Fabrication of a Z-Scheme g-C3N4/Fe-TiO2 Photocatalytic Composite with Enhanced Photocatalytic Activity under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
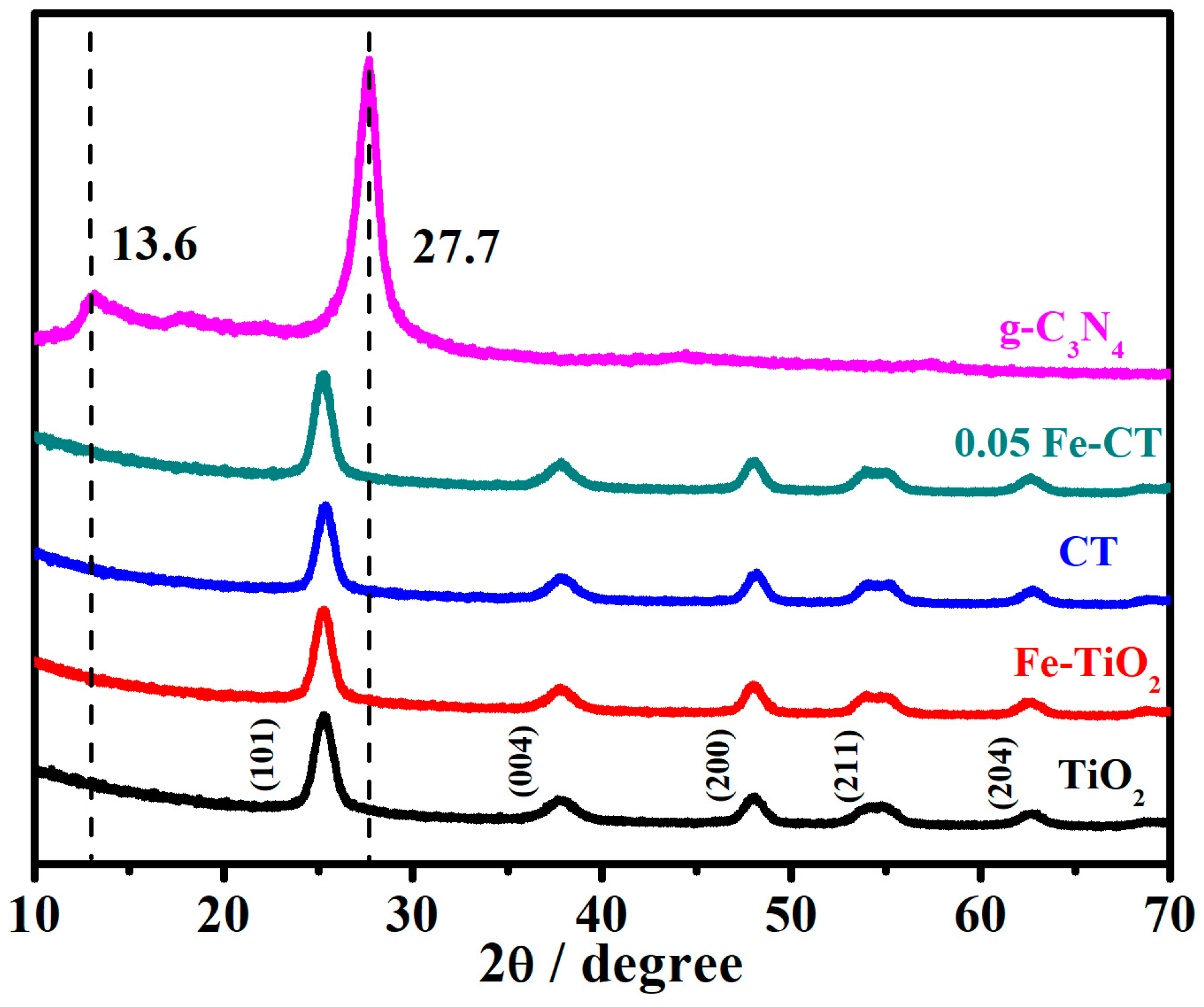
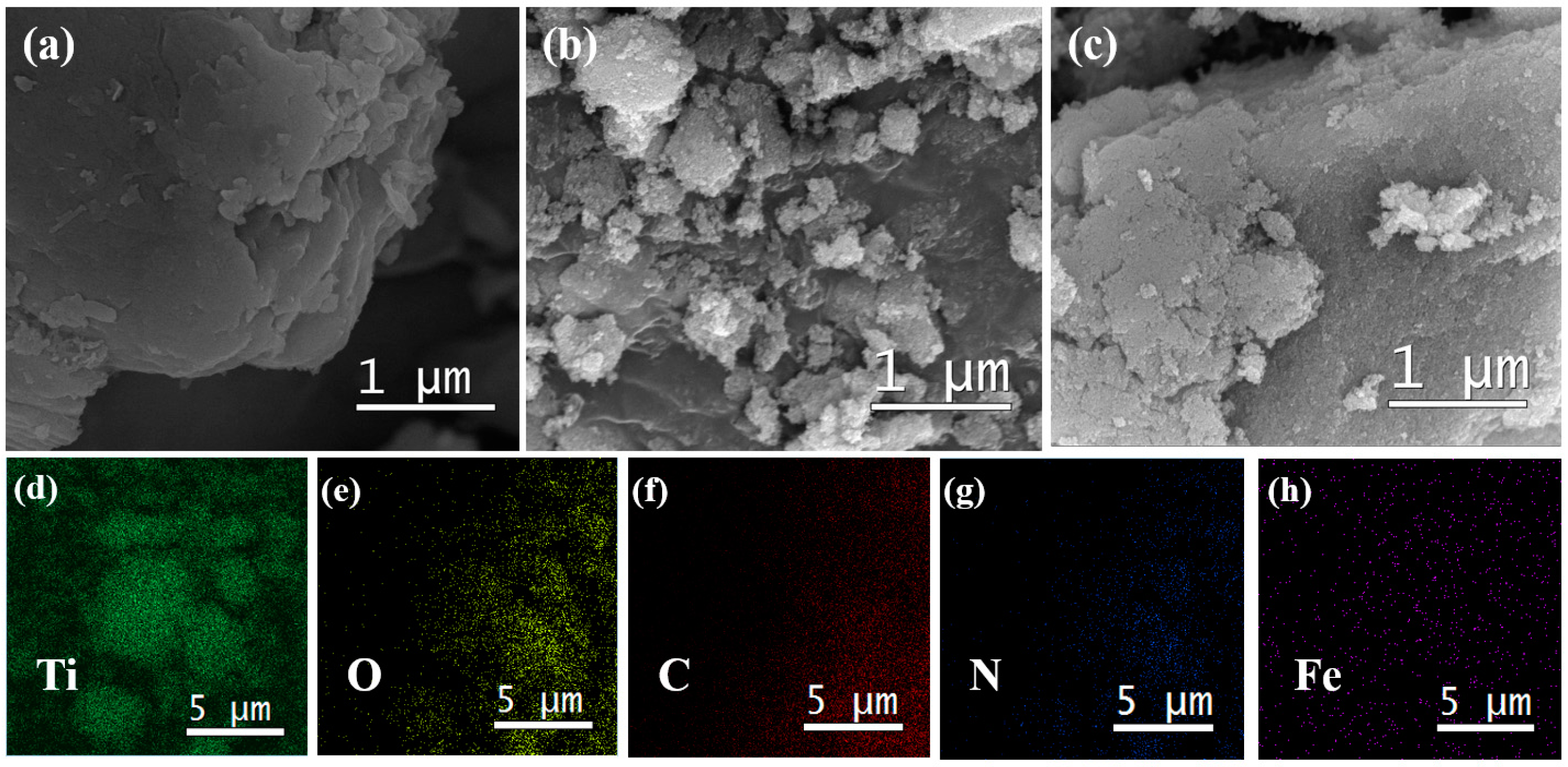
