Synthesis of Tetrahydropyran from Tetrahydrofurfuryl Alcohol over Cu–Zno/Al2O3 under a Gaseous-Phase Condition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. BET Characterization
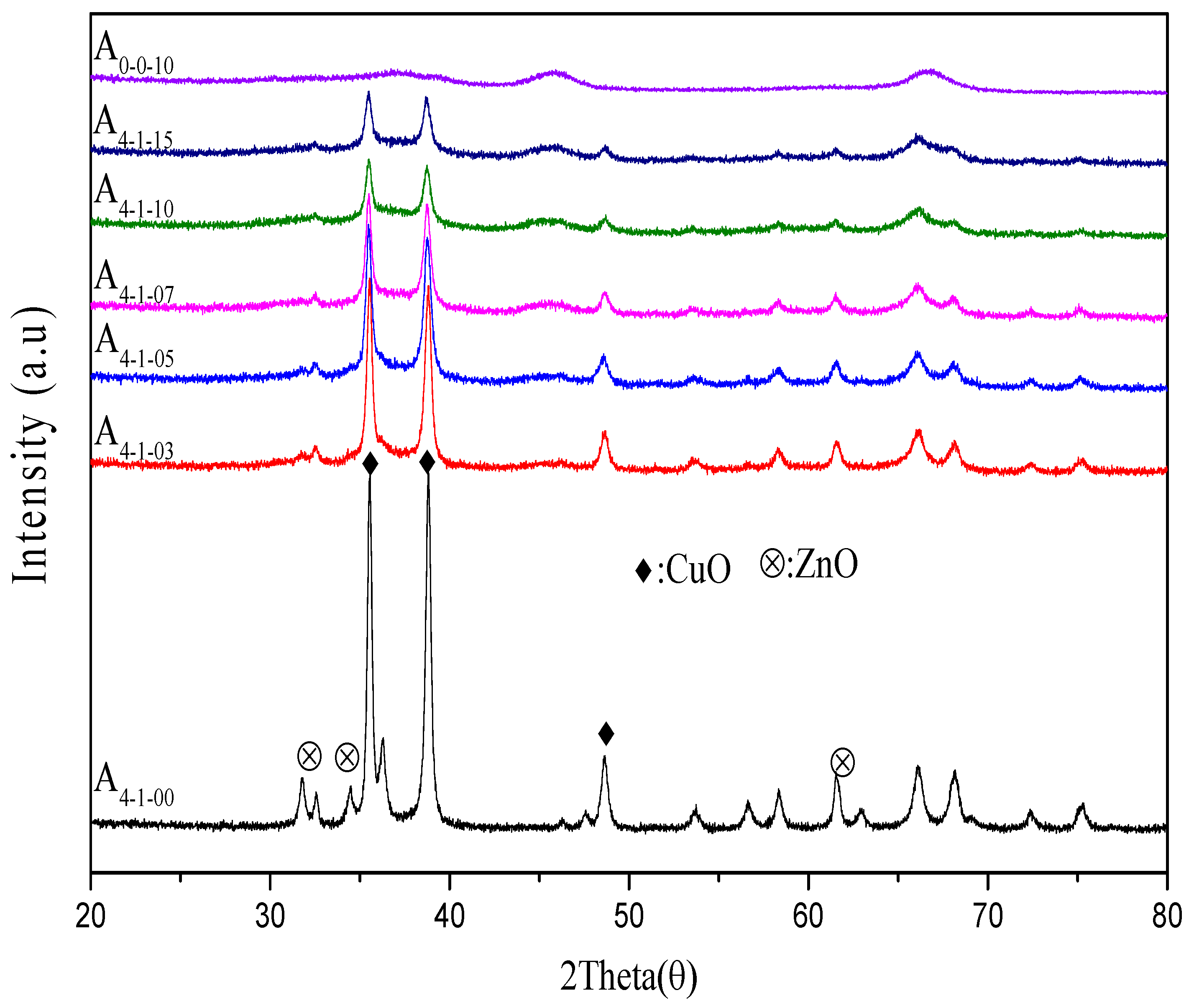
2.1.2. XRD Characterization
2.1.3. NH3-TPD Characterization
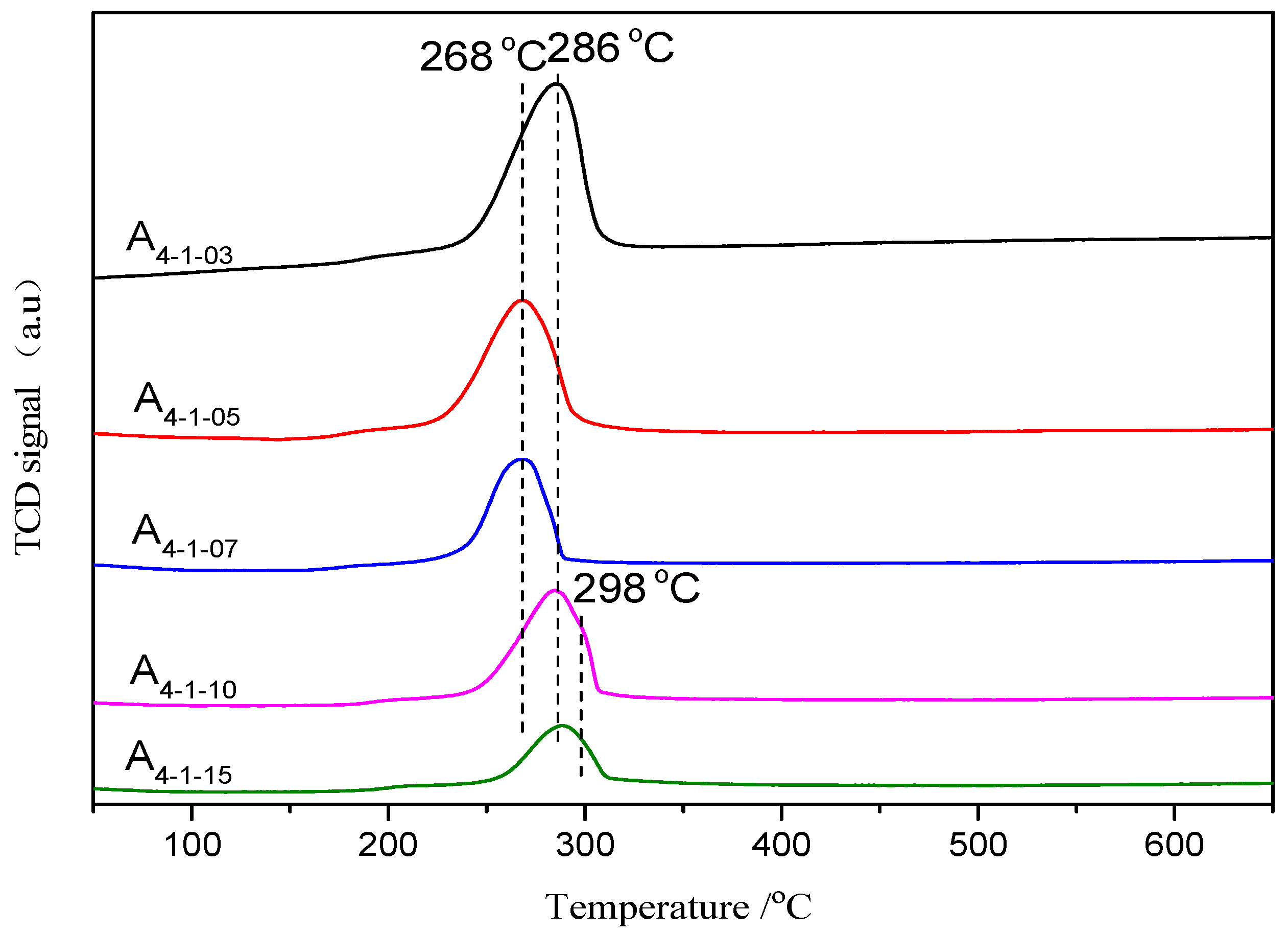
2.1.4. H2-TPR Characterization
2.1.5. HRTEM Characterization
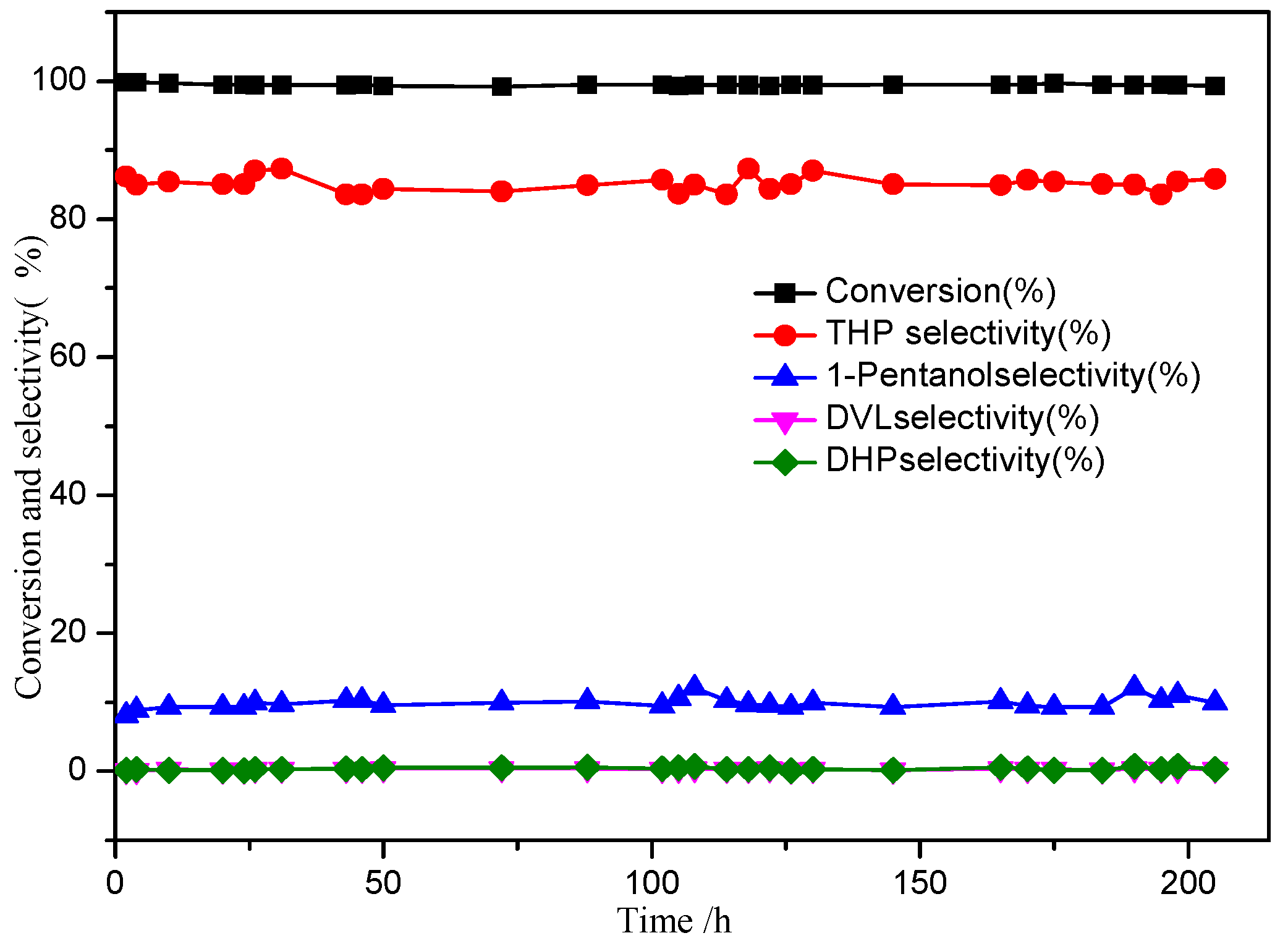
2.2. Catalysts Performance
2.2.1. Effect of Cu/Zn/Al Ratio
2.2.2. Effect of Reaction Temperature and H2 Pressure
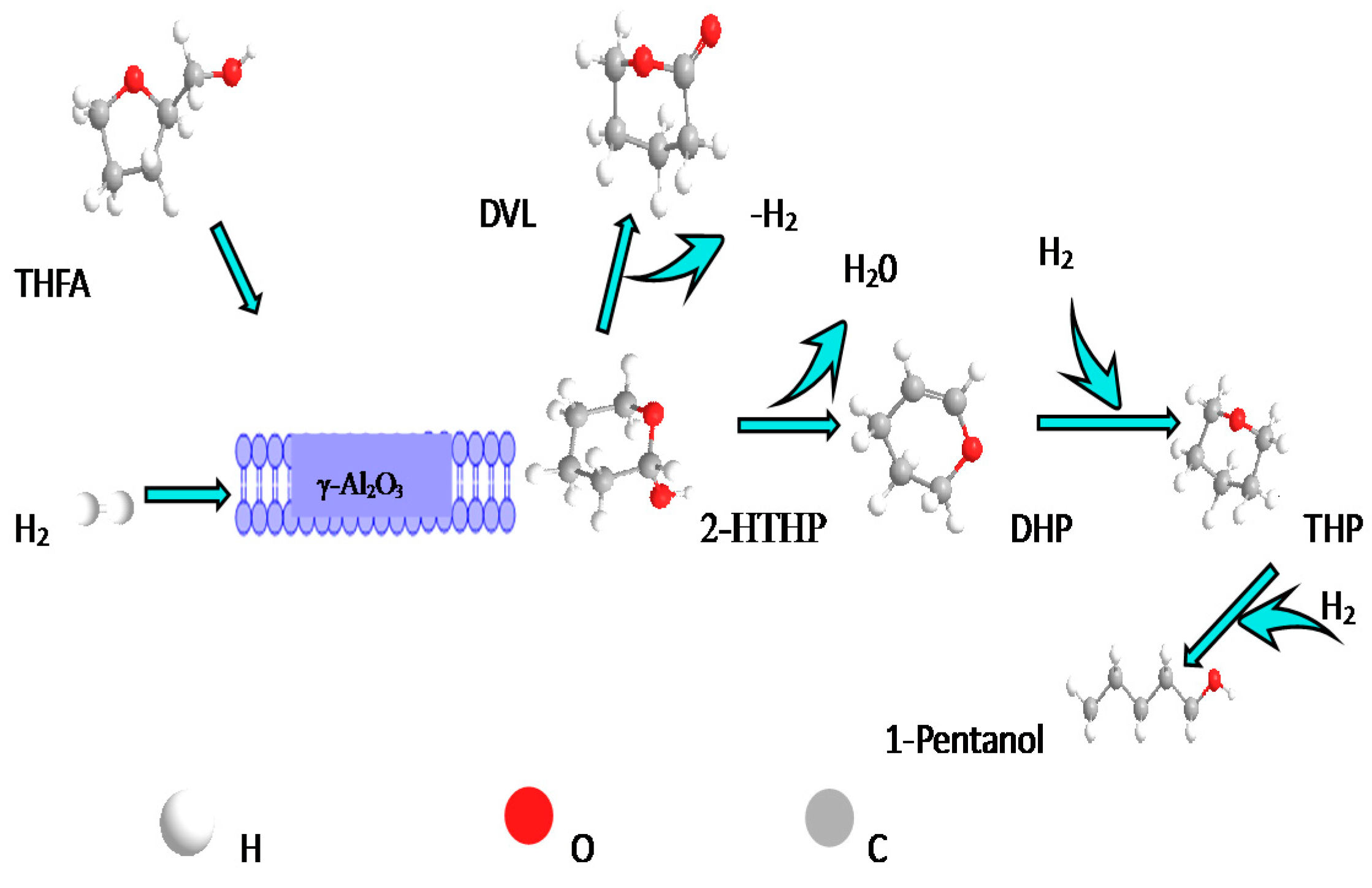
2.2.3. Reaction Paths
3. Experimental
3.1. Catalyst Preparation
3.2. Catalytic Reaction
3.3. Characterization of Catalyst
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corma, A.; Sara Iborra, A.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559. [Google Scholar] [CrossRef] [PubMed]
- Karinen, R.; Vilonen, K.; Niemelä, M. Biorefining: Heterogeneously Catalyzed Reactions of Carbohydrates for the Production of Furfural and Hydroxymethylfurfural. ChemSusChem 2011, 4, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabal-Telleria, I.; Hemmann, F.; Jäger, C.; Arias, P.L.; Kemnitz, E. Functionalized partially hydroxylated MgF2, as catalysts for the dehydration of d-xylose to furfural. J. Catal. 2013, 305, 81–91. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tomishige, K. Catalyst Development for the Hydrogenolysis of Biomass-Derived Chemicals to Value-Added Ones. Catal. Surv. Asia 2011, 15, 111–116. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Production of 1, 5-pentanediol from biomass via furfural and tetrahydrofurfuryl alcohol. Catal. Today 2012, 195, 136–143. