A Comparative Study of Mn/Co Binary Metal Catalysts Supported on Two Commercial Diatomaceous Earths for Oxidation of Benzene
Abstract
:1. Introduction
2. Results and Discussion
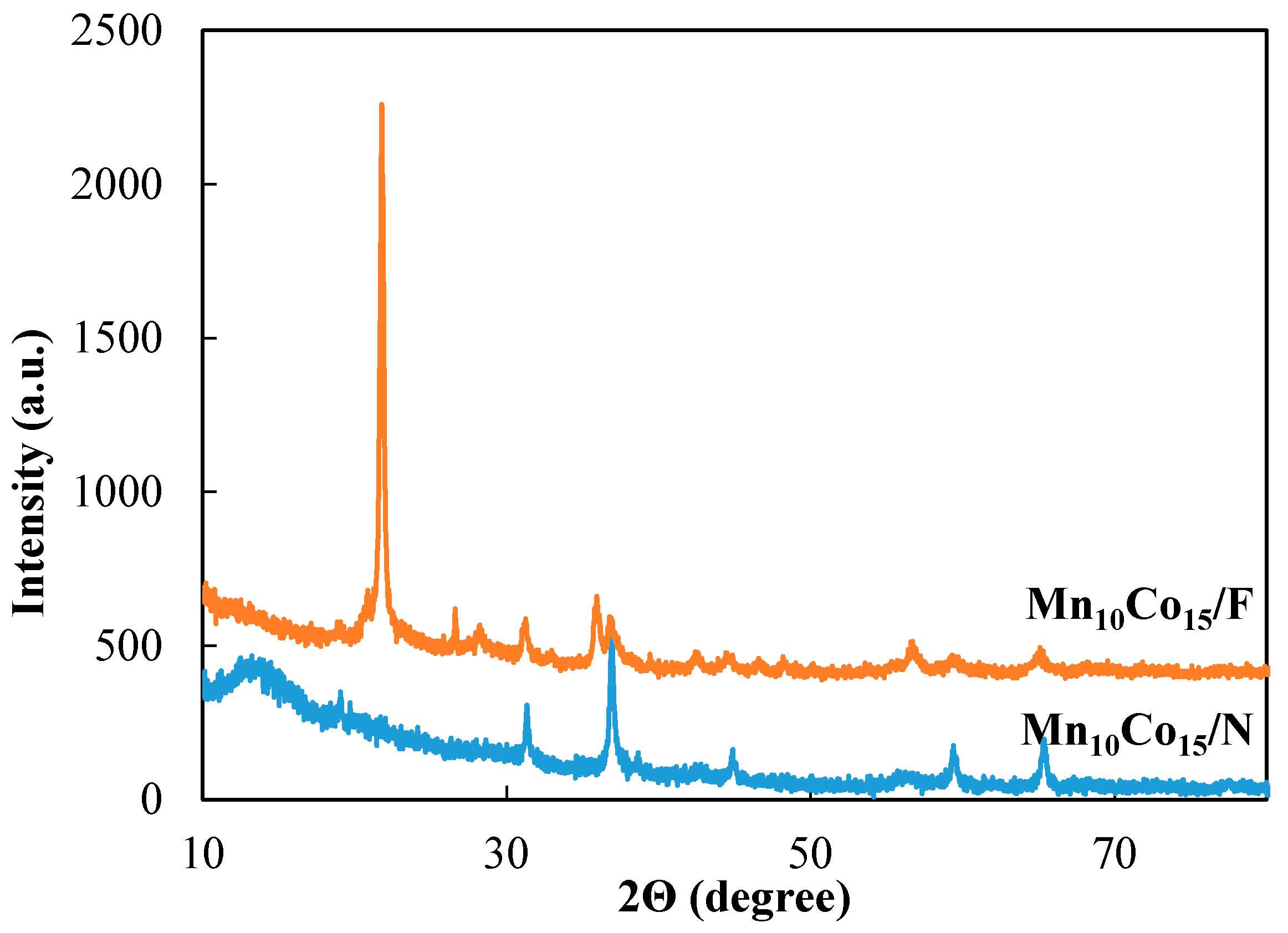
2.1. XRD Characterization
2.2. Morphological and Textural Analysis
2.3. TPR Studies
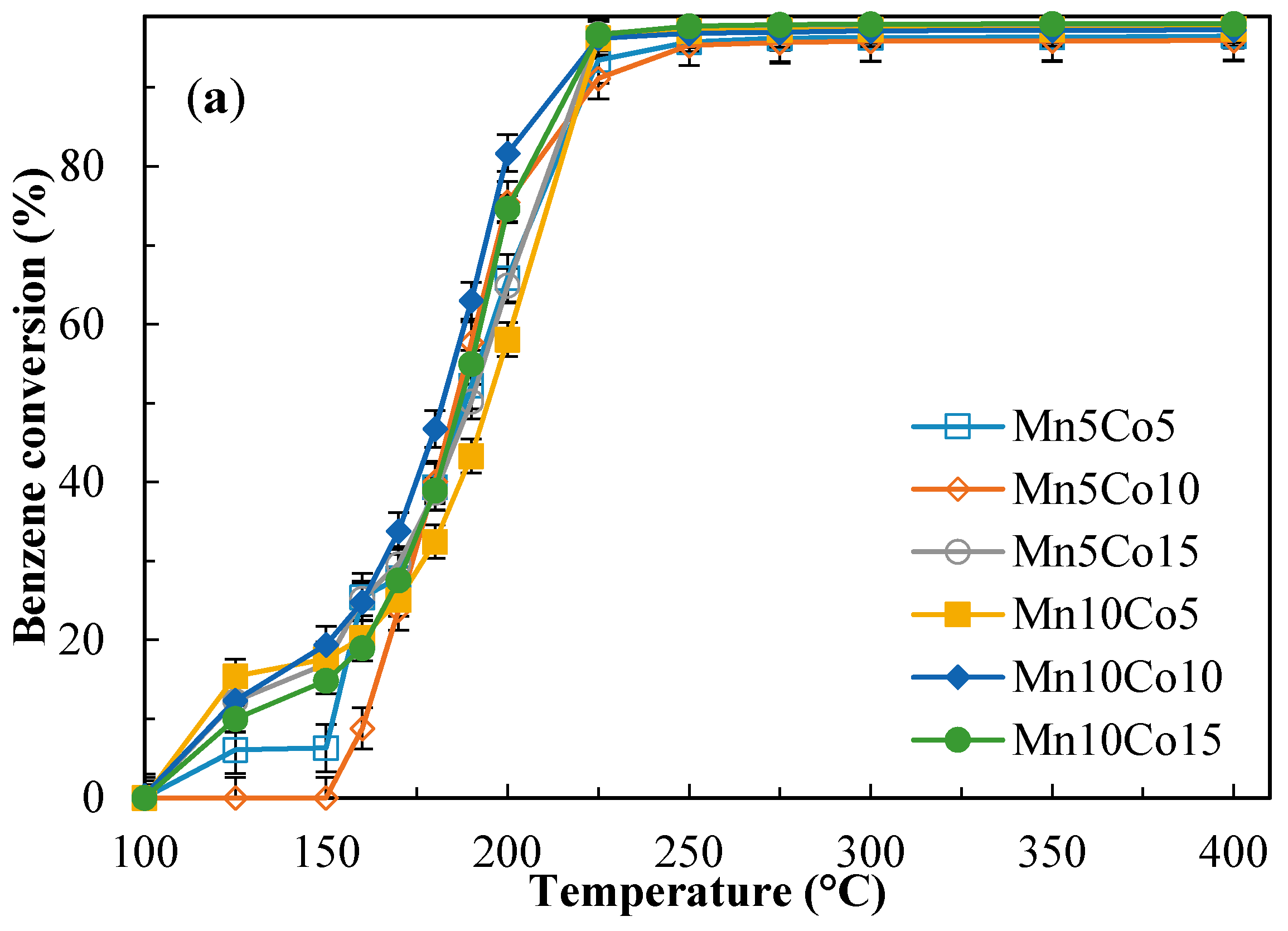
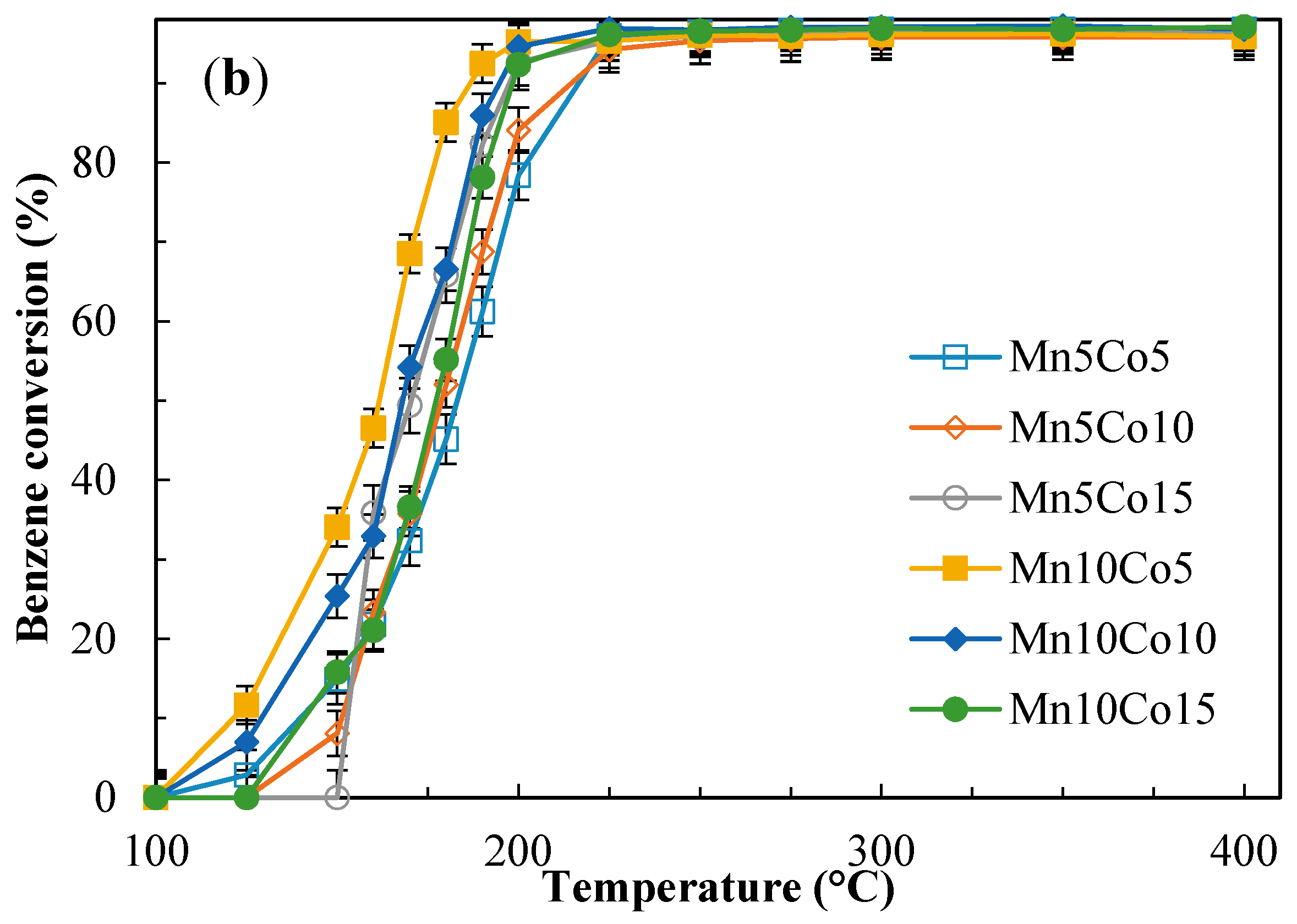
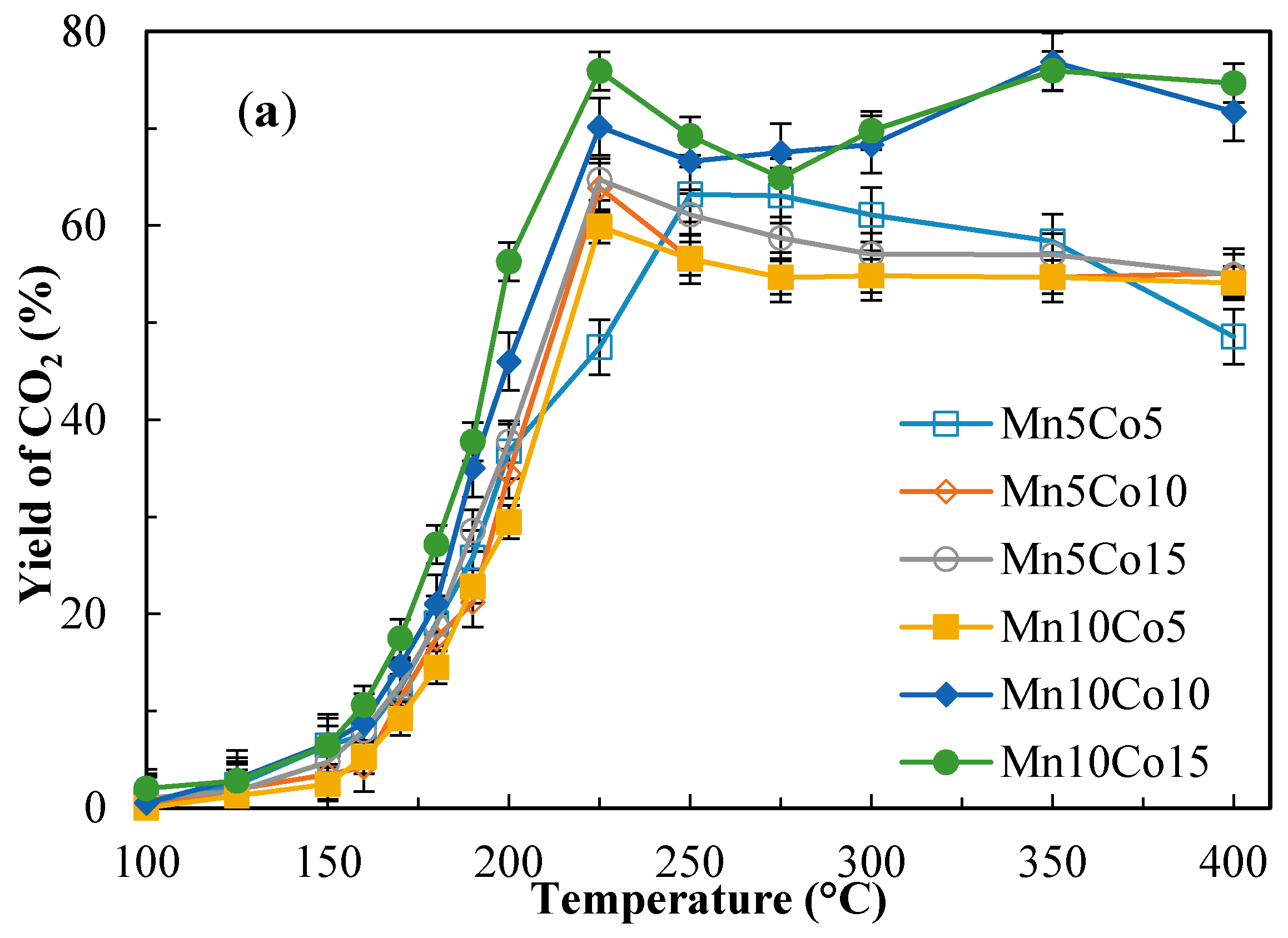
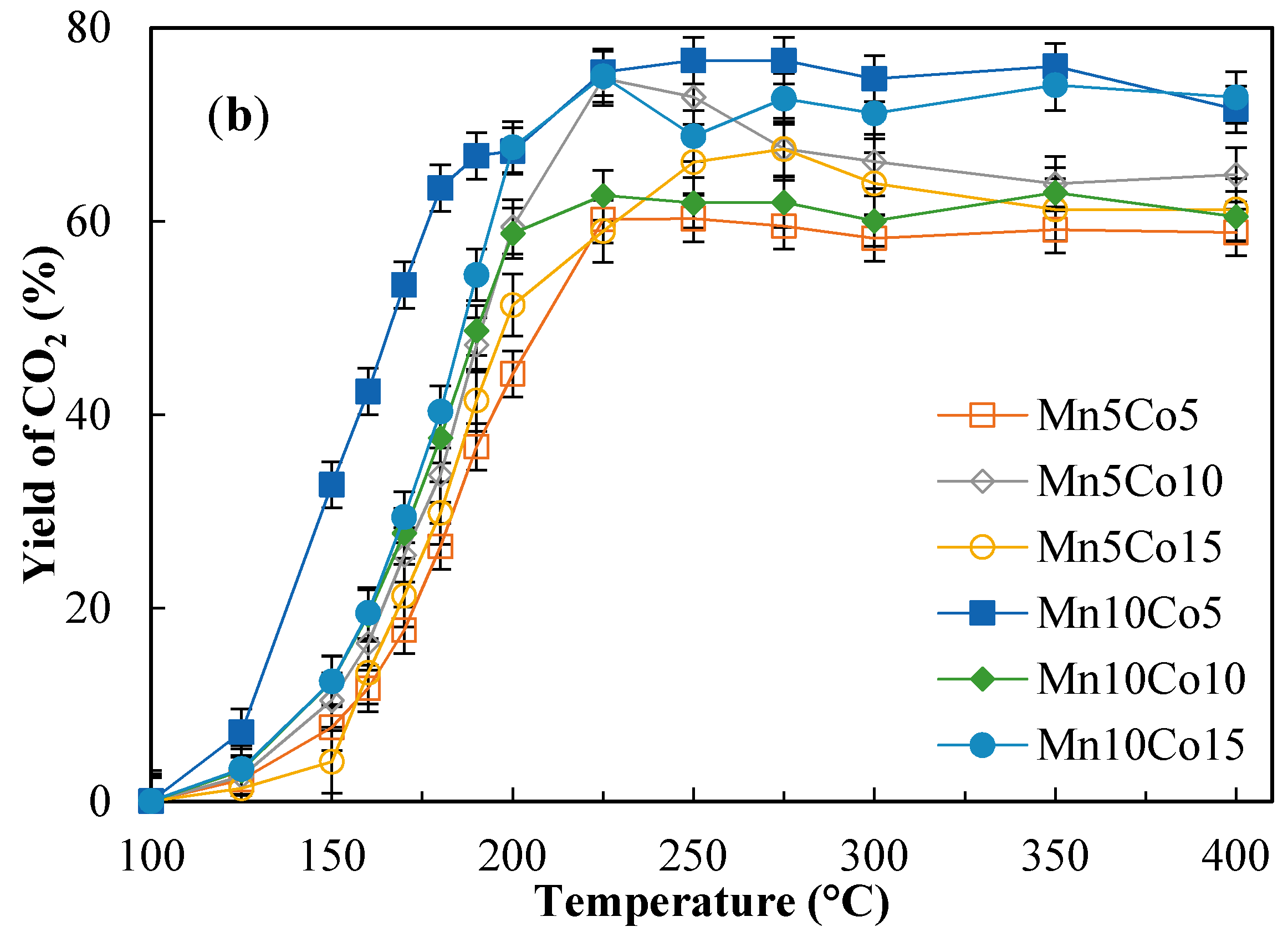
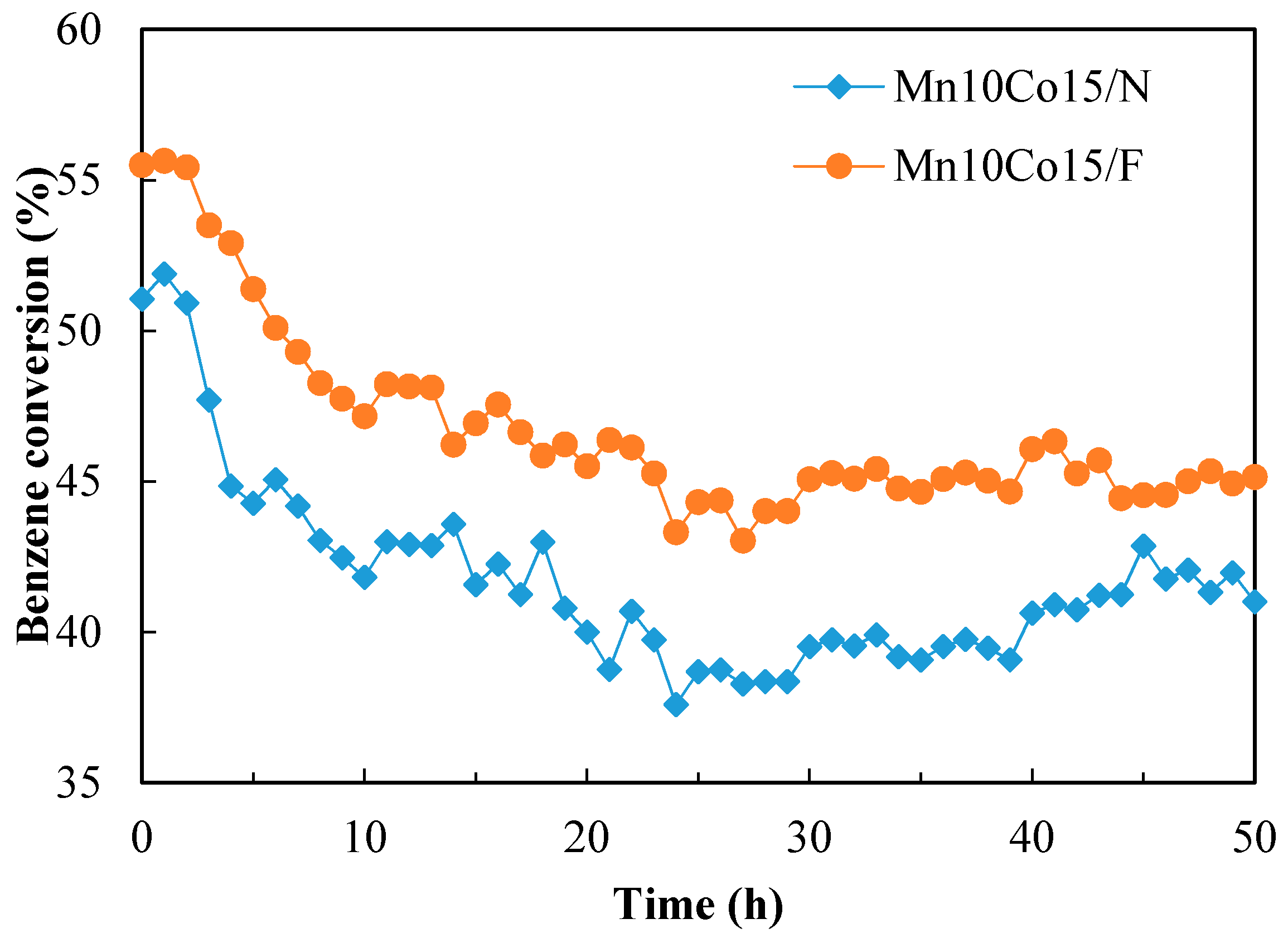
2.4. Catalytic Activity Tests
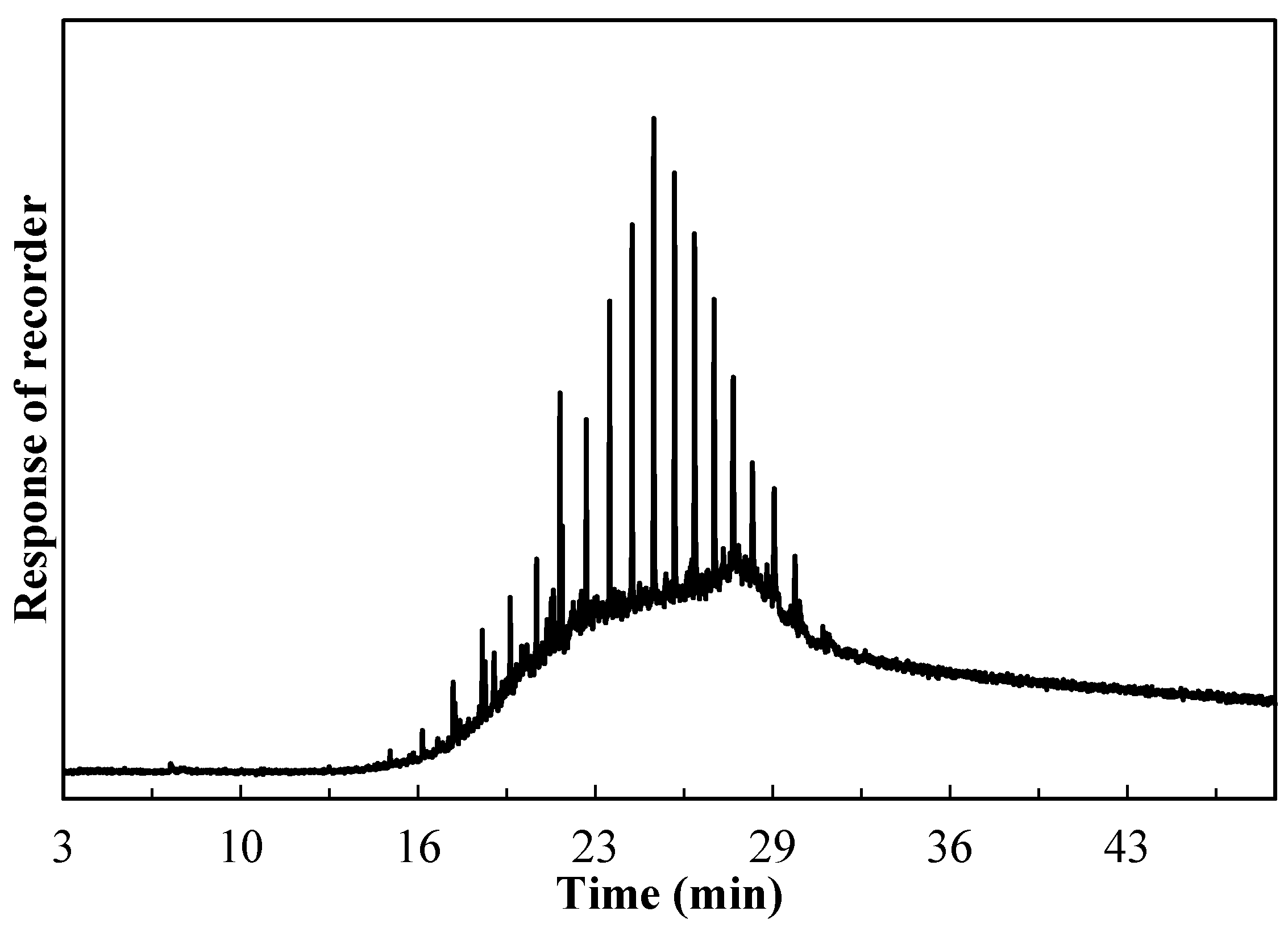
2.5. Characterization of the Used Catalyst
3. Experimental
3.1. Catalyst Preparation
3.2. Characterization of Catalysts
3.3. Determination of the Catalytic Activity
3.4. Coke Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, J.; Liu, X.; Wang, J.; Lv, H.; Zhu, T. Catalytic oxidation of benzene over MnOx/TiO2 catalysts and the mechanism study. J. Mol. Catal. A Chem. 2015, 408, 221–227. [Google Scholar] [CrossRef]
- Yu, W.; Deng, L.; Yuan, P.; Liu, D.; Yuan, W.; Liu, P.; He, H.; Li, Z.; Chen, F. Surface silylation of natural mesoporous/macroporous diatomite for adsorption of benzene. J. Colloid Interface Sci. 2015, 448, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere; Elsevier Inc.: Amsterdam, The Netherlands, 2000; ISBN 978-0-12-257060-5. [Google Scholar]
- Gilbert, M.; Masters, W.P.E. Introduction to Environmental Engineering and Science, 3rd ed.; Prentice-Hall: London, UK, 2008; pp. 327–452. ISBN 0131481932. [Google Scholar]
- United States Environmental Protection Agency. Clean Air Act Text. Available online: https://www.epa.gov/clean-air-act-overview/clean-air-act-text (accessed on 10 March 2018).
- Zhao, X.; Cai, Q.; Ma, C.; Hu, Y.; Luo, K.; Li, W. Economic evaluation of environmental externalities in China’s coal-fired power generation. Energy Policy 2017, 102, 307–317. [Google Scholar] [CrossRef]
- Berenjian, A.; Chan, N.; Malmiri, H.J. Volatile Organic Compounds Removal Methods: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 220–229. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Yao, W.; Wu, Z. One-step synthesis of bimetallic Pt-Pd/MCM-41 mesoporous materials with superior catalytic performance for toluene oxidation. Catal. Commun. 2016, 83, 22–26. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.H.; He, J.; Zhang, X.D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 8324826. [Google Scholar] [CrossRef]
