High Performance of Manganese Porphyrin Sensitized p-Type CuFe2O4 Photocathode for Solar Water Splitting to Produce Hydrogen in a Tandem Photoelectrochemical Cell
Abstract
:1. Introduction
2. Results
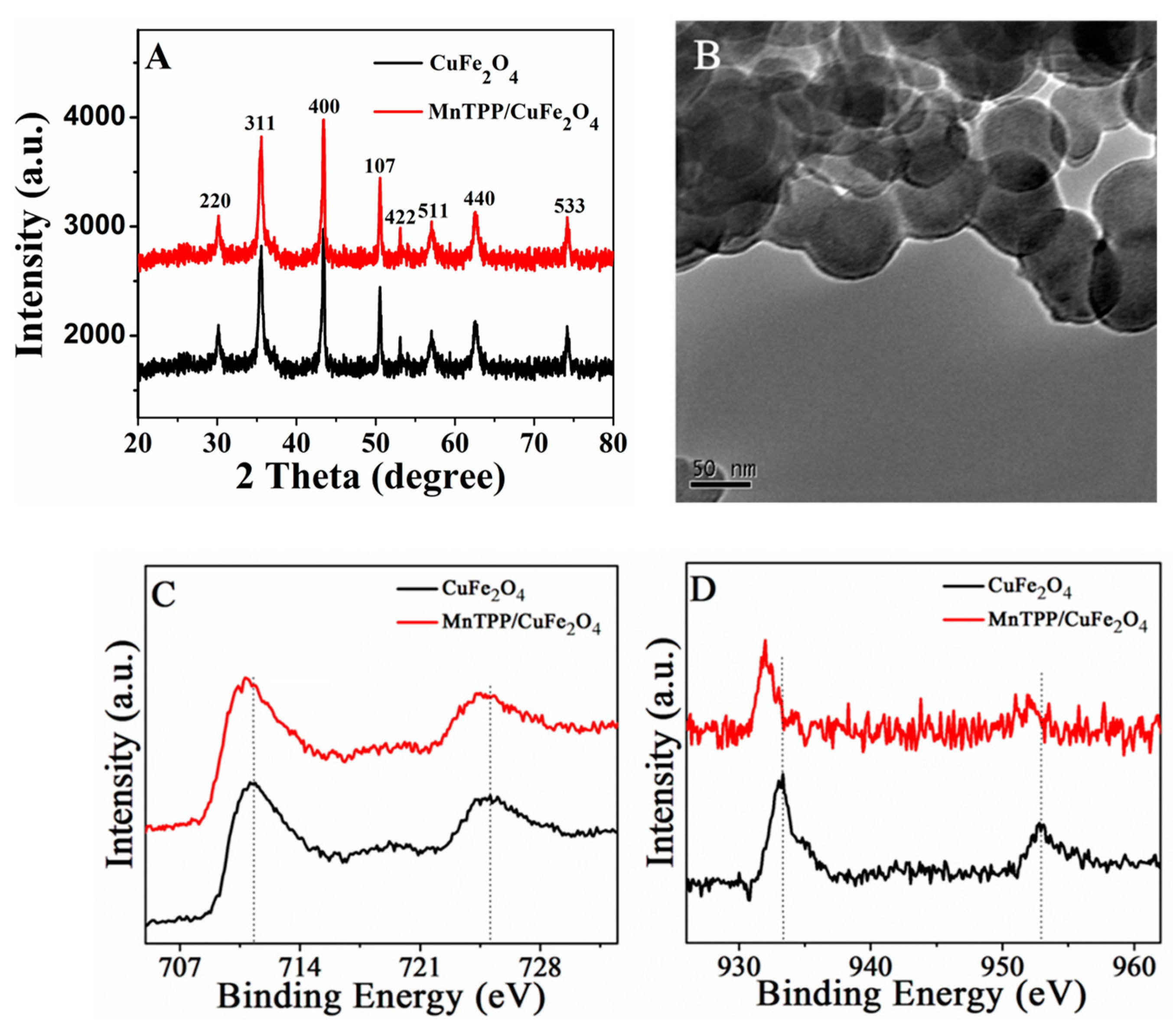
2.1. Morphology and Structure
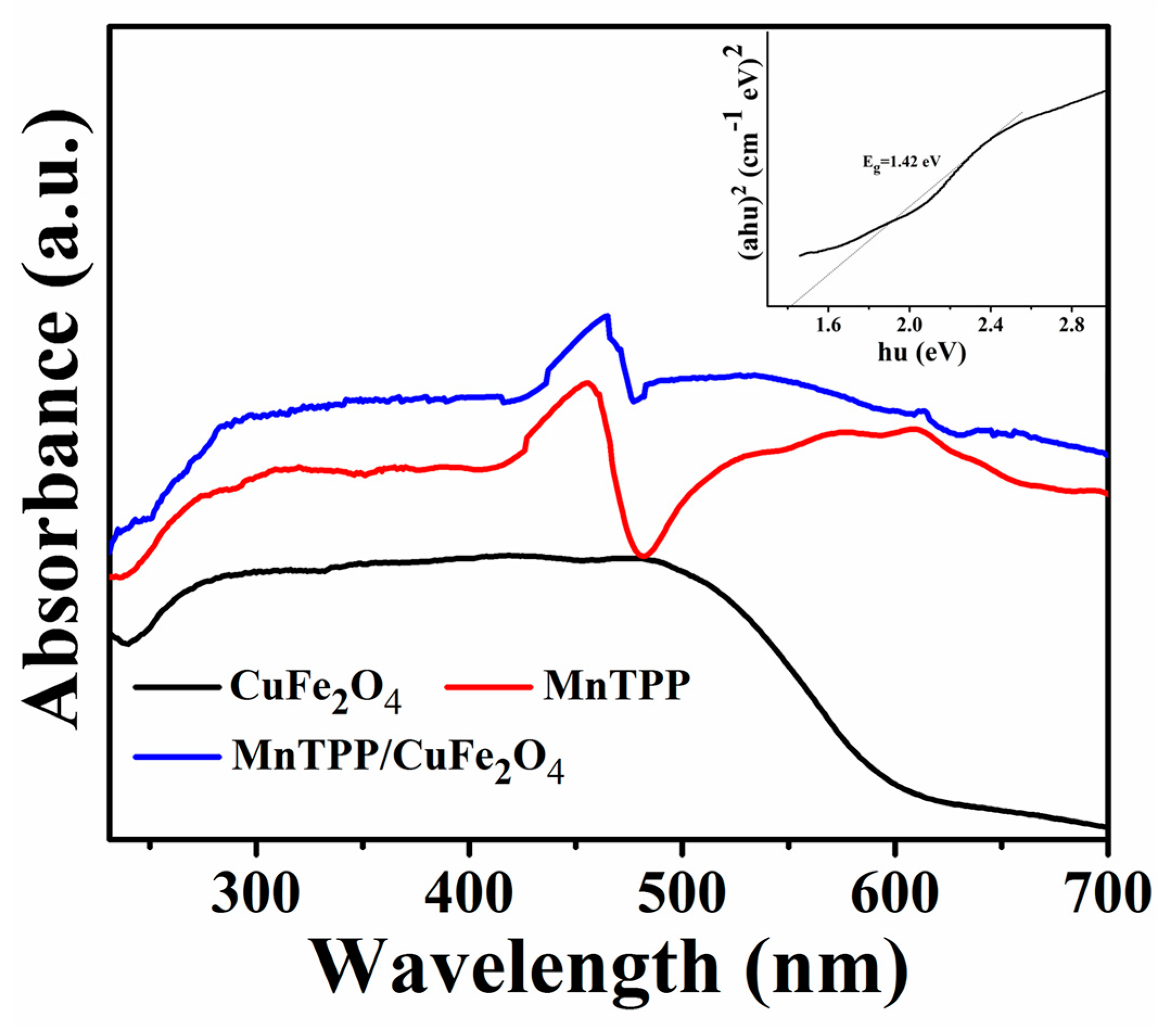
2.2. Optical and Photoelectrochemical Properties
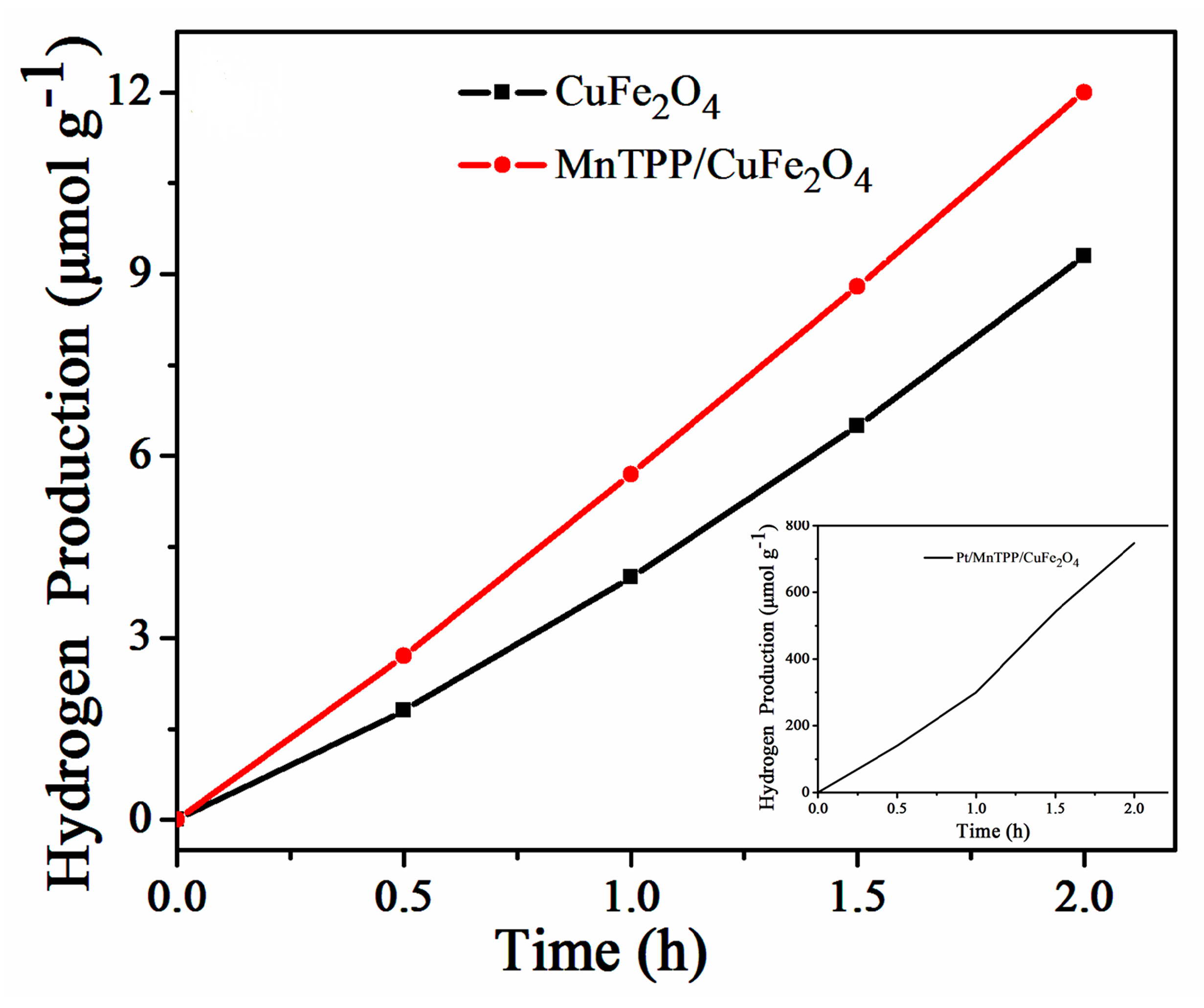
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Photoelectrode Preparation
3.3. Characterization
3.4. Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, W.; Lai, W.; Cao, R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, J.; Li, X.; Yang, P.; Du, Y.; Goh, C.; Lu, C. Photocatalytic H2 production under visible-light irradiation based on covalent attachment of manganese phthalocyanine to grapheme. J. Mater. Chem. A 2015, 3, 4195–4202. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Zhang, R.; Zou, B.; Shi, H. Enhanced photoelectrochemical water splitting with TiO2@Ag2O nanowire arrays via p-n heterojunction formation. Sol. Energy Mater. Sol. Cells 2018, 174, 132–139. [Google Scholar] [CrossRef]
- She, X.; Zhang, Z.; Baek, M.; Yong, K. Photoelectrochemical enhancement of ZnO/BiVO4/ZnFe2O4/rare earth oxide hetero-nanostructures. Appl. Surf. Sci. 2017, 429, 29–36. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal engineering of sputter-grown CuO photocathode for visible-light-driven electrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Kushwaha, A.; Dalapati, G.K. Stable and efficient CuO based photocathode through oxygen-rich composition and Au-Pd nanostructure incorporation for solar-hydrogen production. ACS Appl. Mater. Interfaces 2017, 9, 27596–27606. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Kushwaha, A.; Wong, T.I.; Dalapati, G.K. Rapid thermal annealing assisted stability and efficiency enhancement in a sputter deposited CuO photocathode. RSC Adv. 2016, 6, 29383–29390. [Google Scholar] [CrossRef]