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Tomishige, K.; Tamura, M.; Nakagawa, Y. Role of Re species and acid cocatalyst on Ir-ReOx/SiO2 in the C–O hydrogenolysis of biomass-derived substrates. Chem. Rec. 2014, 14, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Koso, S.; Furikado, I.; Shimao, A.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol. Chem. Commun. 2009, 15, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Koso, S.; Ueda, N.; Shinmi, Y.; Okumura, K.; Kizuka, T.; Tomishige, K. Promoting effect of Mo on the hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Rh/SiO2. J. Catal. 2009, 267, 89–92. [Google Scholar] [CrossRef]
- Koso, S.; Nakagawa, Y.; Tomishige, K. Mechanism of the hydrogenolysis of ethers over silica-supported rhodium catalyst modified with rhenium oxide. J. Catal. 2011, 280, 221–229. [Google Scholar] [CrossRef]
- Chia, M.; Pagán-Torres, Y.J.; Hibbitts, D.; Tan, Q.; Pham, H.N.; Datye, A.K.; Neurock, M.; Davis, R.J.; Dumesic, J.A. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts. J. Am. Chem. Soc. 2011, 133, 12675–12689. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mori, K.; Watanabe, H.; Nakagawa, Y.; Tomishige, K. C–O bond hydrogenolysis of cyclic ethers with OH groups over rhenium-modified supported iridium catalysts. J. Catal. 2012, 294, 171–183. [Google Scholar] [CrossRef]