- Ryoo, M.-W.; Chung, S.-G.; Kim, J.-H.; Song, Y.S.; Seo, G. The effect of mass transfer on the catalytic combustion of benzene and methane over palladium catalysts supported on porous materials. Catal. Today 2003, 83, 131–139. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, C.; Chen, B.; Wang, Y.; Shi, C.; Guo, X. Nanoscale HZSM-5 supported PtAg bimetallic catalysts for simultaneous removal of formaldehyde and benzene. Catal. Today 2015, 258, 616–626. [Google Scholar] [CrossRef]
- Azalim, S.; Franco, M.; Brahmi, R.; Giraudon, J.M.; Lamonier, J.F. Removal of oxygenated volatile organic compounds by catalytic oxidation over Zr-Ce-Mn catalysts. J. Hazard. Mater. 2011, 188, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Song, S.Q. Enhancing the performance of Co3O4/CNTs for the catalytic combustion of toluene by tuning the surface structures of CNTs. Appl. Catal. B 2013, 140, 1–8. [Google Scholar] [CrossRef]
- Urbutis, A.; Kitrys, S. Dual function adsorbent-catalyst CuO-CeO2/NaX for temperature swing oxidation of benzene, toluene and xylene. Cent. Eur. J. Chem. 2014, 12, 492–501. [Google Scholar] [CrossRef]
- Zhou, G.L.; Gui, B.G.; Xie, H.M.; Yang, F.; Chen, Y.; Chen, S.M.; Zheng, X.X. Influence of CeO2 morphology on the catalytic oxidation of ethanol in air. J. Ind. Eng. Chem. 2014, 20, 160–165. [Google Scholar] [CrossRef]
- Yang, P.; Shi, Z.; Yang, S.; Zhou, R. High catalytic performances of CeO2–CrOx catalysts for chlorinated VOCs elimination. Chem. Eng. Sci. 2015, 126, 361–369. [Google Scholar] [CrossRef]
- Rotter, H.; Landau, M.V.; Herskowitz, M. Combustion of Chlorinated VOC on Nanostructured Chromia Aerogel as Catalyst and Catalyst Support. Environ. Sci. Technol. 2005, 39, 6845–6850. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, K.; Cherif, L.; Siffert, S.; Tidahy, H.L.; Benaissa, H.; Aboukais, A. The effect of the use of lanthanum-doped mesoporous SBA-15 on the performance of Pt/SBA-15 and Pd/SBA-15 catalysts for total oxidation of toluene. Appl. Catal. A 2008, 351, 82–87. [Google Scholar] [CrossRef]