- Minami, T.; Sato, H.; Nanto, H.; Takata, S. Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Films 1993, 236, 27–31. [Google Scholar]
- Dong, Y.; Chen, Y.; Jiang, P.; Wang, G.; Wu, X.; Wu, R.; Zhang, C. Efficient and stable MoS2/CdSe/NiO photocathode for photoelectrochemical hydrogen generation from water. Chem. Asian J. 2015, 10, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.S.; Oh, S.Y. Characterization of a photoelectrochemical cell using porous TiO2 and its application to a z-scheme system for water splitting in combination with a p-type InP photocathode. J. Nanosci. Nanotechnol. 2017, 17, 5593–5596. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, M.; Zhong, M.; Ma, L.; Wang, F.; Ma, L.; Shen, W. Engineering MoSx/Ti/InP hybrid photocathode for improved solar hydrogen production. Sci. Rep. 2016, 6, 29738. [Google Scholar] [CrossRef] [PubMed]
- Mckone, J.R.; Pieterick, A.P.; Gray, H.B.; Lewis, N.S. Hydrogen evolution from Pt/Ru-coated p-type WSe2 photocathodes. J. Am. Chem. Soc. 2013, 135, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.W.; Lu, H.; Liu, Q.; Cao, F.R.; Guo, J.; Deng, K.M.; Li, L.A. Efficient p-type dye-sensitized solar cells with all-nano-electrodes: NiCo2S4 mesoporous nanosheet counter electrodes directly converted from NiCo2O4 photocathodes. Nanoscale Res. Lett. 2014, 9, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.; Olga, P.; Roman, Y.; Kateřina, P. Hydrogen sensors based on electrophoretically deposited Pd nanoparticles onto InP. Nanoscale Res. Lett. 2011, 6, 392–396. [Google Scholar]
- Gupta, N.; Sharma, C.; Kumar, M.; Kumar, R. Synthesis and comparative charge transfer studies in porphyrin—Fullerene dyads: Mode of attachment effect. New J. Chem. 2017, 41, 13276–13286. [Google Scholar] [CrossRef]