- Koso, S.; Watanabe, H.; Okumura, K.; Nakagawa, Y.; Tomishige, K. Comparative study of Rh–MoOx and Rh–ReOx supported on SiO2 for the hydrogenolysis of ethers and polyols. Appl. Catal. B Environ. 2012, 111, 27–37. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mori, K.; Chen, K.; Amada, Y.; Tamura, M.; Tomishige, K. Hydrogenolysis of C, O bond over Re-modified Ir catalyst in alkane solvent. Appl. Catal. A Gen. 2013, 468, 418–425. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Performance and characterization of rhenium-modified Rh–Ir alloy catalyst for one-pot conversion of furfural into 1,5-pentanediol. Catal. Sci. Technol. 2014, 4, 2535–2549. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. One-pot selective conversion of furfural into 1, 5-pentanediol over a Pd-added Ir–ReOx/SiO2 bifunctional catalyst. Green Chem. 2014, 16, 617–626. [Google Scholar] [CrossRef]
- Pholjaroen, B.; Li, N.; Huang, Y.; Li, L.; Wang, A.; Zhang, T. Selective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over vanadium modified Ir/SiO2, catalyst. Catal. Today 2015, 245, 93–99. [Google Scholar] [CrossRef]
- Feng, S.; Nagao, A.; Aihara, T.; Miura, H.; Shishido, T. Selective hydrogenolysis of tetrahydrofurfuryl alcohol on Pt/WO3/ZrO2, catalysts: Effect of WO3, loading amount on activity. Catal. Today 2017, 303, 207–212. [Google Scholar] [CrossRef]
- Wan, W.; Jenness, G.R.; Xiong, K.; Vlachos, D.G.; Chen, J.G. Ring-Opening Reaction of Furfural and Tetrahydrofurfuryl Alcohol on Hydrogen-Predosed Iridium(III) and Cobalt/Iridium(III) Surfaces. ChemCatChem 2017, 9, 1701–1707. [Google Scholar] [CrossRef]
- Brentzel, Z.J.; Barnett, K.J.; Huang, K.