- Liu, Z.S.; Chen, J.Y.; Peng, Y.H. Activated carbon fibers impregnated with Pd and Pt catalysts for toluene removal. J. Hazard. Mater. 2013, 256–257, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Salameh, S.I.Y.; Khalili, F.I.; Al-Dujaili, A.H. Removal of U(VI) and Th(IV) from aqueous solutions by organically modified diatomaceous earth: Evaluation of equilibrium, kinetic and thermodynamic data. Int. J. Miner. Process. 2017, 168, 9–18. [Google Scholar] [CrossRef]
- Yusan, S.; Bampaiti, A.; Aytas, S.; Erenturk, S.; Aslani, M.A.A. Synthesis and structural properties of ZnO and diatomite-supported ZnO nanostructures. Ceram. Int. 2016, 42, 2158–2163. [Google Scholar] [CrossRef]
- Huang, X.L.; Catignani, G.L.; Swaisgood, H.E. Comparison of the properties of trypsin immobilized on 2 Celite™ derivatives1Paper No. FSR 95-4 of the Journal Series of the Department of Food Science, North Carolina State University, Raleigh, NC 27695-7624. The use of trade names in this publication does not imply endorsement by the North Carolina Agricultural Research Service of the products named, nor criticism of similar ones not mentioned. J. Biotechnol. 1997, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-F.; Chang, S.-W.; Yen, Y.-H.; Shieh, C.-J. Optimum immobilization of Candida rugosa lipase on Celite by RSM. Appl. Clay Sci. 2007, 37, 67–73. [Google Scholar] [CrossRef]
- Giton, F.; Guechot, J.; Fiet, J. New reusable Celite/ethylene glycol cartridges for selective chromatography of steroids before immunoassay. Clin. Biochem. 2009, 42, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, E.R.; Huff, E.M.; Hamilton, D.W.; Jones, J.L. The evaluation of hollow-fiber ultrafiltration and celite concentration of enteroviruses, adenoviruses and bacteriophage from different water matrices. J. Virol. Methods 2016, 228, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; He, X.; Liu, S.; Xie, H.; Fu, M. Phenyl VOCs catalytic combustion on supported CoMn/AC oxide catalyst. J. Ind. Eng. Chem. 2015, 21, 932–941. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, X.; Yang, P.; Qi, C. Preparation and high performance of rare earth modified Pt/MCM-41 for benzene catalytic combustion. Catal. Commun. 2017, 94, 52–55. [Google Scholar] [CrossRef]