- Joshi, U.A.; Maggard, P.A. CuNb3O8: A p-type semiconducting metal oxide photoelectrode. J. Phys. Chem. Lett. 2012, 3, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Fukuda, M.; Nakabayashi, Y.; Saito, N.; Ogawa, N.; Nakajima, T.; Shinoda, K.; Tsuchiya, T.; Nosaka, Y. A method to give chemically stabilities of photoelectrodes for water splitting: Compositing of a highly crystalized TiO2 layer on a chemically unstable Cu2O photocathode using laser-induced crystallization process. Appl. Surf. Sci. 2016, 363, 173–180. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhang, W.; Zhang, J.; Wang, B.; Xia, C.; Afzal, M.; Li, J.; Singh, M.; Zhu, B. Natural CuFe2O4 mineral for solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 17514–17521. [Google Scholar] [CrossRef]
- Chen, Y.; Mou, Z.; Yin, S.; Huang, H.; Yang, P.; Wang, X.; Du, Y. Graphene enhanced photocatalytic hydrogen evolution performance of dye-sensitized TiO2 under visible light irradiation. Mater. Lett. 2013, 107, 31–34. [Google Scholar] [CrossRef]
- Diez-Garcia, M.; Lana-Villarreal, T.; Gomez, R. Study of copper ferrite as a novel photocathode for water reduction: Improving its photoactivity by electrochemical pretreatment. ChemSusChem 2016, 9, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Hasselman, G.M.; Watson, D.F.; Stromberg, J.R.; Bocian, D.F.; Holten, D.; Lindsey, J.S.; Meyer, G.J. Theoretical solar-to-electrical energy-conversion efficiencies of perylene-porphyrin light-harvesting arrays. J. Phys. Chem. B 2006, 110, 25430–25440. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Jiang, J. Recent advances in phthalocyanine-based functional molecular materials. In 50 Years of Structure and Bonding—The Anniversary Volume; Springer: Heidelberg, Germany, 2015; Volume 172, pp. 159–199. [Google Scholar]
- Hagiwara, H.; Higashi, K.; Watanabe, M.; Kakigi, R.; Ida, S.; Ishihara, T. Effect of porphyrin molecular structure on water splitting activity of a KTaO3 Photocatalyst. Catalysts 2016, 6, 42. [Google Scholar] [CrossRef]