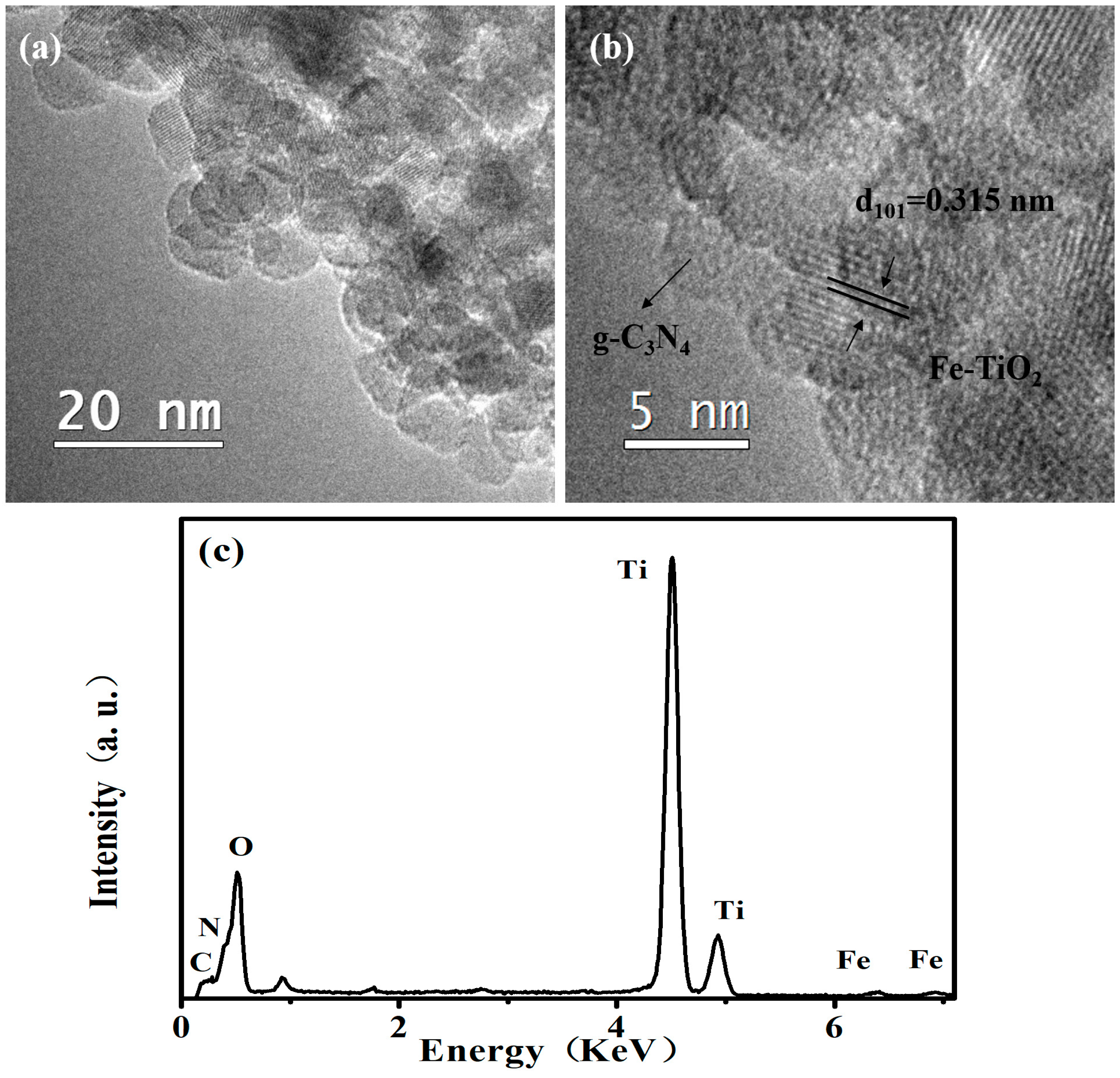
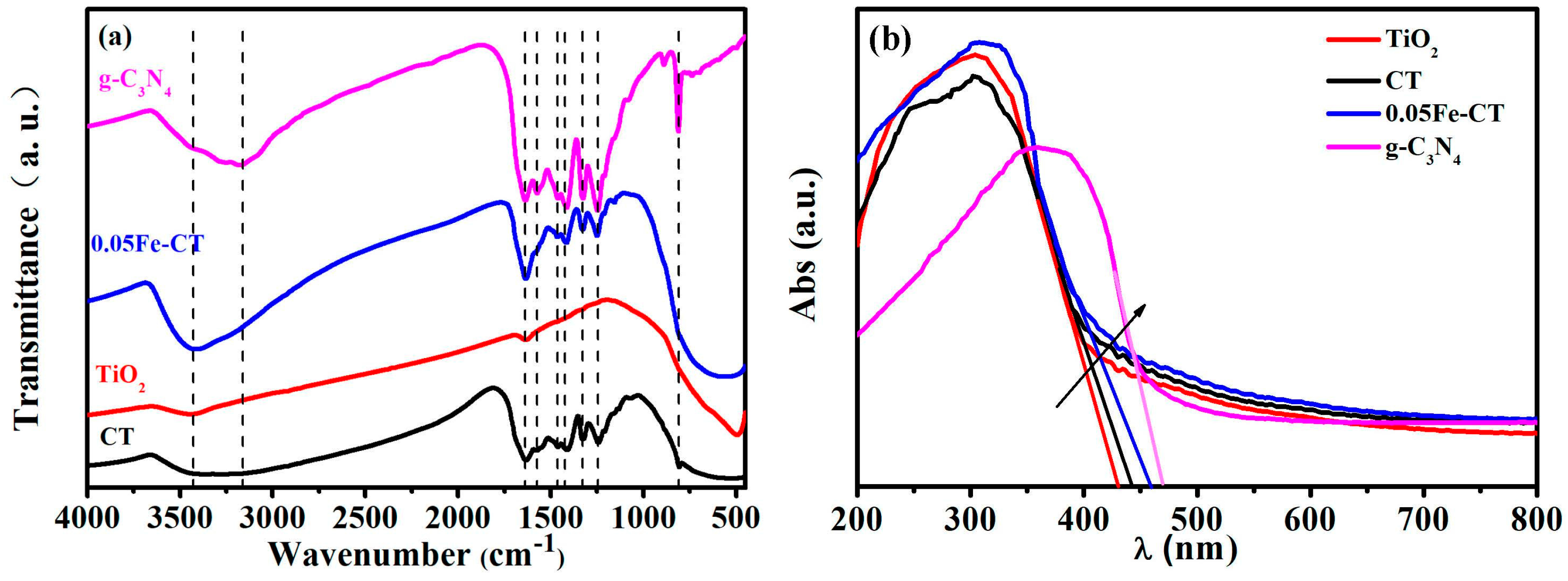
