Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials
Abstract
:1. Introduction
2. Downsizing
2.1. Effect of Downsizing on Desorption Thermodynamics
 | (1) |
 | (2) |
 | (3) |
2.2. Effect of Downsizing on Kinetics
3. Catalysis
3.1. The Doping of Transition Metals
3.2. Introduction of Metal Oxides
3.3. Halides Doped
3.4. Other Additives
4. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic tuning of Mg-based hydrogen storage alloys: A review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; He, L.; Lin, H.; Li, H.-W. Progress and trends in magnesium-based materials for energy-storage research: A review. Energy Technol. 2018. [Google Scholar] [CrossRef]
- Trimm, D.; Önsan, Z.I. Onboard fuel conversion for hydrogen-fuel-cell-driven vehicles. Catal. Rev. 2001, 43, 31–84. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Venkateswaran, P.S.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrog. Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Hudson, M.S.L.; Pukazhselvan, D.; Sheeja, G.I.; Srivastava, O.N. Studies on synthesis and dehydrogenation behavior of magnesium alanate and magnesium–sodium alanate mixture. Int. J. Hydrog. Energy 2007, 32, 4933–4938. [Google Scholar]
- Sadhasivama, T.; Kimb, H.; Jungc, S.; Rohd, S.; Parka, J.; Junga, H. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Sandí, G. Hydrogen storage and its limitations. Electrochem. Soc. Interface 2004, 13, 40–44. [Google Scholar]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse magnesium hydride nanoparticles uniformly self-assembled on graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.F.; Liu, Y.; Cho, E.S.; Forster, J.D.; Jeong, S.; Wang, H.; Urban, J.J.; Guo, J.; Prendergast, D. Atomically thin interfacial suboxide key to hydrogen storage performance enhancements of magnesium nanoparticles encapsulated in reduced graphene oxide. Nano Lett. 2017, 17, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Ruminski, A.M.; Aloni, S.; Liu, Y.; Guo, J.; Urban, J.J. Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage. Nat. Commun. 2016, 7, 10804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, X.; Tian, X.; Liu, Y.; Li, X. New approaches for rare earth-magnesium based hydrogen storage alloys. Prog. Nat. Sci. Mater. Int. 2017, 27, 50–57. