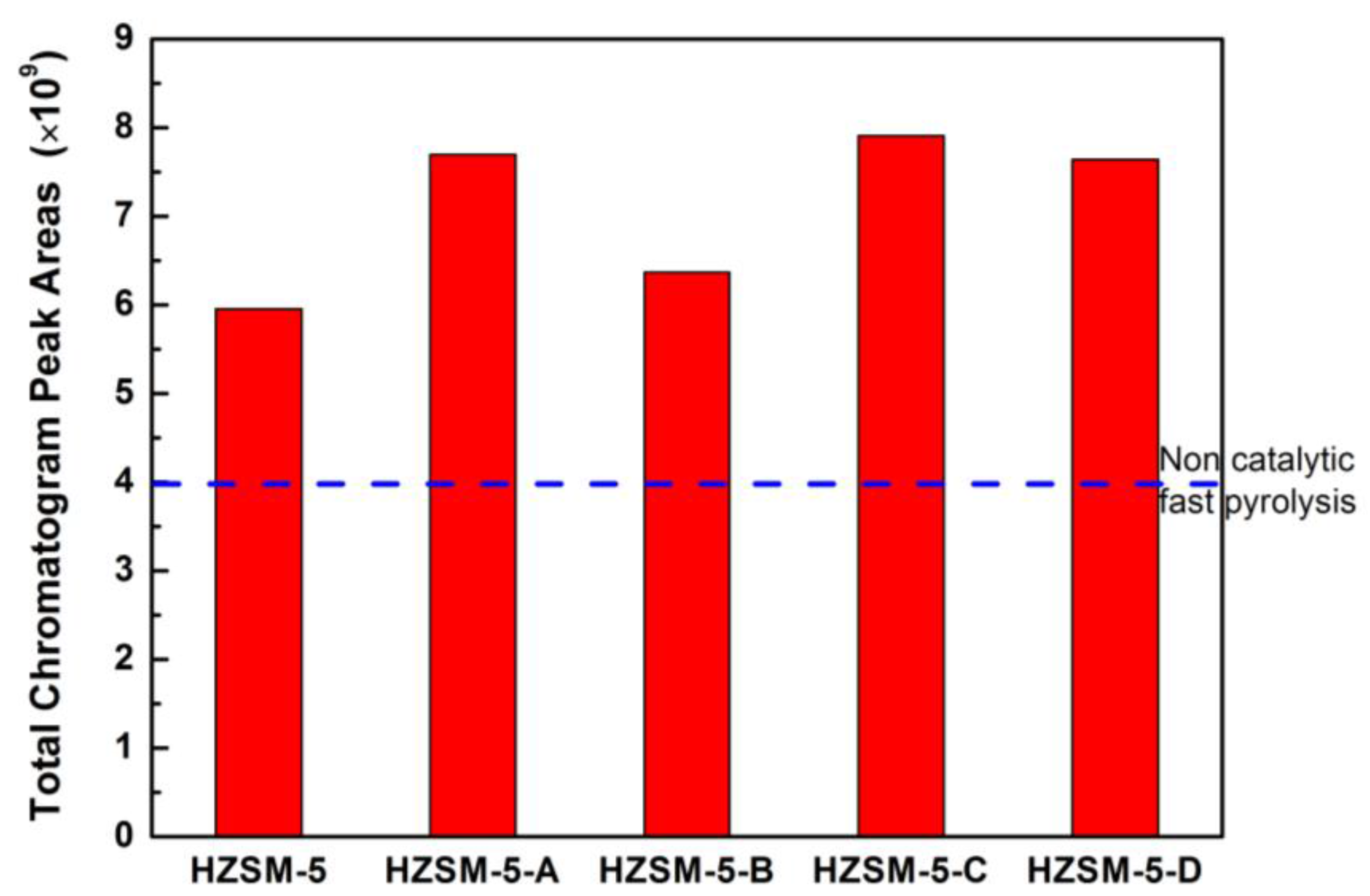
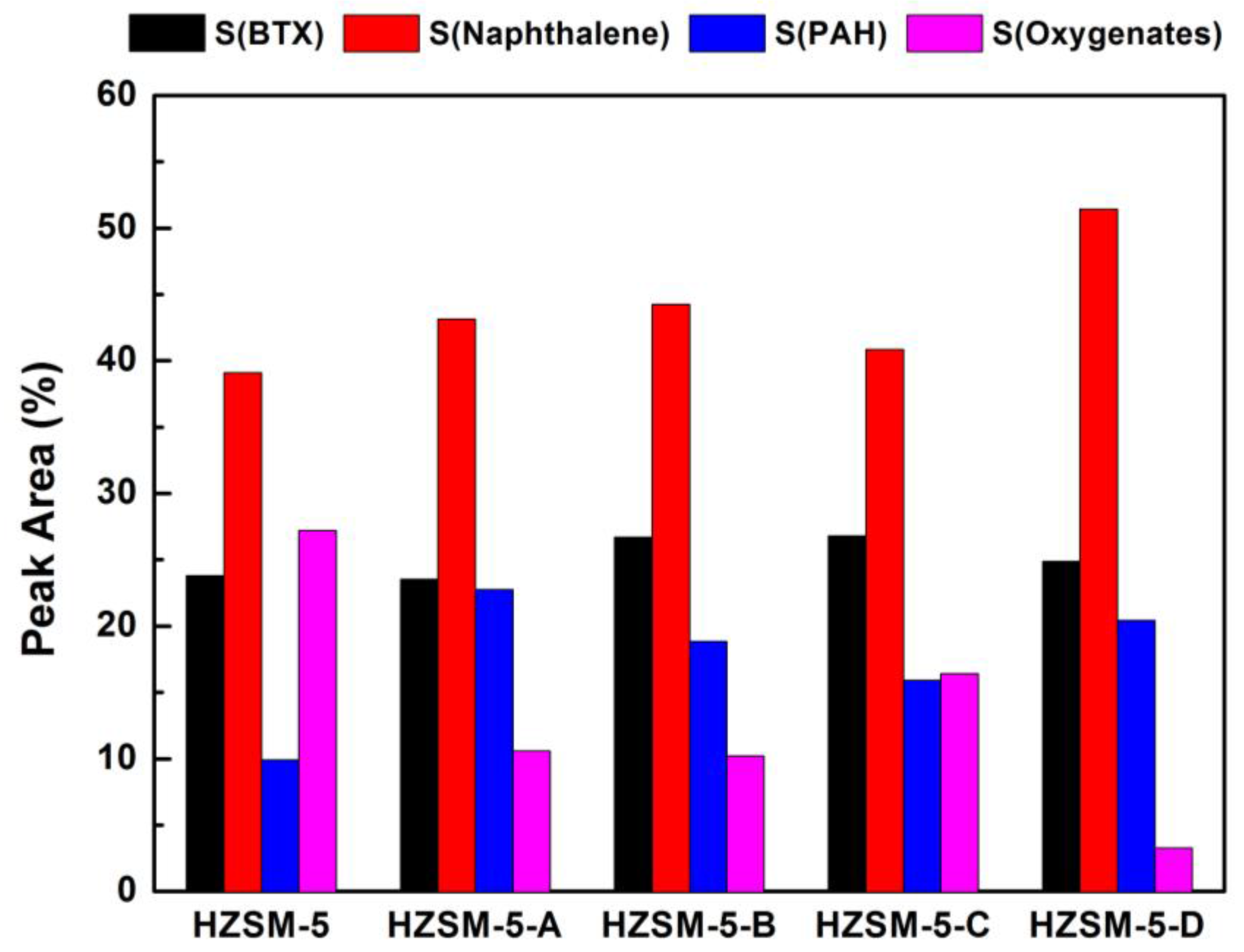
Figure 8 shows the yield of the condensable volatiles for lignin CFP over HZSM-5 zeolites obtained by different post-treatment methods. Compared with parent HZSM-5, the yield of the condensable volatiles on hierarchical HZSM-5 zeolites increased slightly, which is basically in accordance with the increase of mesoporous surface area. As shown in
Figure 9, the alkaline desilication treatment improved considerably the selectivity in aromatics hydrocarbon over hierarchical HZSM-5 zeolites. Compared with pure HZSM-5, the selectivity in naphthalene and PAH for hierarchical HZSM-5 zeolite is increased without affecting the yields in BTX. The most significant distinction for hierarchical HZSM-5 zeolite is the sharp reduction of oxygenates in the condensable volatiles, especially for HZSM-5-D, only 3.3%. Oxygenates are significantly