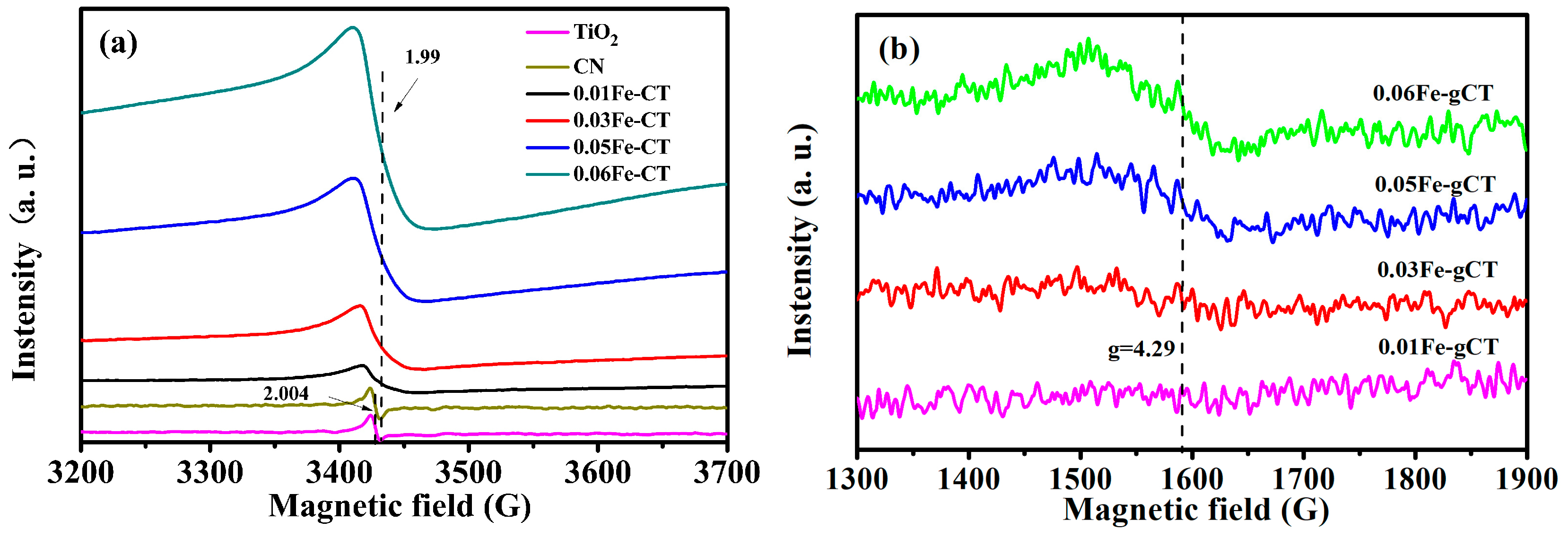
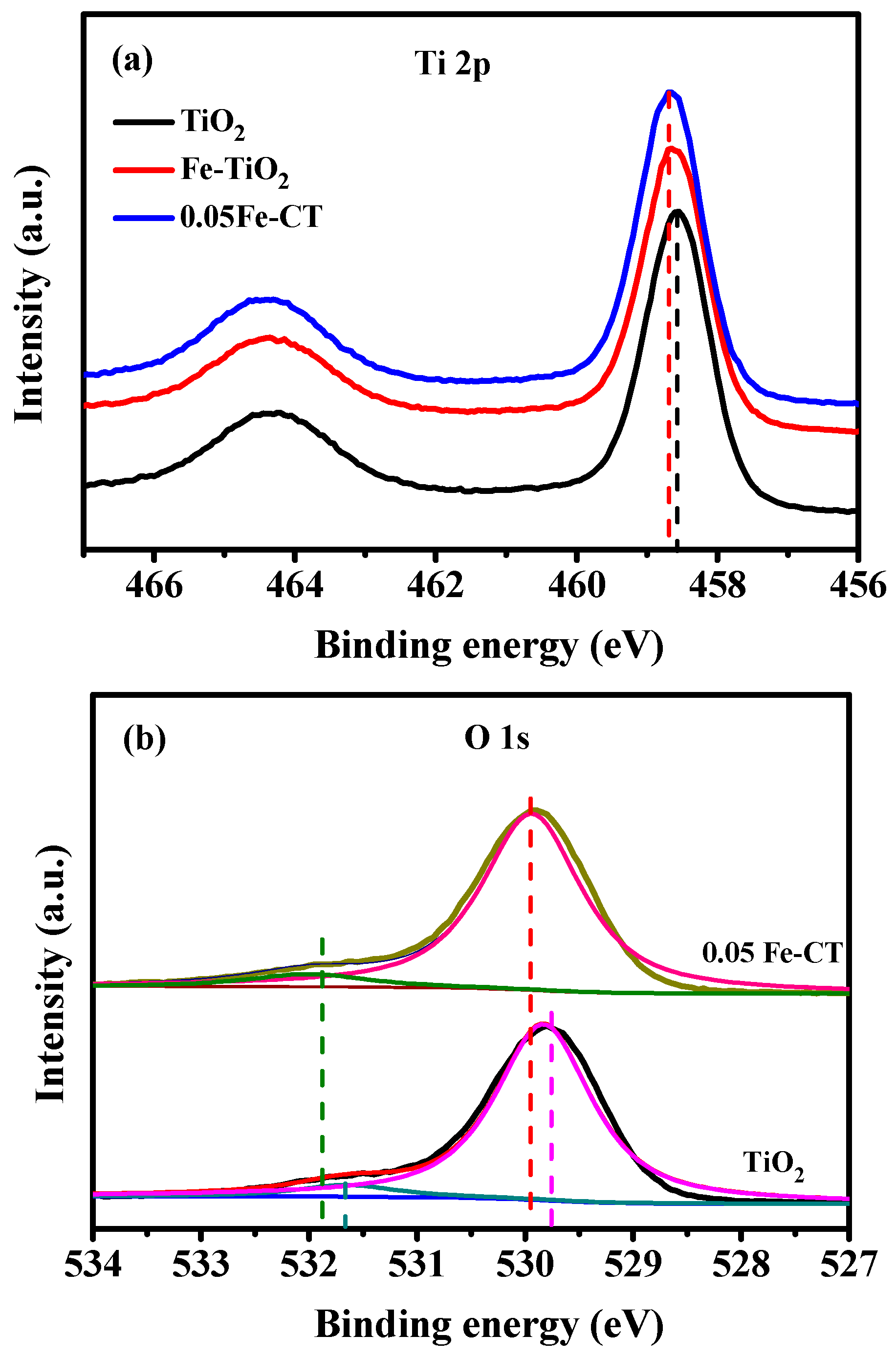