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Cai, W.; Ouyang, L.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloy. Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Li, L.; Peng, B.; Ji, W.; Chen, J. Studies on the hydrogen storage of magnesium nanowires by density functional theory. J. Phys. Chem. C 2009, 113, 3007–3013. [Google Scholar] [CrossRef]
- Wagemans, R.W.P.; van Lenthe, J.H.; de Jongh, P.E.; van Dillen, A.J.; Jong, K.P.D. Hydrogen storage in magnesium clusters quantum chemical study. J. Am. Chem. Soc. 2005, 127, 16675–16680. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Ronnebro, E. Hydrogen storage properties of nanosized MgH2−0.1TiH2 prepared by ultrahigh-energy−high-pressure milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Felderhoff, M.; Schuth, F. Hydrogen storage properties of nanostructured MgH2/TiH2 composite prepared by ball milling under high hydrogen pressure. Int. J. Hydrog. Energy 2011, 36, 10828–10833. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Ma, H.; Chen, J. Magnesium nanowires enhanced kinetics for hydrogen absorption and desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef] [PubMed]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Structure, catalysis and atomic reactions on the nano-scale a systematic approach to metal hydrides for hydrogen storage. Appl. Phys. A Mater. Sci. Process. 2001, 72, 157–165. [Google Scholar] [CrossRef]
- Cheung, S.; Deng, W.; van Duin, A.C.T.; Goddard, W.A. Reaxffmgh reactive force field for magnesium hydride systems. J. Phys. Chem. A 2005, 109, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.F.; Ahn, C.C.; van Atta, S.L.; Liu, P.; Vajo, J.J. Fabrication and hydrogen sorption behaviour of nanoparticulate MgH2 incorporated in a porous carbon host. Nanotechnology 2009, 20, 204005. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, C.; Wang, H.; Ouyang, L.; Zhu, Y.; Li, L.; Wang, W.; Zhu, M. Controlling nanocrystallization and hydrogen storage property of Mg-based amorphous alloy via a gas-solid reaction. J. Alloy. Compd. 2016, 685, 272–277. [Google Scholar] [CrossRef]
- Shao, H.; Wang, Y.; Xu, H.; Li, X. Hydrogen storage properties of magnesium ultrafine particles prepared by hydrogen plasma-metal reaction. Mater. Sci. Eng. B 2004, 110, 221–226. [Google Scholar] [CrossRef]