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W. Chemicals from Biomass: Combining Ring-Opening Tautomerization and Hydrogenation Reactions to Produce 1, 5-Pentanediol from Furfural. ChemSusChem 2017, 10, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, L.E.; Geller, H.H. Preparation of Dihydropyran, δ-Hydroxyvaleraldehyde and 1,5-Pentanediol from Tetrahydrofurfuryl Alcohol. J. Am. Chem. Soc. 1946, 68, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L. Reactions of Furan Compounds. VII. Thermal Interconversion of 2,3-Dihydrofuran and Cyclopropane Aldehyde. J. Am. Chem. Soc. 1947, 69, 3002–3004. [Google Scholar] [CrossRef]
- Sato, S.; Igarashi, J.; Yamada, Y. Stable vapor-phase conversion of tetrahydrofurfuryl alcohol into 3, 4–2 H -dihydropyran. Appl. Catal. A Gen. 2013, 453, 213–218. [Google Scholar] [CrossRef]
- Thomas, H.P.; Wilson, C.L. Reactions of Furan Compounds. XV. Behavior of Tetrahydrofurfuryl Alcohol over Iron-Copper Catalysts. J. Am. Chem. Soc. 2002, 73, 4803–4805. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Yamamoto, N.; Kaneko, E.; Inoue, H. Vapor-phase dehydration of 1,5-pentanediol into 4-penten-1-ol. Appl. Catal. A Gen. 2008, 334, 84–91. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Bando, K.K.; Shirai, M. Enhancement of cyclic ether formation from polyalcohol compounds in high temperature liquid water by high pressure carbon dioxide. Green Chem. 2009, 11, 48–52. [Google Scholar] [CrossRef]
- Agrawal, O. Organic Chemistry Reactions and Reagents; Goel Publishing House: New Delhi, India, 2008; Volume 627, pp. 686–687. [Google Scholar]
- Falbe, J. Carbon Monoxide in Organic Synthesis; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Müller, S.P.; Kucher, M.; Ohlinger, C.; Kraushaar-Czarnetzki, B. Extrusion of Cu/ZnO catalysts for the single-stage gas-phase processing of dimethyl maleate to tetrahydrofuran. J. Catal. 2003, 218, 419–426. [Google Scholar] [CrossRef]