reduced, while the amount of aromatic hydrocarbons increased. The formation of mesopores after desilication enhances a faster diffusion of reactants into the active surface and of products out of the zeolite particles. The introduction of mesopores in hierarchical HZSM-5 zeolite makes the larger oxygenates molecules diffuse into the pores and become effectively deoxygenated under the catalysis functions of Brønsted acid sites. As the main active sites for biomass pyrolysis vapors conversion, the remained Brønsted acid sites mainly presented in the micropores are more accessible in the hierarchical HZSM-5 zeolite. The deoxidation of oxygenates derived from lignin pyrolysis was effectively improved, which could be tentatively ascribed to the suppression of mass transfer resistance over hierarchical HZSM-5 zeolite. We speculated that the obtained deoxidation product can quickly diffuse out of the pore, avoiding the formation of unnecessary coke due to molecules condensation.
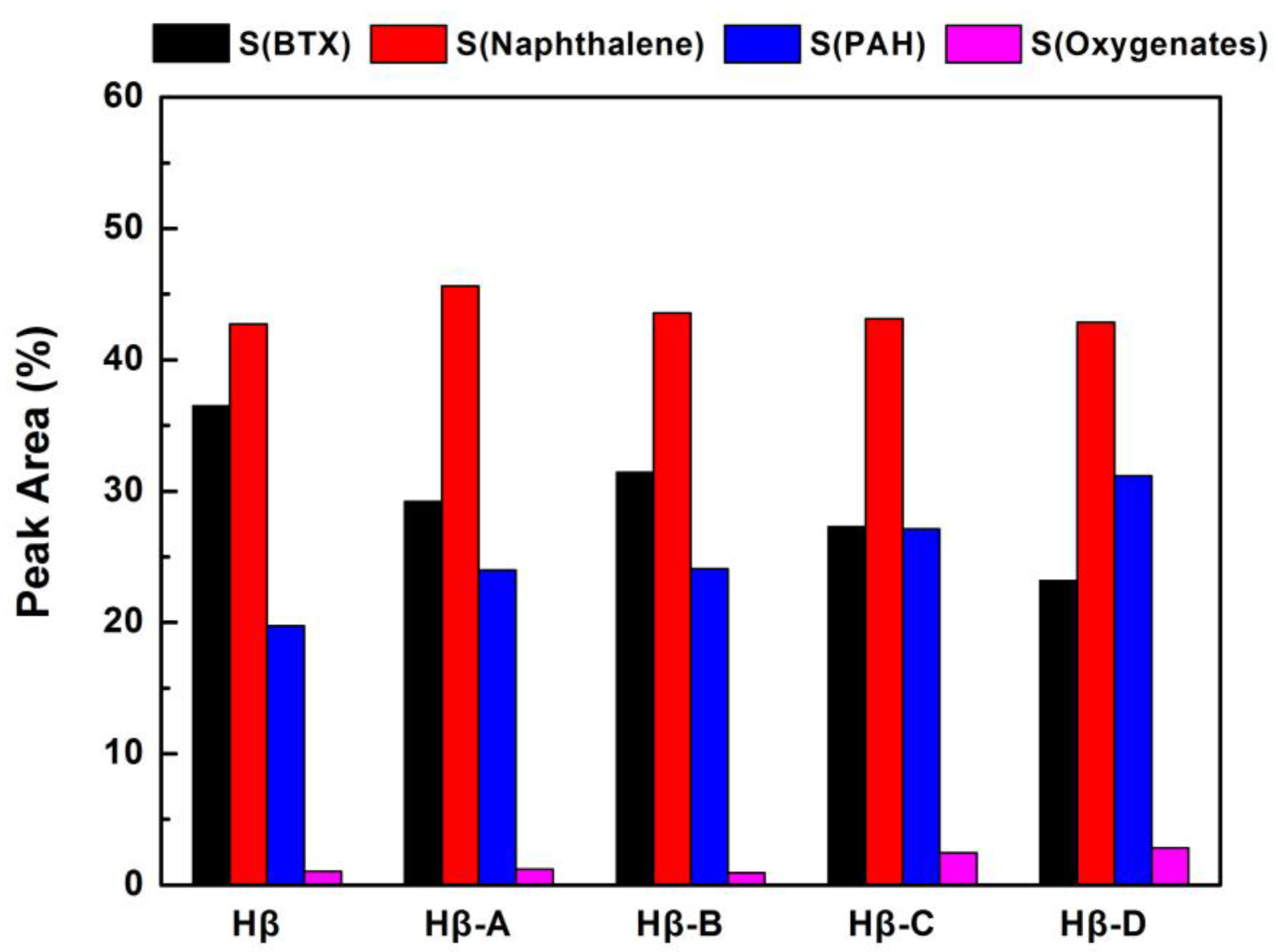
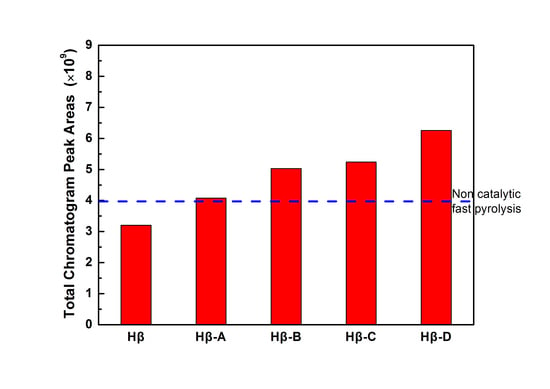
Figure 10 shows the yield of the condensable volatiles for lignin catalytic fast pyrolysis over Hβ zeolites obtained by different post-treatment methods. The hierarchical Hβ zeolite is much more active than the parent one. The yield of the condensable volatiles gradually increases from Hβ-A to Hβ-D, which correlates well with the change of mesoporous surface area. In particular, the condensable product yield for Hβ-D is 2 times that of the parent Hβ. The increase of the yield can also be attributed to the improvement of the mass transfer performance, because the alkaline desilication treatments are more effective for the formation of mesopores on hierarchical Hβ zeolite. Similar to the case of hierarchical zeolite HZSM-5, the introduction of mesopores mainly promotes the diffusion of molecules in and out of the active sites during the pyrolysis process. The yield of condensable volatiles over parent Hβ is lower than that of parent HZSM-5. Considering that the parent HZSM-5 contains hardly any mesopore, the mesopores present in the parent Hβ itself had tiny effect on inhibiting carbon deposition. It is speculated that the additional mesopores in hierarchical Hβ zeolite may be interconnected with micropores. The hierarchical pore structures can improve the utilization of micropores, due to the promoted mass transfer performance and the more accessible surface Brønsted acid sites. As shown in
Figure 11, the selectivity in oxygenates for hierarchical Hβ zeolites is slightly increased without affecting the yields in aromatic hydrocarbons. The improved activity of the hierarchical zeolite is ascribed to the synergistic effect between the presence of Brønsted sites and accessible mesopores.