- Shao, H.; Xu, H.; Wang, Y.; Li, X. Preparation and hydrogen storage properties of Mg2Ni intermetallic nanoparticles. Nanotechnology 2004, 15, 269–274. [Google Scholar] [CrossRef]
- Shao, H.; Felderhoff, M.; Schuth, F.; Weidenthaler, C. Nanostructured Ti-catalyzed MgH2 for hydrogen storage. Nanotechnology 2011, 22, 235401. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Shao, H.; Felderhoff, M.; Weidenthaler, C. Kinetics enhancement, reaction pathway change, and mechanism clarification in LiBH4 with Ti-catalyzed nanocrystalline MgH2 composite. J. Phys. Chem. C 2015, 119, 2341–2348. [Google Scholar]
- Stampfer, J.F.; Holley, J.R.C.E.; Suttle, J.J.F. The magnesium-hydrogen system1-3. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Liu, P.; Shang, J.; Li, X.; Liu, T. Formation of multiple-phase catalysts for the hydrogen storage of mg nanoparticles by adding flowerlike NiS. ACS Appl. Mater. Interfaces 2017, 9, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.S.; Kjoller, J.; Larsen, B.; Vigeholm, B. Magnesium for hydrogen storage. Int. J. Hydrog. Energy 1983, 8, 205–211. [Google Scholar] [CrossRef]
- Konarova, M.; Tanksale, A.; Beltramini, J.N.; Lu, G.Q. Effects of nano-confinement on the hydrogen desorption properties of MgH2. Nano Energy 2013, 2, 98–104. [Google Scholar] [CrossRef]
- Kumar, S.; Tiwari, G.P. Thermodynamics and kinetics of MgH2–nfTa2O5 composite for reversible hydrogen storage application. J. Mater. Sci. 2017, 52, 6962–6968. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Liu, Y.; Shea, P.T.; Kang, S.; Zaia, E.W.; Park, J.Y.; Chuang, Y.; Yuk, J.M.; Zhou, X.; et al. Hierarchically controlled inside-out doping of Mg nanocomposites for moderate temperature hydrogen storage. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, X.; Zhou, D.; Lu, Y.; Gao, M.; Pan, H. An ultrasound-assisted wet-chemistry approach towards uniform Mg(BH4)2·6NH3 nanoparticles with improved dehydrogenation properties. J. Mater. Chem. A 2016, 4, 8366–8373. [Google Scholar] [CrossRef]