- Roy, D.R.; Shah, E.V.; Roy, S.M. Optical activity of Co-porphyrin in the light of IR and Raman spectroscopy: A critical DFT investigation. Spectrochim. Acta Part A 2018, 190, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.A.; Song, T.; Sun, Q.; Liu, H.; Wang, T.; Zeng, H. Plasmon mediated Fe-O in octahedral site of cuprospinel by Cu NPs for photocatalytic hydrogen evolution. Nanascale 2017, 9, 15760–15765. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, A.V.; Yadav, G.D. Solvothermal synthesis of CuFe2O4@rGO: Efficient catalyst for C-O cross coupling and N-arylation reaction under ligand-free condition. ChemistrySelect 2017, 2, 7150–7159. [Google Scholar] [CrossRef]
- Wu, L.-K.; Wu, H.; Zhang, H.-B.; Cao, H.-Z.; Hou, G.-Y.; Tang, Y.-P.; Zheng, G.-Q. Graphene oxide/CuFe2O4 foam as an efficient absorbent for arsenic removal from water. Chem. Eng. J. 2018, 334, 1808–1819. [Google Scholar] [CrossRef]
- Zhang, W.; Quan, B.; Lee, C.; Park, S.K.; Li, X.; Choi, E.; Diao, G.; Piao, Y. One-step facile solvothermal synthesis of copper ferrite-graphene composite as a high-performance supercapacitor material. ACS Appl. Mater. Interfaces 2015, 7, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Park, J.; Kim, D.-W.; Kim, H.; Kang, Y.-C. Controlling the antibacterial activity of CuSn thin films by varying the contents of Sn. Appl. Surf. Sci. 2016, 389, 1012–1016. [Google Scholar] [CrossRef]
- Nayak, J.; Maniraj, M.; Gloskovskii, A.; Krajčí, M.; Sebastian, S.; Fisher, I.R.; Horn, K.; Barman, S.R. Bulk electronic structure of Zn-Mg-Y and Zn-Mg-Dy icosahedral quasicrystals. Phys. Rev. B 2015, 91. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Sites for high efficient photocatalytic hydrogen evolution on a limited-layered MoS2 cocatalyst confined on graphene sheets-the role of grapheme. J. Phys. Chem. C 2012, 116, 25415–25424. [Google Scholar] [CrossRef]