- Tamaddon, F.; Nasiri, A.; Farokhi, S. CsF–Celite as an efficient heterogeneous catalyst for sulfonylation and desulfonylation of heteroatoms. Catal. Commun. 2011, 12, 1477–1482. [Google Scholar] [CrossRef]
- Kumar, A.; Kanwar, S.S. Synthesis of ethyl ferulate in organic medium using celite-immobilized lipase. Bioresour. Technol. 2011, 102, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wu, D.Q.; He, H.P.; Lin, Z.Y. The hydroxyl species and acid sites on diatomite surface: a combined IR and Raman study. Appl. Surf. Sci. 2004, 227, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yang, Y.; Qu, X.; Zhang, J.; Chen, Y.; Nie, L. Decolouring mechanism of Zhejiang diatomite. Application to printing and dyeing wastewater. Environ. Chem. Lett. 2005, 3, 33–37. [Google Scholar] [CrossRef]
- Dehestaniathar, S.; Khajelakzay, M.; Ramezani-Farani, M.; Ijadpanah-Saravi, H. Modified diatomite-supported CuO-TiO2 composite: Preparation, characterization and catalytic CO oxidation. J. Taiwan Inst. Chem. Eng. 2016, 58, 252–258. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Zheng, S.; Sun, Z. Characterizations of nano-TiO2/diatomite composites and their photocatalytic reduction of aqueous Cr(VI). Appl. Surf. Sci. 2014, 311, 369–376. [Google Scholar] [CrossRef]
- Beauchet, R.; Magnoux, P.; Mijoin, J. Catalytic oxidation of volatile organic compounds (VOCs) mixture (isopropanol/o-xylene) on zeolite catalysts. Catal. Today 2007, 124, 118–123. [Google Scholar] [CrossRef]
- Pinard, L.; Tayeb, K.B.; Hamieh, S.; Vezin, H.; Canaff, C.; Maury, S.; Delpoux, O.; Pouilloux, Y. On the involvement of radical “coke” in ethanol conversion to hydrocarbons over HZSM-5 zeolite. Catal. Today 2013, 218–219, 57–64. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Coking and deactivation of zeolites. Appl. Catal. 1989, 54, 1–27. [Google Scholar] [CrossRef]
- Wyrwalski, F.; Lamonier, J.F.; Siffert, S.; Aboukaïs, A. Additional effects of cobalt precursor and zirconia support modifications for the design of efficient VOC oxidation catalysts. Appl. Catal. B 2007, 70, 393–399. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Integrated Risk Information System (IRIS) on Benzene. In Office of Research and Development; National Center for Environmental, Ed.; United States Environmental Protection Agency: Washington, DC, USA, 2009. [Google Scholar]