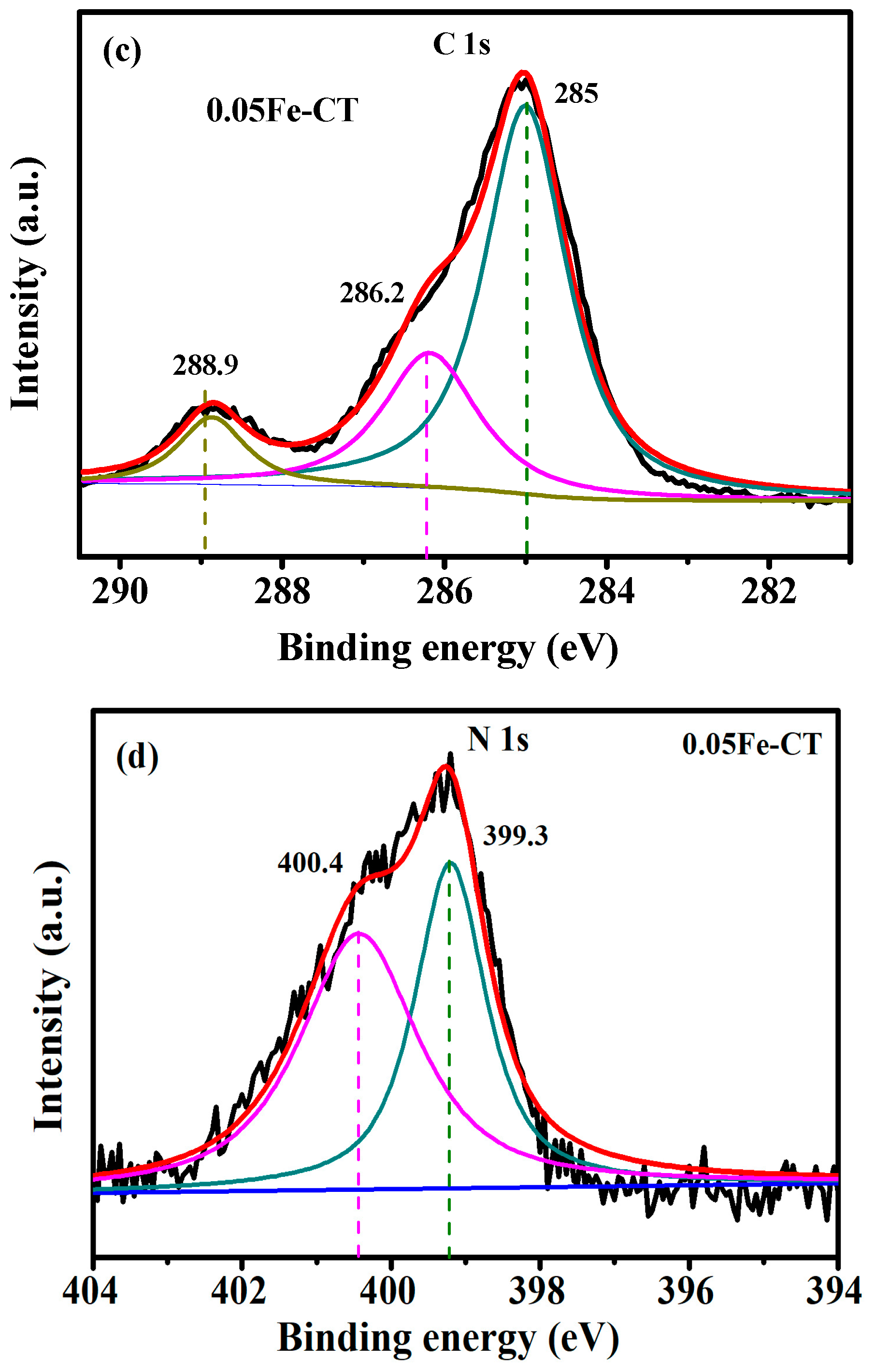
2.1. Phase Structures and Morphology