- Baldé, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; Jong, K.P.D. Sodium alanate nanoparticles—Linking size to hydrogen storage properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, Y.; Gao, M.; Ouyang, L.; Liu, J.; Wang, H.; Zhu, M.; Pan, H. A mechanical-force-driven physical vapour deposition approach to fabricating complex hydride nanostructures. Nat. Commun. 2014, 5, 3519. [Google Scholar] [CrossRef] [PubMed]
- Norberg, N.S.; Arthur, T.S.; Fredrick, S.J.; Prieto, A.L. Size-dependent hydrogen storage properties of Mg nanocrystals prepared from solution. J. Am. Chem. Soc. 2011, 133, 10679–10681. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Shao, H. Heat modeling and material development of Mg-based nanomaterials combined with solid oxide fuel cell for stationary energy storage. Energies 2017, 10, 1767. [Google Scholar] [CrossRef]
- Shao, H.; Ma, W.; Kohno, M.; Takata, Y.; Xin, G.; Fujikawa, S.; Fujino, S.; Bishop, S.; Li, X. Hydrogen storage and thermal conductivity properties of Mg-based materials with different structures. Int. J. Hydrog. Energy 2014, 39, 9893–9898. [Google Scholar] [CrossRef]
- Shao, H.; Asano, K.; Enoki, H.; Akiba, E. Fabrication, hydrogen storage properties and mechanistic study of nanostructured Mg50Co50 body-centered cubic alloy. Scr. Mater. 2009, 60, 818–821. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 1–17. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Huot, J.; Boily, S.; van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni). J. Alloy. Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Schulz, R. Hydrogen desorption kinetics of a mechanically milled MgH2+5at.%v nanocomposite. J. Alloy. Compd. 2000, 305, 239–245. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- Lu, C.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of core-shell structured Mg@TM (TM = Co., V) composites. Int. J. Hydrog. Energy 2017, 42, 15246–15255. [Google Scholar] [CrossRef]
- Zou, J.; Guo, H.; Zeng, X.; Zhou, S.; Chen, X.; Ding, W. Hydrogen storage properties of Mg–Tm–La (Tm = Ti, Fe, Ni) ternary composite powders prepared through arc plasma method. Int. J. Hydrog. Energy 2013, 38, 8852–8862. [Google Scholar] [CrossRef]
- Yu, H.; Bennici, S.; Auroux, A. Hydrogen storage and release: Kinetic and thermodynamic studies of MgH2 activated by transition metal nanoparticles. Int. J. Hydrog. Energy 2014, 39, 11633–11641. [Google Scholar] [CrossRef]