- Zhang, Z.; Shi, D. Purifying Process of Diatomaceous Earth through Flocculation and Magneto-Seperation by Specific Gravity. Google Patent CN 1,045,252 A, 12 September 1990. [Google Scholar]
- Wang, B. Diatomaceous Earth Products, Processes for Preparing them, and Methods of Their Use. Google Patent WO 2013096578 A1, 27 June 2013. [Google Scholar]
- Sarangi, M.; Nayak, P.; Tiwari, T.N. Effect of temperature on nano-crystalline silica and carbon composites obtained from rice-husk ash. Compos. Part B 2011, 42, 1994–1998. [Google Scholar] [CrossRef]
- Li, W.B.; Zhuang, M.; Wang, J.X. Catalytic combustion of toluene on Cu-Mn/MCM-41 catalysts: Influence of calcination temperature and operating conditions on the catalytic activity. Catal. Today 2008, 137, 340–344. [Google Scholar] [CrossRef]
- Anderson, J.R. Particle-Size Effects in Metal-Catalysts. Sci. Prog. 1985, 69, 461–484. [Google Scholar]
- Isaifan, R.J.; Ntais, S.; Baranova, E.A. Particle size effect on catalytic activity of carbon-supported Pt nanoparticles for complete ethylene oxidation. Appl. Catal. A 2013, 464–465, 87–94. [Google Scholar] [CrossRef]
- Ustinov, E.A. Nitrogen adsorption on silica surfaces of nonporous and mesoporous materials. Langmuir 2008, 24, 6668–6675. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, L.; Dai, R.; Lu, M.; Sun, T.; Liu, Q.; Huang, X.; Huang, Z. Mesoporous Mn promoted Co3O4 oxides as an efficient and stable catalyst for low temperature oxidation of CO. Solid State Sci. 2017, 71, 69–74. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hou, F.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.; Cui, L. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl. Surf. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic Activity of Supported Platinum and Metal Oxide Catalysts for Toluene Oxidation. Top. Catal. 2009, 52, 517–527. [Google Scholar] [CrossRef]
- Basińska, A.; Jóźwiak, W.K.; Góralski, J.; Domka, F. The behaviour of Ru/Fe2O3 catalysts and Fe2O3 supports in the TPR and TPO conditions. Appl. Catal. A 2000, 190, 107–115. [Google Scholar] [CrossRef]