- Guo, P.J.; Chen, L.F.; Yan, S.R.; Dai, W.L.; Qiao, M.H.; Xu, H.L.; Fan, K.N. One-step hydrogenolysis of dimethyl maleate to tetrahydrofuran over chromium-modified Cu–B/γ-Al2O3, catalysts. J. Mol. Catal. A Chem. 2006, 256, 164–170. [Google Scholar] [CrossRef]
- Soghrati, E.; Choong, C.; Poh, C.K.; Kawi, S.; Borgna, A. Single-Pot Conversion of Tetrahydrofurfuryl Alcohol into Tetrahydropyran over a Ni/HZSM—5 Catalyst under Aqueous-Phase Conditions. ChemCatChem 2017, 9, 1402–1408. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922. [Google Scholar] [CrossRef] [PubMed]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, X.; Li, X.; Ding, G.; Zhu, Y.; Li, Y. Cu Nanoparticles Inlaid Mesoporous Al2O3 As a High-Performance Bifunctional Catalyst for Ethanol Synthesis via Dimethyl Oxalate Hydrogenation. ACS Catal. 2014, 4, 3612–3620. [Google Scholar] [CrossRef]
- Huang, Z.W.; Cui, F.; Xue, J.J.; Zuo, J.; Chen, J.; Xia, C. Cu/SiO2 catalysts prepared by hom- and heterogeneous deposition-precipitation methods: Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Today 2012, 183, 42–51. [Google Scholar] [CrossRef]
- Burattin, P.; Che, M.; Louis, C. Molecular Approach to the Mechanism of Deposition−Precipitation of the Ni(II) Phase on Silica. J. Phys. Chem. B 1998, 102, 2722–2732. [Google Scholar] [CrossRef]
- Herrmann, U.; Emig, G. Liquid phase hydrogenation of maleic anhydride and intermediates on copper-based and noble metal catalysts. Ind. Eng. Chem. Res. 1997, 36, 2885–2896. [Google Scholar] [CrossRef]