- Kuji, T.; Nakayama, S.; Hanzawa, N.; Tabira, Y. Synthesis of nano-structured b.c.c. Mg–Tm–V (Tm = Ni, Co., Cu) alloys and their hydrogen solubility. J. Alloy. Compd. 2003, 356–357, 456–460. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Zhang, X.; Qu, J.; Wang, Y.; Li, X. Catalytic effect of Ni nanoparticles on the desorption kinetics of MgH2 nanoparticles. J. Alloy. Compd. 2009, 482, 388–392. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Rontzsch, L.; Kieback, B. Structural and hydrogen storage properties of melt-spun Mg–Ni–Y alloys. Int. J. Hydrog. Energy 2009, 34, 7749–7755. [Google Scholar] [CrossRef]
- Shao, H.; Matsuda, J.; Li, H.; Akiba, E.; Jain, A.; Ichikawa, T.; Kojima, Y. Phase and morphology evolution study of ball milled Mg–Co. hydrogen storage alloys. Int. J. Hydrog. Energy 2013, 38, 7070–7076. [Google Scholar] [CrossRef]
- Shao, H.; Liu, T.; Li, X.; Zhang, L. Preparation of Mg2Ni intermetallic compound from nanoparticles. Scr. Mater. 2003, 49, 595–599. [Google Scholar] [CrossRef]
- Shao, H.; Xu, H.; Wang, Y.; Li, X. Synthesis and hydrogen storage behavior of Mg–Co.–H system at nanometer scale. J. Solid State Chem. 2004, 177, 3626–3632. [Google Scholar] [CrossRef]
- Shao, H.; Wang, Y.; Xu, H.; Li, X. Preparation and hydrogen storage properties of nanostructured Mg2Cu alloy. J. Solid State Chem. 2005, 178, 2211–2217. [Google Scholar] [CrossRef]
- Shao, H.; Asano, K.; Enoki, H.; Akiba, E. Preparation and hydrogen storage properties of nanostructured Mg–Ni BCC alloys. J. Alloy. Compd. 2009, 477, 301–306. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Lin, H.; Liu, Y.; Zhang, Y.; Li, S.; Ma, Z.; Li, L. Metal hydride nanoparticles with ultrahigh structural stability and hydrogen storage activity derived from microencapsulated nanoconfinement. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloy. Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. J. Alloy. Compd. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Fernandez, J.R.A.; Klassen, T.; Bormann, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrog. Energy 2007, 32, 2400–2407. [Google Scholar] [CrossRef]