- Nogueira, F.G.E.; Lopes, J.H.; Silva, A.C.; Lago, R.M.; Fabris, J.D.; Oliveira, L.C.A. Catalysts based on clay and iron oxide for oxidation of toluene. Appl. Clay Sci. 2011, 51, 385–389. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Suzuki, K. Catalytic oxidation of VOCs over Al- and Fe-pillared montmorillonite. Appl. Clay Sci. 2013, 77–78, 56–60. [Google Scholar] [CrossRef]
- Liang, X.; Qi, F.; Liu, P.; Wei, G.; Su, X.; Ma, L.; He, H.; Lin, X.; Xi, Y.; Zhu, J.; et al. Performance of Ti-pillared montmorillonite supported Fe catalysts for toluene oxidation: The effect of Fe on catalytic activity. Appl. Clay Sci. 2016, 132–133, 96–104. [Google Scholar] [CrossRef]
- Sexton, B.A.; Hughes, A.E.; Turney, T.W. An Xps and Tpr Study of the Reduction of Promoted Cobalt Kieselguhr Fischer-Tropsch Catalysts. J. Catal. 1986, 97, 390–406. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Stobbe, E.R.; de Boer, B.A.; Geus, J.W. The reduction and oxidation behaviour of manganese oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Brummer, V.; David, J.; Martinec, J.; Lestinsky, P.; Skryja, P.; Stehlík, P. Impact of catalytic oxidation operating conditions on VOC and Co conversions on the Pt-Pd/Al2O3 catalyst. Chem. Eng. Trans. 2015, 45, 1009–1014. [Google Scholar] [CrossRef]
- Pinard, L.; Hamieh, S.; Canaff, C.; Ferreira Madeira, F.; Batonneau-Gener, I.; Maury, S.; Delpoux, O.; Ben Tayeb, K.; Pouilloux, Y.; Vezin, H. Growth mechanism of coke on HBEA zeolite during ethanol transformation. J. Catal. 2013, 299, 284–297. [Google Scholar] [CrossRef]
- Rojo-Gama, D.; Signorile, M.; Bonino, F.; Bordiga, S.; Olsbye, U.; Lillerud, K.P.; Beato, P.; Svelle, S. Structure–deactivation relationships in zeolites during the methanol–to-hydrocarbons reaction: Complementary assessments of the coke content. J. Catal. 2017, 351, 33–48. [Google Scholar] [CrossRef]
- Schulz, H. “Coking” of zeolites during methanol conversion: Basic reactions of the MTO-, MTP- and MTG processes. Catal. Today 2010, 154, 183–194. [Google Scholar] [CrossRef]