| Catalysts | Cu/Zn/Al Molar Ratio | SBET (m2·g−1) | Pore Volume (cc⋅g−1) | Copper Crystalline Size (nm) | Average Pore Size (nm) |
|---|---|---|---|---|---|
| A4-1-00 | 4:1:00 | 41.3 | 0.18 | 34.4 | 8.8 |
| A0-0-01 | 0:0:10 | 310.3 | 0.94 | - | 6.1 |
| A4-1-03 | 4:1:03 | 196.4 | 0.54 | 26.6 | 4.7 |
| A4-1-05 | 4:1:05 | 199.3 | 0.61 | 25.8 | 4.5 |
| A4-1-07 | 4:1:07 | 203.5 | 0.63 | 25.0 | 3.9 |
| A4-1-10 | 4:1:10 | 223.8 | 0.68 | 24.3 | 3.8 |
| A4-1-15 | 4:1:15 | 261.7 | 0.70 | 22.3 | 3.6 |
| Samples | Temperature of NH3 Desportion Peaks (°C) | Acid Account for Percentage Acid Amount (μmol·g−1) | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | Weak (%) | Medium (%) | Strong (%) | Total | |
| A0-0-01 | 108 | 275 | 324 | 50.0 | 36.1 | 13.9 | 7.2 |
| A4-1-00 | 103 | 225 | 333 | 63.6 | 31.8 | 4.5 | 2.2 |
| A4-1-03 | 103 | 224 | 332 | 66.7 | 30.4 | 2.9 | 10.2 |
| A4-1-05 | 102 | 227 | 335 | 51.9 | 45.7 | 4.9 | 8.1 |
| A4-1-07 | 106 | 225 | 336 | 47.1 | 48.2 | 4.7 | 8.5 |
| A4-1-10 | 105 | 226 | 334 | 46.5 | 48.8 | 4.7 | 8.6 |
| A4-1-15 | 103 | 225 | 330 | 46.7 | 47.8 | 5.4 | 9.2 |
| Catalysts | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| THP | 1-Pentanol | DVL | DHP | ||
| A4-1-00 | 11.5 | 31.3 | 2.1 | 2.1 | 34.5 |
| A0-0-01 | 23.1 | 1.5 | 0.1 | 18.0 | 74.4 |
| A4-1-03 | 38.9 | 51.8 | 4.2 | 16.4 | 13.0 |
| A4-1-05 | 68.1 | 48.7 | 12.8 | 14.3 | 12.4 |
| A4-1-07 | 66.6 | 69.3 | 10.1 | 12.6 | 2.5 |
| A4-1-10 | 78.6 | 77.8 | 8.6 | 8.1 | 1.6 |
| A4-1-15 | 57.4 | 63.6 | 3.5 | 21.1 | 4.6 |
| Catalysts | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| THP | 1-Pentanol | DVL | DHP | ||
| A a | 57.7 | 68.8 | 4.6 | 18.2 | 3.2 |