- Jing, P.; Li, J.; Pan, L.; Wang, J.; Sun, X.; Liu, Q. Efficient photocatalytic degradation of acid fuchsin in aqueous solution using separate porous tetragonal-CuFe2O4 nanotubes. J. Hazard. Mater. 2015, 284, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Nawle, A.C.; Humbe, A.V.; Babrekar, M.K.; Deshmukh, S.S.; Jadhav, K.M. Deposition, characterization, magnetic and optical properties of Zn doped CuFe2O4 thin films. J. Alloys Compd. 2017, 695, 1573–1582. [Google Scholar] [CrossRef]
- Zakeri, M.; Moghadam, M.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Mirkhani, V.; Khosropour, A.R. Multi-wall carbon nanotube supported manganese(III) tetraphenylporphyrin: Efficient catalysts for epoxidation of alkenes with NaIO4 under various reaction conditions. J. Coord. Chem. 2012, 65, 1144–1157. [Google Scholar] [CrossRef]
- Kezzim, A.; Nasrallah, N.; Abdi, A.; Trari, M. Visible light induced hydrogen on the novel hetero-system CuFe2O4/TiO2. Energy Convers. Manag. 2011, 52, 2800–2806. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, C.; Yang, P.; Du, Y.; Lu, C. WS2 as an effective noble-metal free cocatalyst modified TiSi2 for enhanced photocatalytic hydrogen evolution under visible light irradiation. Catalysts 2016, 6, 136. [Google Scholar] [CrossRef]
- Xiao, P.; Guang, W.; Meng, W.; Liu, X.; Wen, S. Electrochemical synthesis of p-type Zn-doped α-Fe2O3 nanotube arrays for photoelectrochemical water splitting. Chem. Commun. 2013, 44, 5742–5744. [Google Scholar]
- Wu, Y.; Yue, Z.; Liu, A.; Yang, P.; Zhu, M. P-type Cu-doped Zn0.3Cd0.7S/Graphene photocathode for efficient water splitting in a photoelectrochemical tandem cell. ACS Sustain. Chem. Eng. 2016, 4, 2569–2577. [Google Scholar] [CrossRef]
- Chen, X.; Xia, N.; Yang, Z.; Gong, F.; Wei, Z.; Wang, D.; Tang, J.; Fang, X.; Fang, D.; Liao, L. Analysis of the influence and mechanism of sulfur passivation on the dark current of a single GaAs nanowire photodetector. Nanotechnology 2018, 29, 095201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wun, J.