- Li, W.B.; Zhuang, M.; Xiao, T.C.; Green, M.L. MCM-41 supported Cu-Mn catalysts for catalytic oxidation of toluene at low temperatures. J. Phys. Chem. B 2006, 110, 21568–21571. [Google Scholar] [CrossRef] [PubMed]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; He, J.; Peng, C.; Wu, T. Recovery of elemental sulphur via selective catalytic reduction of SO2 over sulphided CoMo/γ-Al2O3 catalysts. Fuel 2015, 147, 67–75. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.Q.; Tian, S.; Zhao, E.H.; Zhang, J.C.; Liu, C.G. Preparation of bifunctional NiPb/ZnO-diatomite-ZSM-5 catalyst and its reactive adsorption desulfurization coupling aromatization performance in FCC gasoline upgrading process. Fuel 2013, 104, 201–207. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on γ-Al2O3. Appl. Catal. B 2011, 103, 275–286. [Google Scholar] [CrossRef]
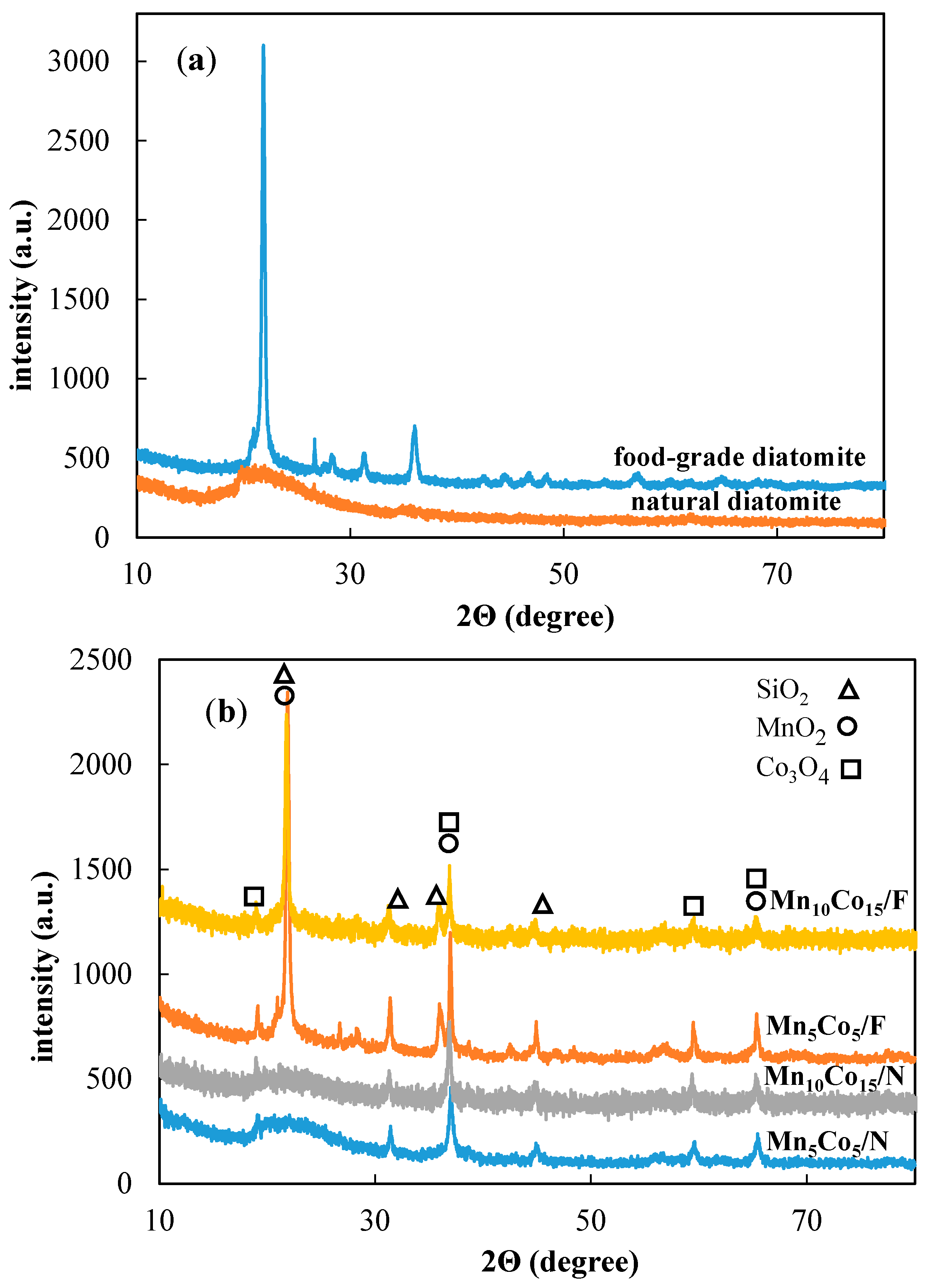
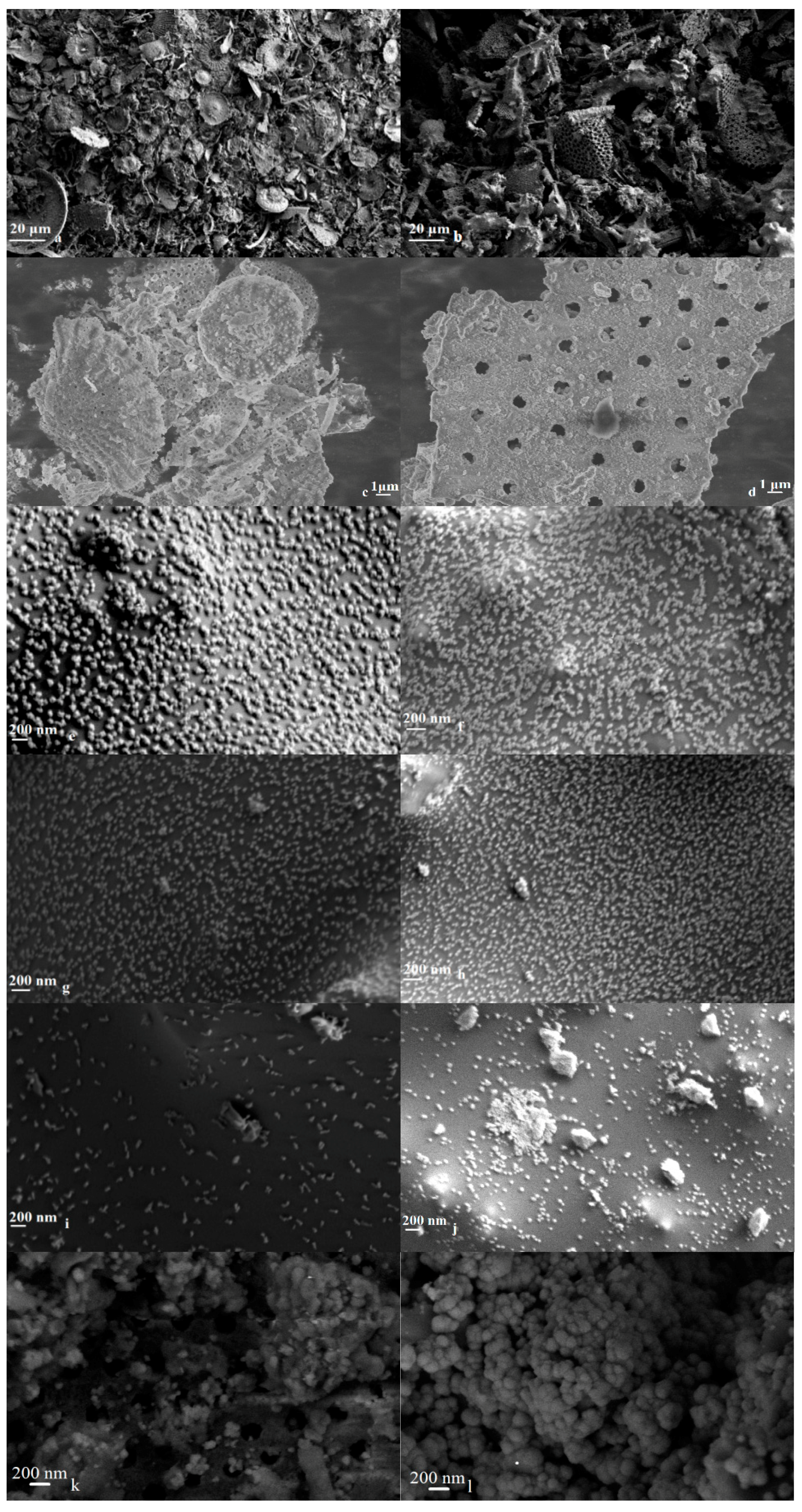

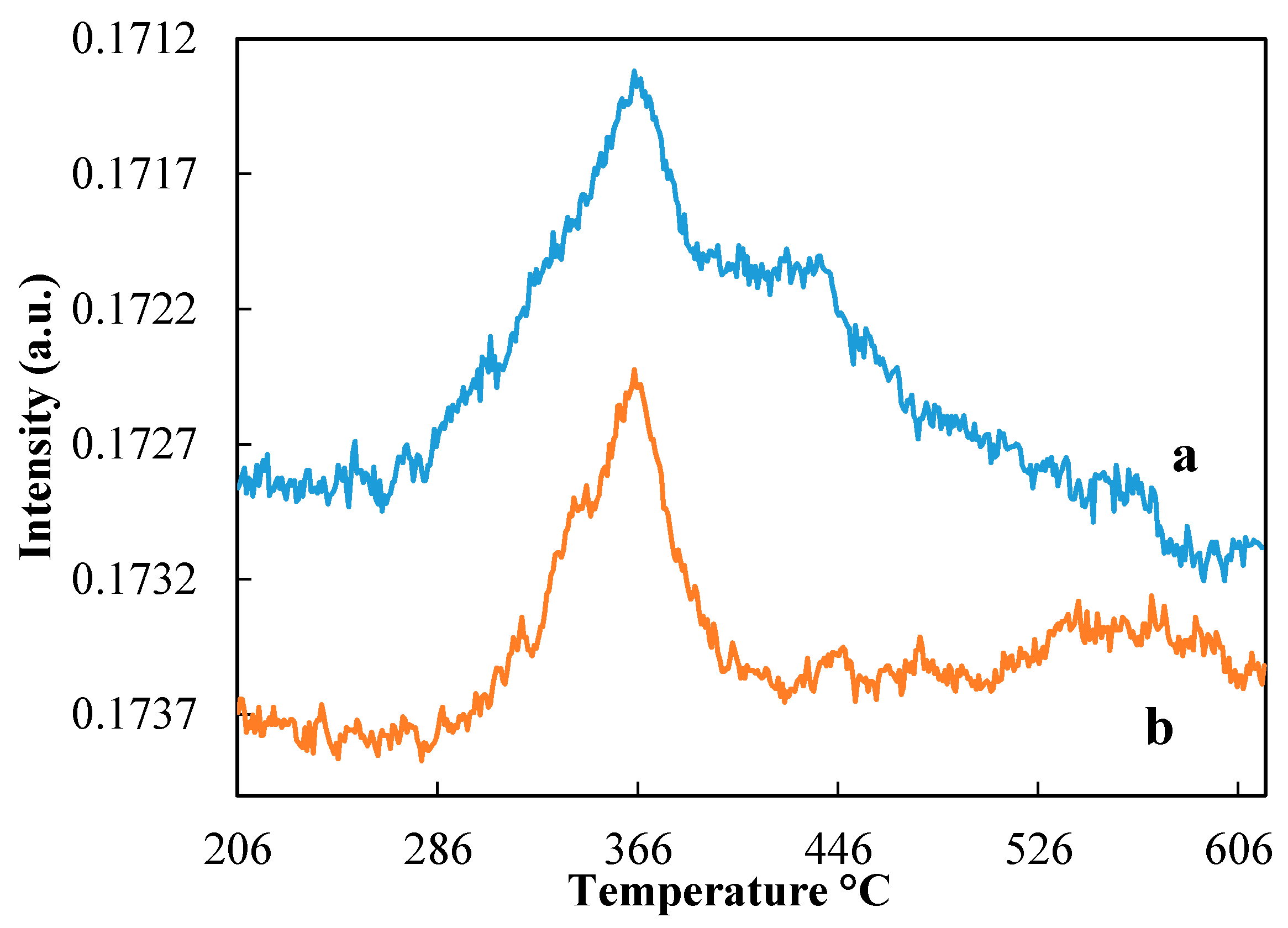
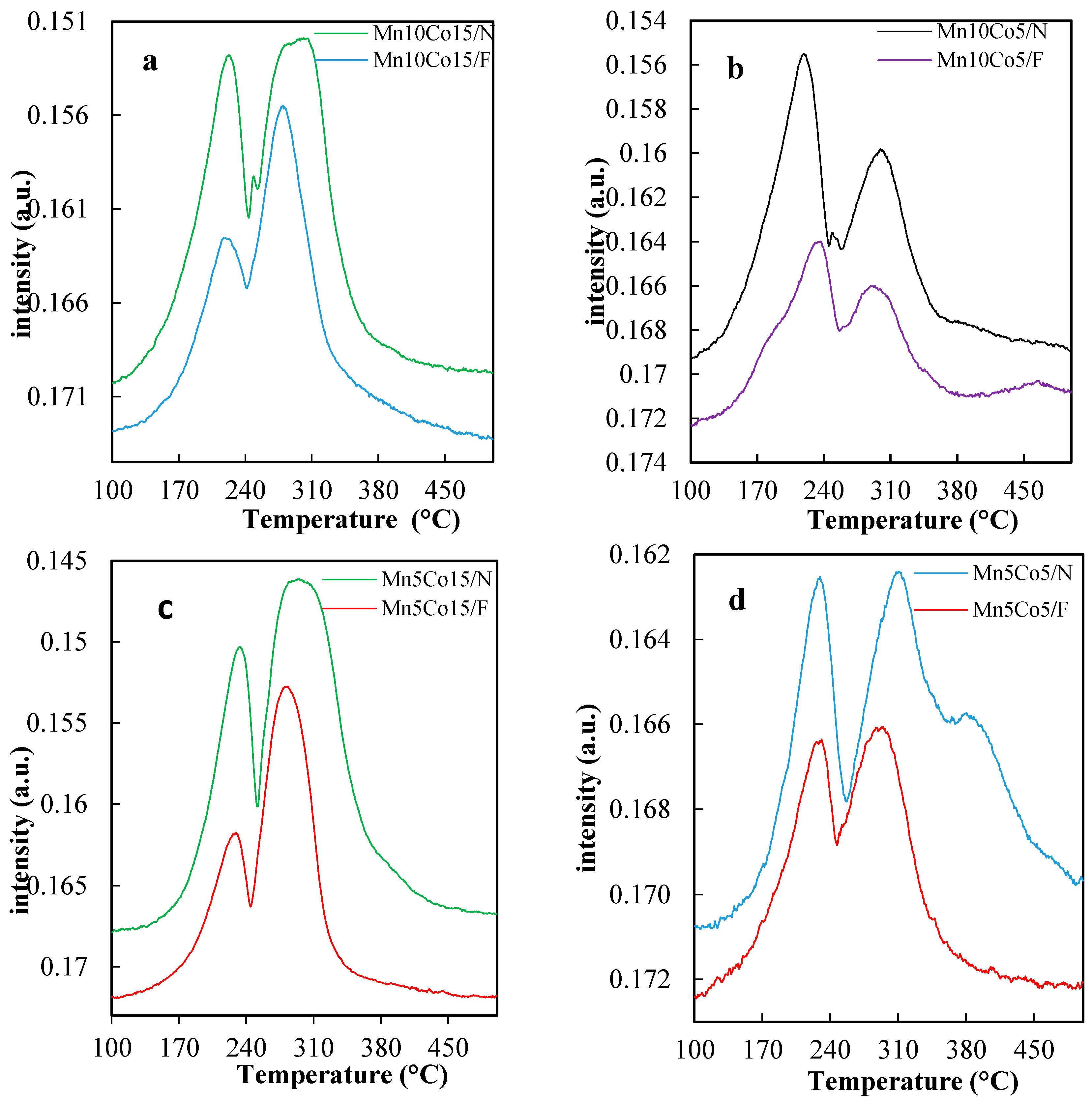
| Sample | Mn wt % | Co wt % | BET SSA (m2/g) a | Total Pore Volume (cm3/g) b |
|---|---|---|---|---|
| Natural diatomite | n.d. | n.d. | 51.3 | 1.73 × 10−1 |
| Food-grade diatomite | n.d. | n.d. | 1.35 | 6.84 × 10−4 |
| Mn10Co15/N | 9.22 | 13.5 | 38.0 | 6.14 × 10−2 |
| Mn10Co15/F | 8.98 | 13.8 | 14.1 | 2.09 × 10−2 |
| Mn10Co5/N | 9.13 | 4.42 | 46.2 | 1.09 × 10−1 |
| Mn10Co5/F | 9.18 | 4.74 | 10.8 | 1.99 × 10−2 |
| Mn5Co15/N | 4.02 | 14.0 | 46.0 | 1.17 × 10−1 |
| Mn5Co15/F | 4.15 | 13.9 | 8.84 | 1.44 × 10−2 |
| Mn5Co5/N | 4.11 | 4.52 | 47.5 | 1.18 × 10−1 |
| Mn5Co5/F | 3.82 | 4.26 | 5.45 | 7.70 × 10−3 |
| Sample | Natural Diatomite Total H2 Consumption (mmol/g) | Food-Grade Diatomite Total H2 Consumption (mmol/g) |
|---|---|---|
| Mn10Co15 | 5.56 | 3.85 |
| Mn10Co5 | 3.29 | 2.71 |
| Mn5Co15 | 5.00 | 3.20 |
| Mn5Co5 | 2.73 | 1.40 |
| Sample | Benzene Conversion (%) at 190 °C | Benzene Conversion (%) at 225 °C | Yield to CO2 (%) at 190 °C | Yield to CO2 (%) at 225 °C | ||||
|---|---|---|---|---|---|---|---|---|
| N a | F b | N a | F b | N a | F b | N a | F b | |
| Mn10Co15 | 55.0 | 78.1 | 96.7 | 96.1 | 37.8 | 54.5 | 75.9 | 74.9 |
| Mn10Co10 | 63.0 | 85.9 | 96.3 | 96.7 | 35.0 | 48.7 | 70.1 | 62.7 |
| Mn10Co5 | 43.3 | 92.5 | 96.3 | 95.3 | 22.8 | 66.8 | 60.0 | 75.4 |
| Mn5Co15 | 50.2 | 82.2 | 96.8 | 95.3 | 28.6 | 41.5 | 64.8 | 59.0 |