| B a | 36.4 | 77.9 | 0.6 | 7 | 0.3 |
| A b | 98 | 89.5 | 7 | 0.6 | 0 |
| B b | 90.4 | 74.9 | 12 | 2.6 | 0.8 |
| T(°C) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| THP | 1-Pentanol | DVL | DHP | ||
| 230 | 37.7 | 55.2 | 2.5 | 29 | 3.8 |
| 240 | 57.7 | 68.8 | 4.6 | 18.2 | 3.2 |
| 250 | 78.6 | 77.8 | 8.6 | 8.1 | 1.6 |
| 260 | 91.9 | 86.8 | 7.3 | 3 | 0.8 |
| 270 | 95.2 | 91.4 | 5.6 | 1.3 | 0.4 |
| 280 | 98 | 89.5 | 7 | 0.6 | 0 |
| 290 | 99.3 | 85.9 | 7.5 | 0.2 | 0.1 |
| P(MPa) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| THP | 1-Pentanol | DVL | DHP | ||
| 0.2 | 68.9 | 45.5 | 8.4 | 17.7 | 8.8 |
| 0.4 | 76.5 | 59.4 | 6.0 | 15.8 | 8.4 |
| 0.6 | 88.3 | 81.6 | 6.5 | 5.9 | 2.8 |
| 0.8 | 91.7 | 90.5 | 5.0 | 2.6 | 1.3 |
| 1.0 | 98.8 | 89.1 | 7.8 | 0.7 | 0.6 |
| 1.5 | 97.8 | 89.1 | 7.9 | 1.2 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, B.; Wang, X.; Huang, L.; Ji, D.; Du, S.; Ma, L.; Lin, S. Synthesis of Tetrahydropyran from Tetrahydrofurfuryl Alcohol over Cu–Zno/Al2O3 under a Gaseous-Phase Condition. Catalysts 2018, 8, 105. https://doi.org/10.3390/catal8030105
Zhang F, Zhang B, Wang X, Huang L, Ji D, Du S, Ma L, Lin S. Synthesis of Tetrahydropyran from Tetrahydrofurfuryl Alcohol over Cu–Zno/Al2O3 under a Gaseous-Phase Condition. Catalysts. 2018; 8(3):105. https://doi.org/10.3390/catal8030105
Chicago/Turabian StyleZhang, Fengyuan, Bin Zhang, Xincheng Wang, Long Huang, Dekun Ji, Songsong Du, Lei Ma, and Shijing Lin. 2018. "Synthesis of Tetrahydropyran from Tetrahydrofurfuryl Alcohol over Cu–Zno/Al2O3 under a Gaseous-Phase Condition" Catalysts 8, no. 3: 105. https://doi.org/10.3390/catal8030105