-M.; Wu, S.-L.; Chao, R.-L.; Huang, J.; Jan, Y.-H.; Chen, H.-S.; Chang, H.-S.; Ni, C.-J.; Chou, E.; et al. Top-illuminated In0.52Al0.48As-based avalanche photodiode with dual charge layers for high-speed and low dark current performances. IEEE J. Sel. Top. Quant. 2017, 24, 3800208. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, C.; Bi, H.; Liu, Y.; Yan, Q. Magnetically separable CuFe2O4/AgBr composite photocatalysts: Preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl. Surf. Sci. 2017, 392, 701–707. [Google Scholar] [CrossRef]
- Park, Y.; Kang, S.H.; Choi, W. Exfoliated and reorganized graphite oxide on titania nanoparticles as an auxiliary co-catalyst for photocatalytic solar conversion. Phys. Chem. Chem. Phys. 2011, 13, 9425–9431. [Google Scholar] [CrossRef] [PubMed]
- Krol, R.V.D.; Liang, Y.; Schoonman, J. Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 2008, 18, 2311–2320. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; Mckone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, A.; Chu, D.; Zhang, C.; Du, Y.; Huang, J.; Yang, P. High Performance of Manganese Porphyrin Sensitized p-Type CuFe2O4 Photocathode for Solar Water Splitting to Produce Hydrogen in a Tandem Photoelectrochemical Cell. Catalysts 2018, 8, 108. https://doi.org/10.3390/catal8030108
Li X, Liu A, Chu D, Zhang C, Du Y, Huang J, Yang P. High Performance of Manganese Porphyrin Sensitized p-Type CuFe2O4 Photocathode for Solar Water Splitting to Produce Hydrogen in a Tandem Photoelectrochemical Cell. Catalysts. 2018; 8(3):108. https://doi.org/10.3390/catal8030108
Chicago/Turabian StyleLi, Xia, Aijuan Liu, Dongmei Chu, Chunyong Zhang, Yukou Du, Jie Huang, and Ping Yang. 2018. "High Performance of Manganese Porphyrin Sensitized p-Type CuFe2O4 Photocathode for Solar Water Splitting to Produce Hydrogen in a Tandem Photoelectrochemical Cell" Catalysts 8, no. 3: 108. https://doi.org/10.3390/catal8030108