| Mn5Co10 | 57.7 | 68.8 | 91.1 | 94.2 | 21.1 | 47.2 | 63.9 | 74.7 |
| Mn5Co5 | 52.2 | 61.2 | 93.5 | 95.0 | 25.8 | 36.7 | 47.5 | 60.2 |
| Sample | Reaction Rate (mol/s) | Moles of Available Active Phase (mol) × 10−5 | TOF (s−1) × 10−5 a | Total Moles of Active Phase (mol) × 10−4 | TOF (s−1) × 10−5 b |
|---|---|---|---|---|---|
| Mn10Co15/N | 1.70 × 10−8 | 17.2 | 9.90 | 11.9 | 1.43 |
| Mn5Co10/N | 5.12 × 10−8 | 19.6 | 26.2 | 6.77 | 7.56 |
| Mn5Co5/N | 6.87 × 10−10 | 11.6 | 0.592 | 4.55 | 0.151 |
| Mn10Co15/F | 4.03 × 10−8 | 2.40 | 168 | 11.9 | 3.38 |
| Mn5Co10/F | 3.91 × 10−8 | 1.85 | 210 | 6.94 | 5.63 |
| Mn5Co5/F | 0.999 × 10−8 | 2.63 | 38.0 | 4.25 | 2.35 |
| Sample | Coke Content (wt %) Natural Diatomite | Coke Content (wt %) Food-Grade Diatomite |
|---|---|---|
| Mn10Co15 (50 h) | 8.96 | 1.45 |
| Mn10Co15 | 8.25 | 1.18 |
| Mn10Co10 | 7.21 | 1.26 |
| Mn10Co5 | 10.2 | 1.07 |
| Mn5Co15 | 8.22 | 1.14 |
| Mn5Co10 | 8.26 | 1.18 |
| Mn5Co5 | 7.40 | 1.22 |
| Family | Formula | m/z Range |
|---|---|---|
| Alkylbenzene | CnH2n-6 (n > 6) | 120–238 |
| Alkylnaphthalene | CnH2n-12 (n > 10) | 194–220 |
| Alkylphenanthrene | CnH2n-18 (n > 14) | 206–250 |
| Alkylanthracene | CnH2n-18 (n > 14) | 206–250 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomatis, M.; Xu, H.; Wei, C.; Bishop, M.T.; He, J.; Wang, C.; Zhao, M.; Xiao, H.; Yu, H.; Behera, S.N.; et al. A Comparative Study of Mn/Co Binary Metal Catalysts Supported on Two Commercial Diatomaceous Earths for Oxidation of Benzene. Catalysts 2018, 8, 111. https://doi.org/10.3390/catal8030111
Tomatis M, Xu H, Wei C, Bishop MT, He J, Wang C, Zhao M, Xiao H, Yu H, Behera SN, et al. A Comparative Study of Mn/Co Binary Metal Catalysts Supported on Two Commercial Diatomaceous Earths for Oxidation of Benzene. Catalysts. 2018; 8(3):111. https://doi.org/10.3390/catal8030111
Chicago/Turabian StyleTomatis, Marco, Honghui Xu, Chaohui Wei, Matthew Thomas Bishop, Jun He, Chengjun Wang, Ming Zhao, Hang Xiao, Huan Yu, Sailesh N. Behera, and et al. 2018. "A Comparative Study of Mn/Co Binary Metal Catalysts Supported on Two Commercial Diatomaceous Earths for Oxidation of Benzene" Catalysts 8, no. 3: 111. https://doi.org/10.3390/catal8030111