- Da Conceição, M.O.T.; Brum, M.C.; Santos, D.S.D.; Dias, M.L. Hydrogen sorption enhancement by Nb2O5 and Nb catalysts combined with MgH2. J. Alloy. Compd. 2013, 550, 179–184. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Hydrogen sorption improvement of MgH2 catalyzed by CeO2 nanopowder. J. Alloy. Compd. 2017, 695, 2532–2538. [Google Scholar] [CrossRef]
- Lin, H.; Tang, J.; Yu, Q.; Wang, H.; Ouyang, L.; Zhao, Y.; Liu, J.; Wang, W.; Zhu, M. Symbiotic CeH2.73/CeO2 catalyst: A novel hydrogen pump. Nano Energy 2014, 9, 80–87. [Google Scholar] [CrossRef]
- Sevic, S.M.; Kurko, S.; Pasquini, L.; Matovic, L.; Vujasin, R.; Novakovic, N.; Novakovic, J.G. Fast hydrogen sorption from MgH2–VO2 (b) composite materials. J. Power Sources 2016, 307, 481–488. [Google Scholar]
- Cabo, M.; Garroni, S.; Pellicer, E.; Milanese, C.; Girella, A.; Marini, A.; Rossinyol, E.; Surinach, S.; Baro, M.D. Hydrogen sorption performance of MgH2 doped with mesoporous nickel- and cobalt-based oxides. Int. J. Hydrog. Energy 2011, 36, 5400–5410. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride. J. Alloy. Compd. 2009, 486, 697–701. [Google Scholar] [CrossRef]
- Chen, B.; Chuang, Y.; Chen, C.O.-K. Improving the hydrogenation properties of MgH2 at room temperature by doping with nano-size ZrO2 catalyst. J. Alloy. Compd. 2016, 655, 21–27. [Google Scholar] [CrossRef]
- Ivanov, E.; Konstanchuk, I.; Bokhonov, B.; Boldyrev, V. Hydrogen interaction with mechanically alloyed magnesium–salt composite materials. J. Alloy. Compd. 2003, 359, 320–325. [Google Scholar] [CrossRef]
- Jin, S.; Shim, J.; Cho, Y.W.; Yi, K.-W. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides. J. Power Sources 2007, 172, 859–862. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Wang, Y.; Jiao, L.; Yuan, H. Catalytic effects of different Ti-based materials on dehydrogenation performances of MgH2. J. Alloy. Compd. 2015, 645, S509–S512. [Google Scholar] [CrossRef]
- Danaie, M.; Mitlin, D. TEM analysis of the microstructure in TiF3-catalyzed and pure MgH2 during the hydrogen storage cycling. Acta Mater. 2012, 60, 6441–6456. [Google Scholar] [CrossRef]
- Lin, H.; Matsuda, J.; Li, H.; Zhu, M.; Akiba, E. Enhanced hydrogen desorption property of MgH2 with the addition of cerium fluorides. J. Alloy. Compd. 2015, 645, S392–S396. [Google Scholar] [CrossRef]
- Ismail, M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2. Energy 2015, 79, 177–182. [Google Scholar] [CrossRef]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with magnesium hydride. Int. J. Hydrog. Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Ma, L.; Wang, P.; Cheng, H.-M. Hydrogen sorption kinetics of MgH2 catalyzed with titanium compounds. Int. J. Hydrog. Energy 2010, 35, 3046–3050. [Google Scholar] [CrossRef]
- Putungan, D.B.; Lin, S.; Wei, C.; Kuo, J.-L. Li adsorption, hydrogen storage and dissociation using monolayer MoS2: An ab initio random structure searching approach. Phys. Chem. Chem. Phys. 2015, 17, 11367–11374. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Han, S.; Zhang, W.; Zhao, X.; Sun, P.; Liu, Y.; Shi, H.; Wang, J. Hydrogen absorption and desorption kinetics of MgH2 catalyzed by MoS2 and MoO2. Int. J. Hydrog. Energy 2013, 38, 2352–2356. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Lv, Y.; Yan, Y.; Wu, Y. The hydrogen storage properties of MgH2–Fe3S4 composites. Energy 2015, 93, 625–630. [Google Scholar] [CrossRef]
- Hashmi, A.; Farooq, M.U.; Khan, I.; Son, J.; Hong, J. Ultra-high capacity hydrogen storage in a Li decorated two-dimensional C2N layer. J. Mater. Chem. A 2017, 5, 2821–2828. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y. Effects of additives on the microstructure and hydrogen storage properties of the Li3N-MgH mixture. J. Alloy. Compd. 2014, 613, 199–203. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Lv, Y.; Yan, Y.; Wu, Y. Hydrogen storage properties of the mixtures MgH2–Li3N with different molar ratios. J. Alloy. Compd. 2015, 645, S464–S467. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zang, L.; Chang, X.; Jiao, L.; Yuan, H.; Wang, Y. Core-shell Ni3N@nitrogen-doped carbon: Synthesis and application in MgH2. J. Alloy. Compd. 2017, 703, 381–388. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Hu, J.; Roth, A.; Wang, D.; Kubel, C.; Lohstroh, W.; Fichtner, M. Altered thermodynamic and kinetic properties of MgH2 infiltrated in microporous scaffold. Chem. Commun. 2010, 46, 8353–8355. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sun, C.; Cheng, L.; Wahab, M.A.; Cui, J.; Zou, J.; Zhu, M.; Yao, X. Destabilization of Mg-H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2. Phys. Chem. Chem. Phys. 2013, 15, 5814–5820. [Google Scholar] [CrossRef] [PubMed]
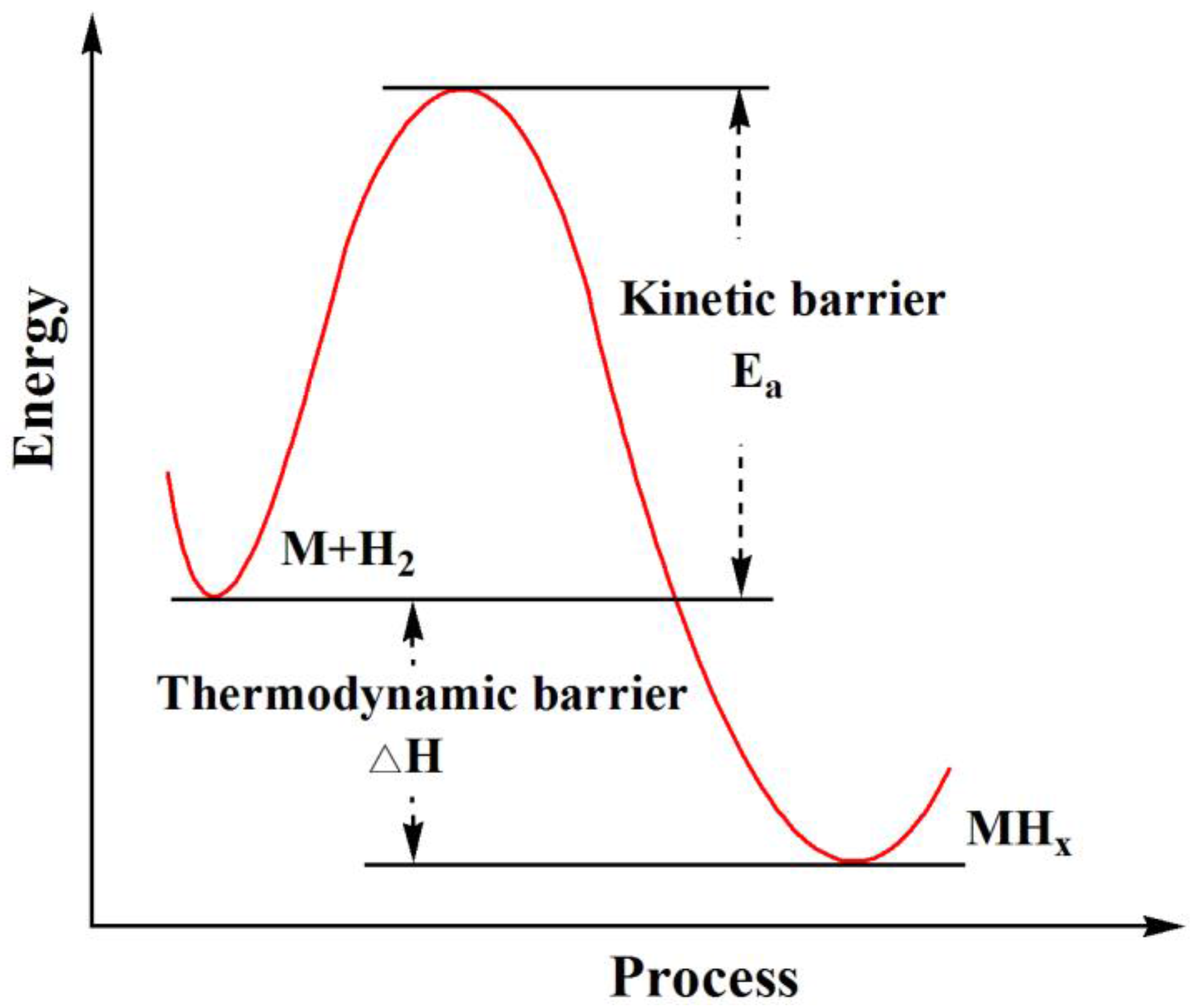
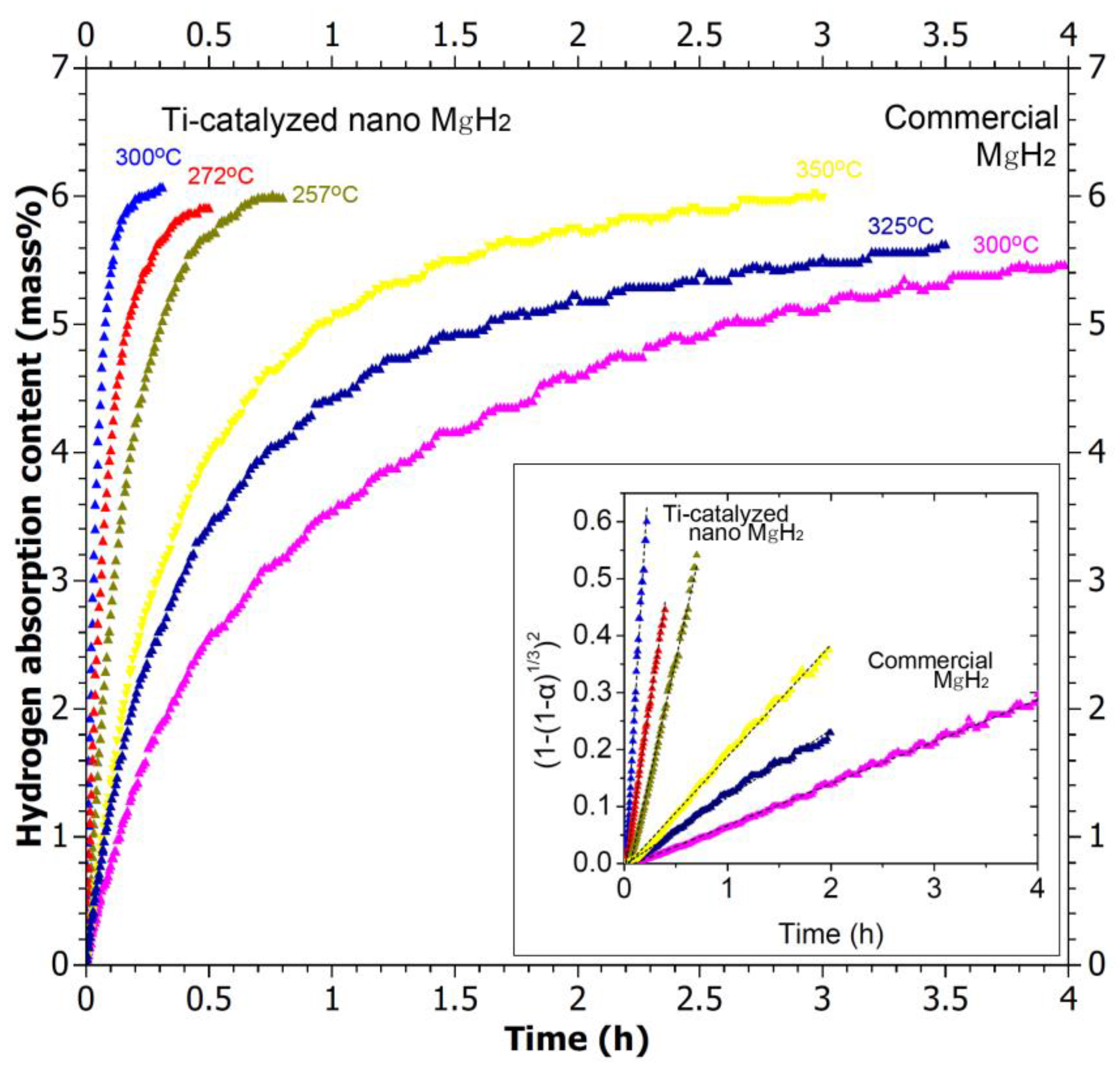
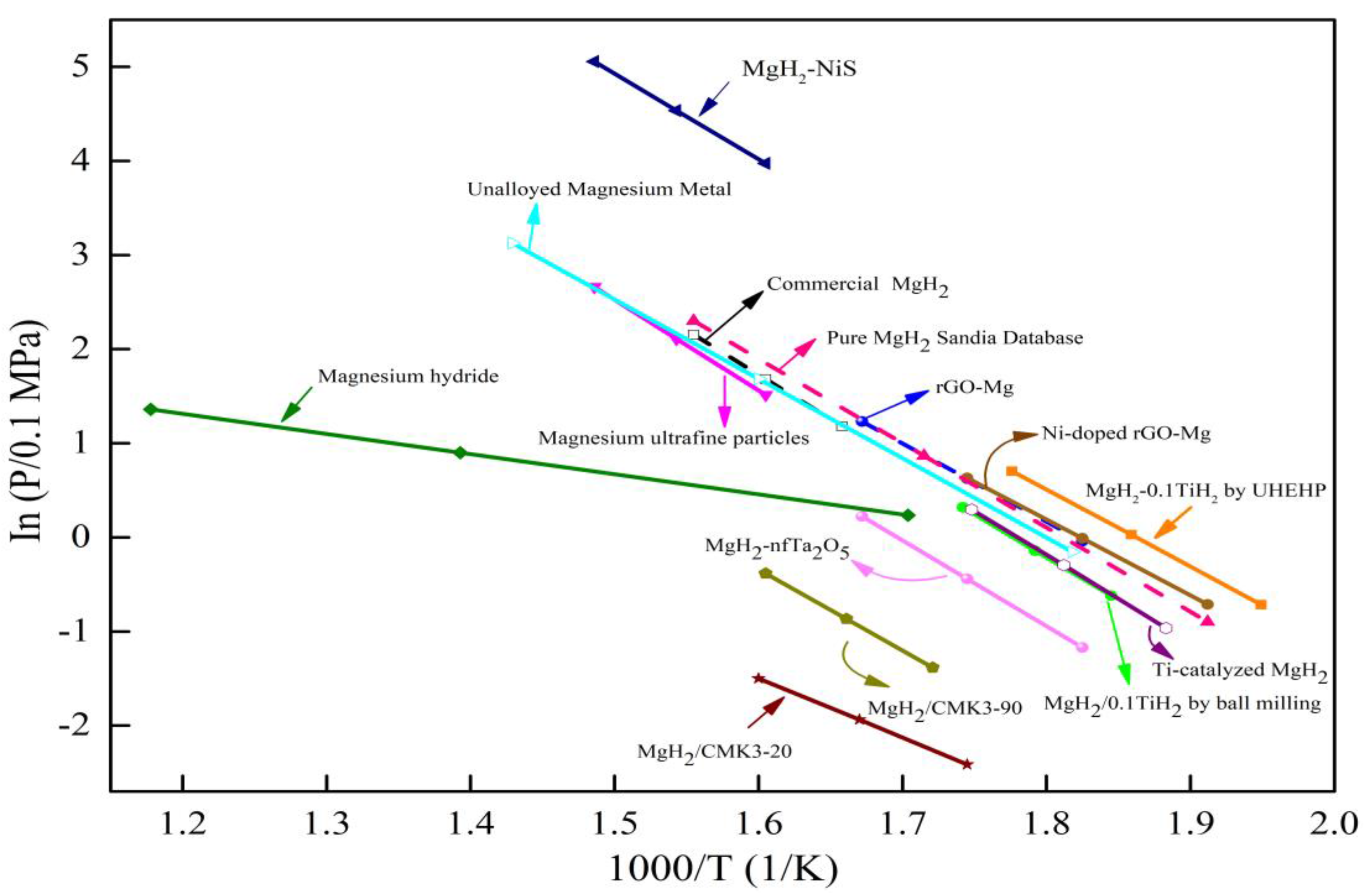

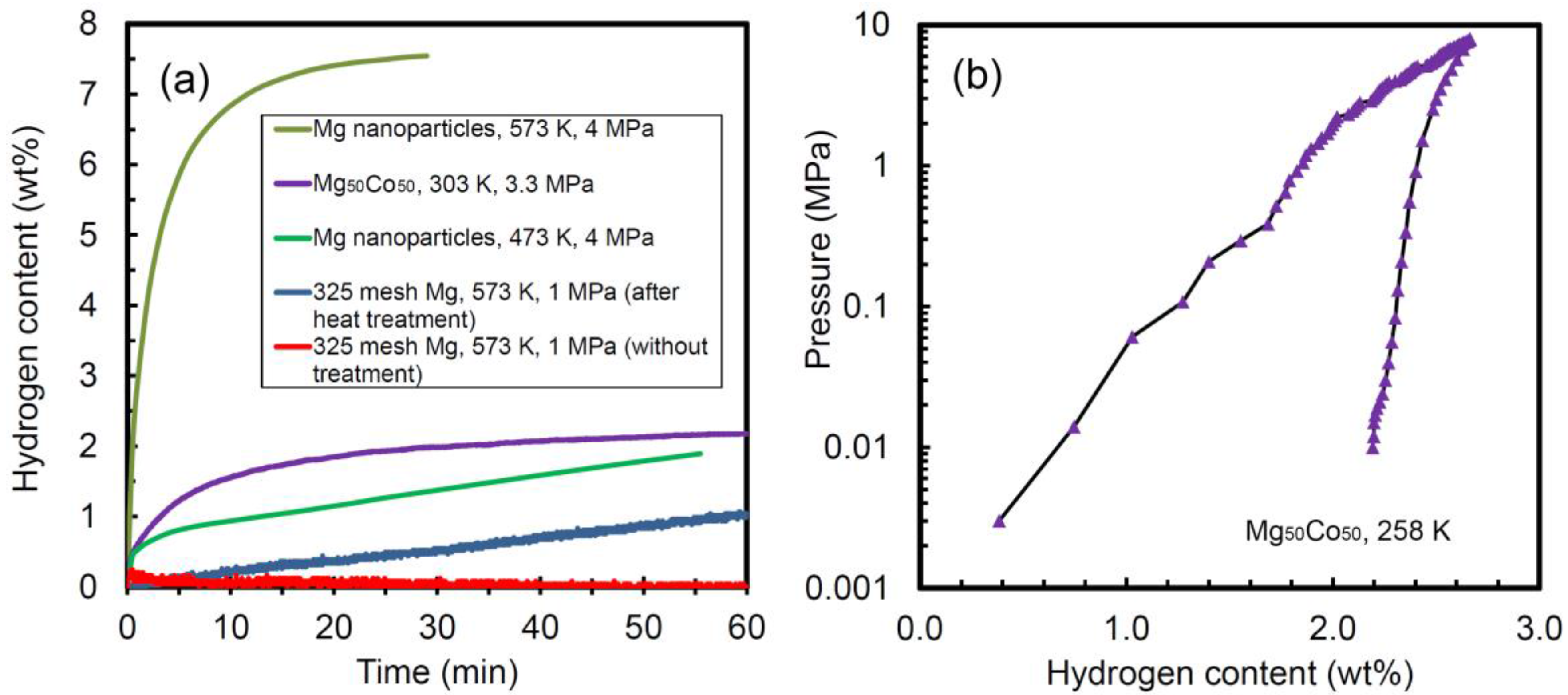

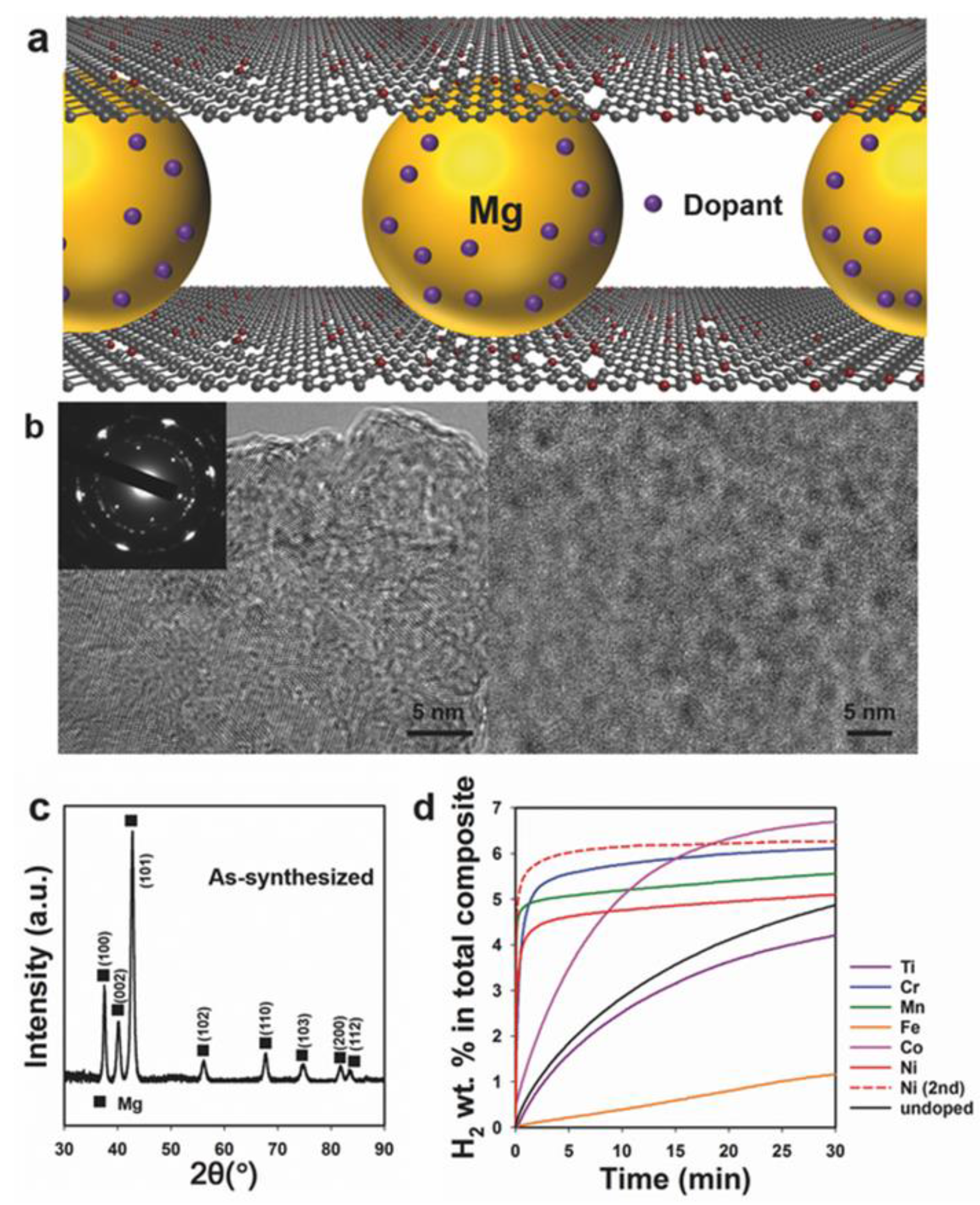
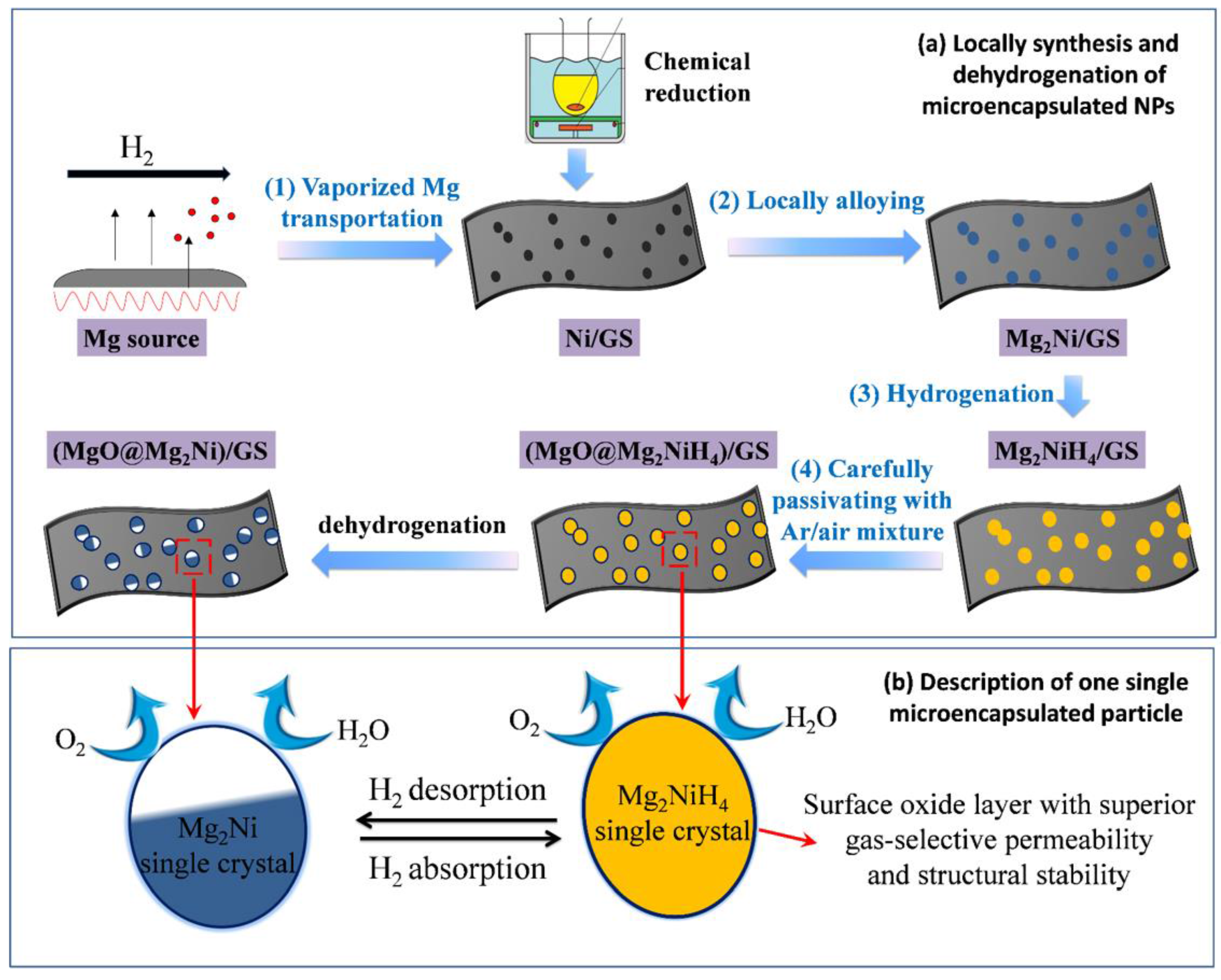
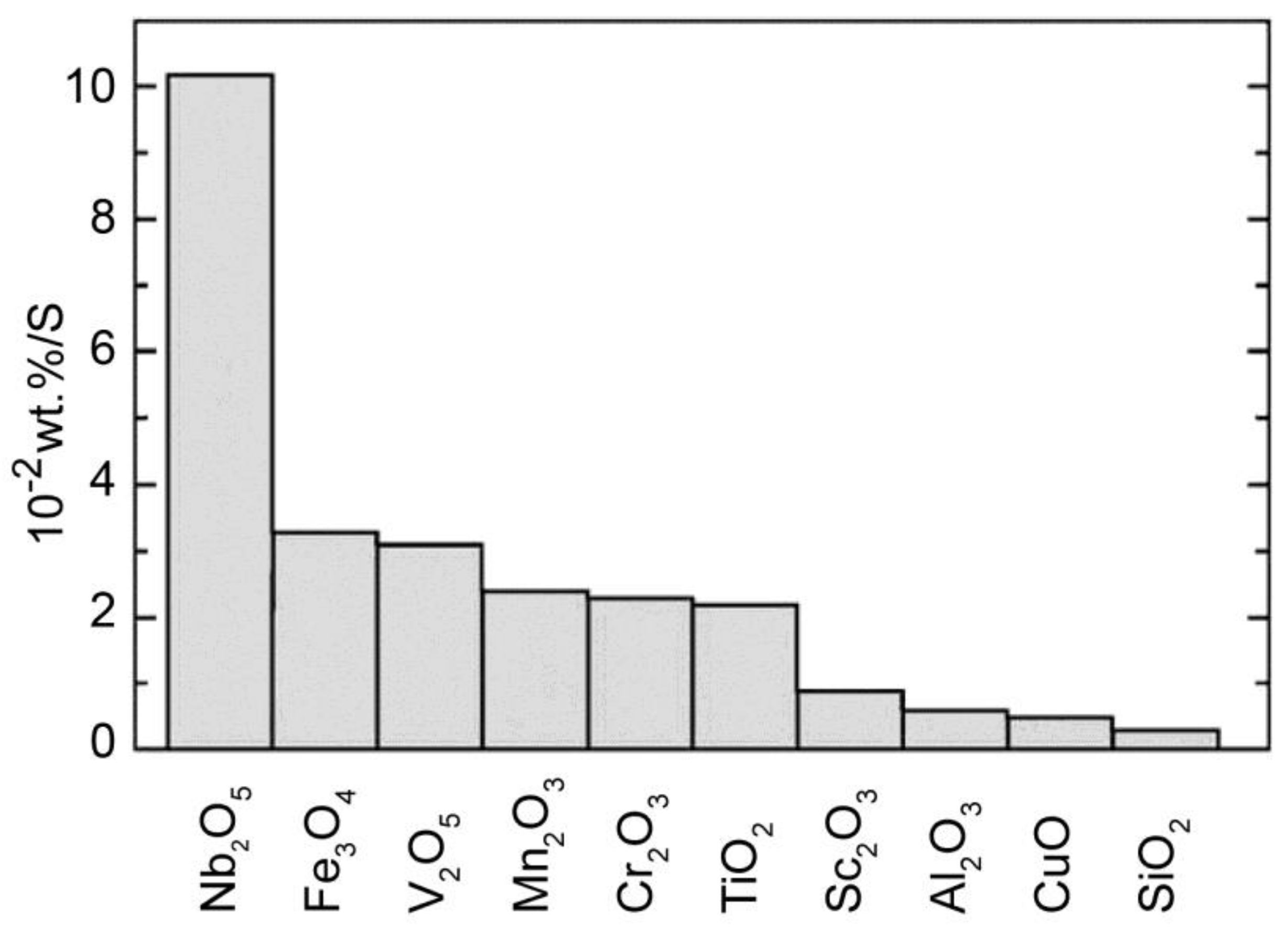
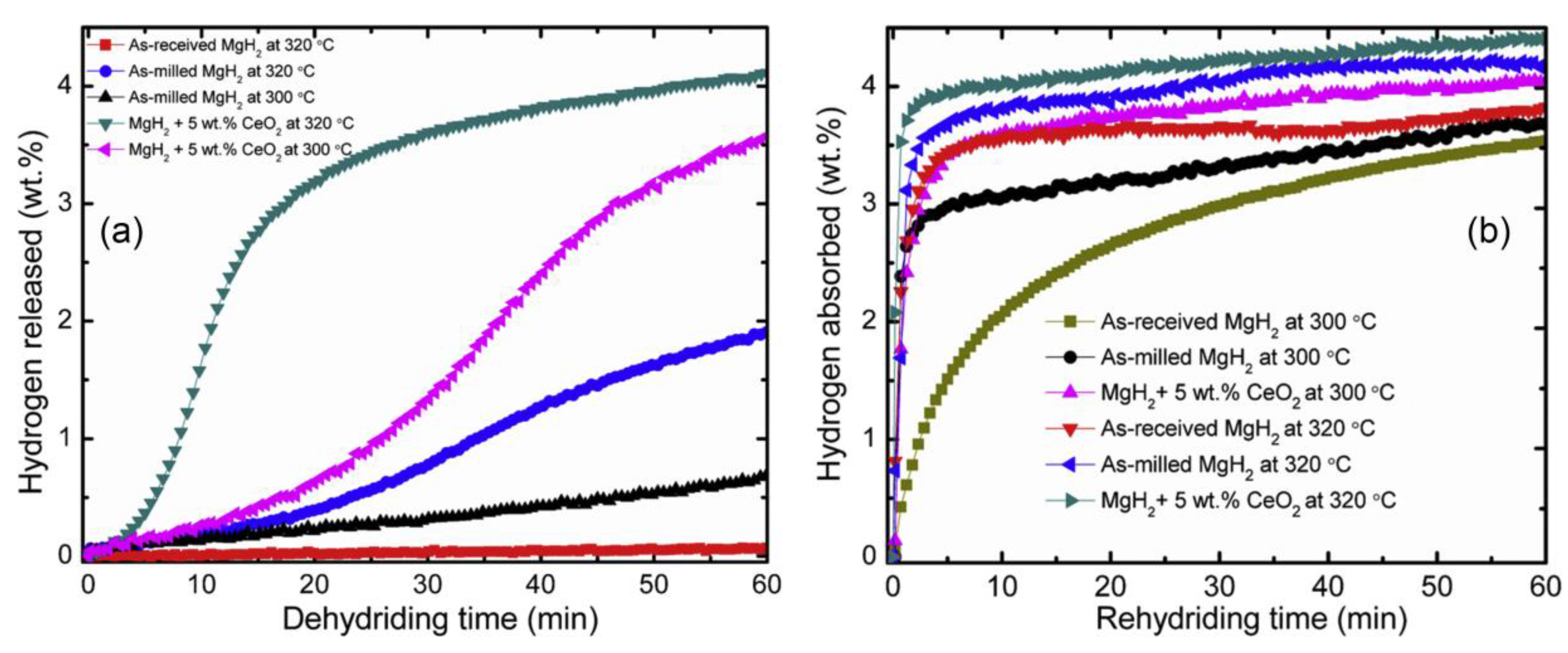
| System | Temperature Range (°C) | Van’t Hoff Equation | ∆H (kJ/mol H2) | ∆S J/(K mol H2) | Reference |
|---|---|---|---|---|---|
| MgH2-0.1TiH2 by UHEHP | 240–290 | logP(bar) = −3560.6/T + 6.6302 | 68.2 | 127 | [20] |
| MgH2/0.1TiH2 by ball milling | 269–301 | ln(P/0.1 MPa) = −9308.8/T + 16.539 | 77.4 | 137.5 | [21] |
| Commercial MgH2 | 330–370 | ln(P/0.1 MPa) = −9445.1/T + 16.844 | 78.5 | 140 | |
| Magnesium Ultrafine Particles | 350–400 | ln(P/bar) = −9604/T + 16.93 | 79.8 | 140.8 | [27] |
| Magnesium hydride | 314–576 | InfH2 = −2139.2/T + 3.88 | 73.1 | 135.8 | [32] |
| MgH2-NiS | 350–400 | lnP = −9061.7/T + 18.5192 | 75.34 | 153.97 | [33] |
| Unalloyed Magnesium Metal | 277–427 | ln(P/0.1MPa) = −8419.5/T + 15.155 | 70 | 126 | [34] |
| Ti-catalyzed MgH2 | 258–299 | ln(P/0.1 MPa) = −9345.7/T + 16.635 | 77.7 | 138.3 | [29] |
| MgH2/CMK3-20 | 300–350 | ln(P/0.1 MPa) = −6300.2/T + 8.580 | 52.38 | 71.33 | [35] |
| MgH2/CMK3-90 | 308–350 | ln(P/0.1 MPa) = −8620.4/T + 13.453 | 71.67 | 111.85 | |
| MgH2–nfTa2O5 | 275–325 | ln(P/0.1 MPa) = −9141.2/T + 15.510 | 76 | 129 | [36] |
| rGO–Mg | 275–325 | ln(P/0.1 MPa) = −8347.4/T + 15.191 | 69.4 | 126.3 | [37] |
| Ni-doped rGO–Mg | 250–300 | ln(P/0.1 MPa) = −8046.7/T + 14.674 | 66.9 | 122 | |
| Pure MgH2 | 250–370 | log(P/0.1 MPa) = −3893.8/T + 7.055 | 74.6 | 135.1 | Sandia National Lab database |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts 2018, 8, 89. https://doi.org/10.3390/catal8020089
Li J, Li B, Shao H, Li W, Lin H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts. 2018; 8(2):89. https://doi.org/10.3390/catal8020089
Chicago/Turabian StyleLi, Jianding, Bo Li, Huaiyu Shao, Wei Li, and Huaijun Lin. 2018. "Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials" Catalysts 8, no. 2: 89. https://doi.org/10.3390/catal8020089