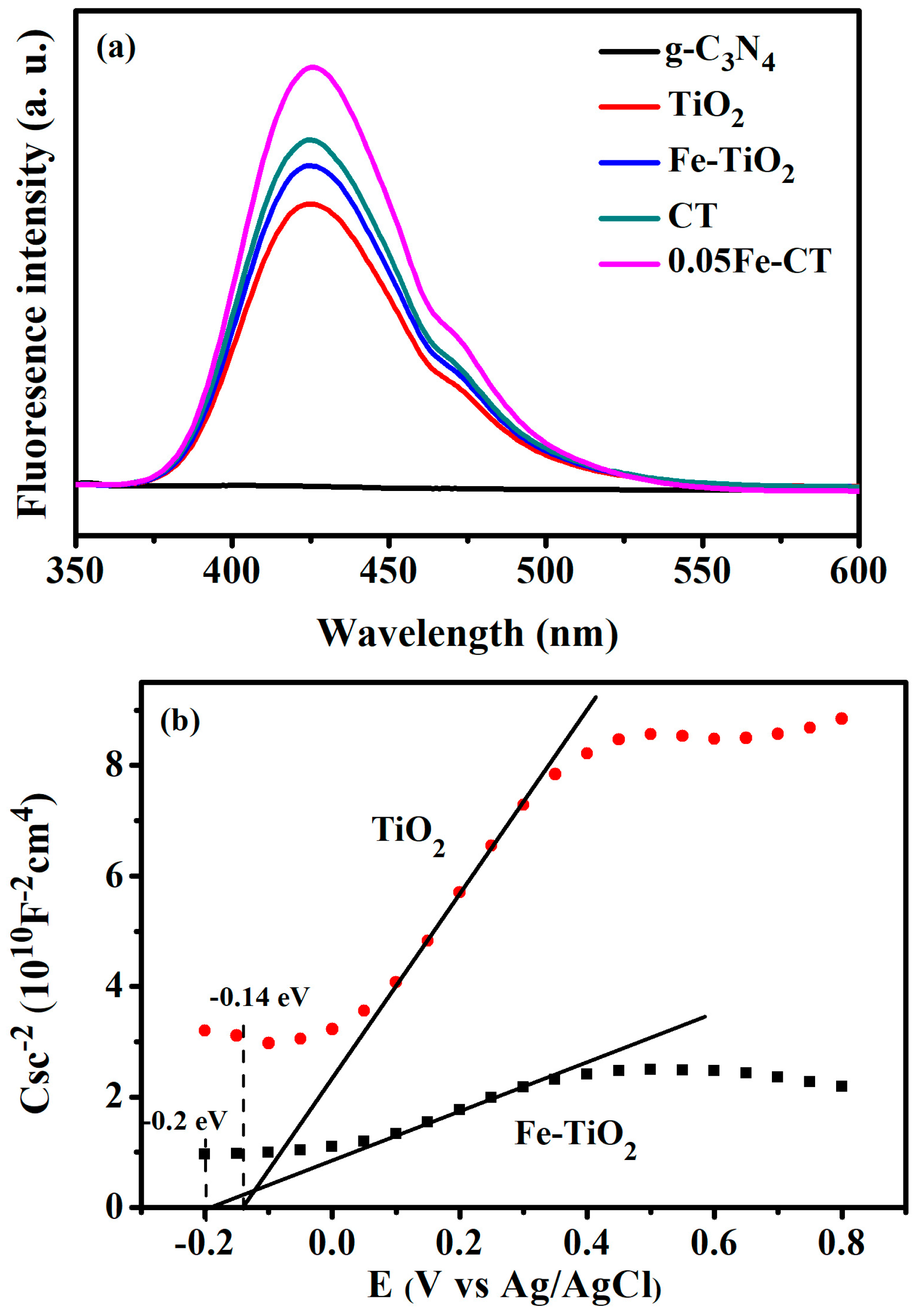
2.2. Photocatalytic Activity Test
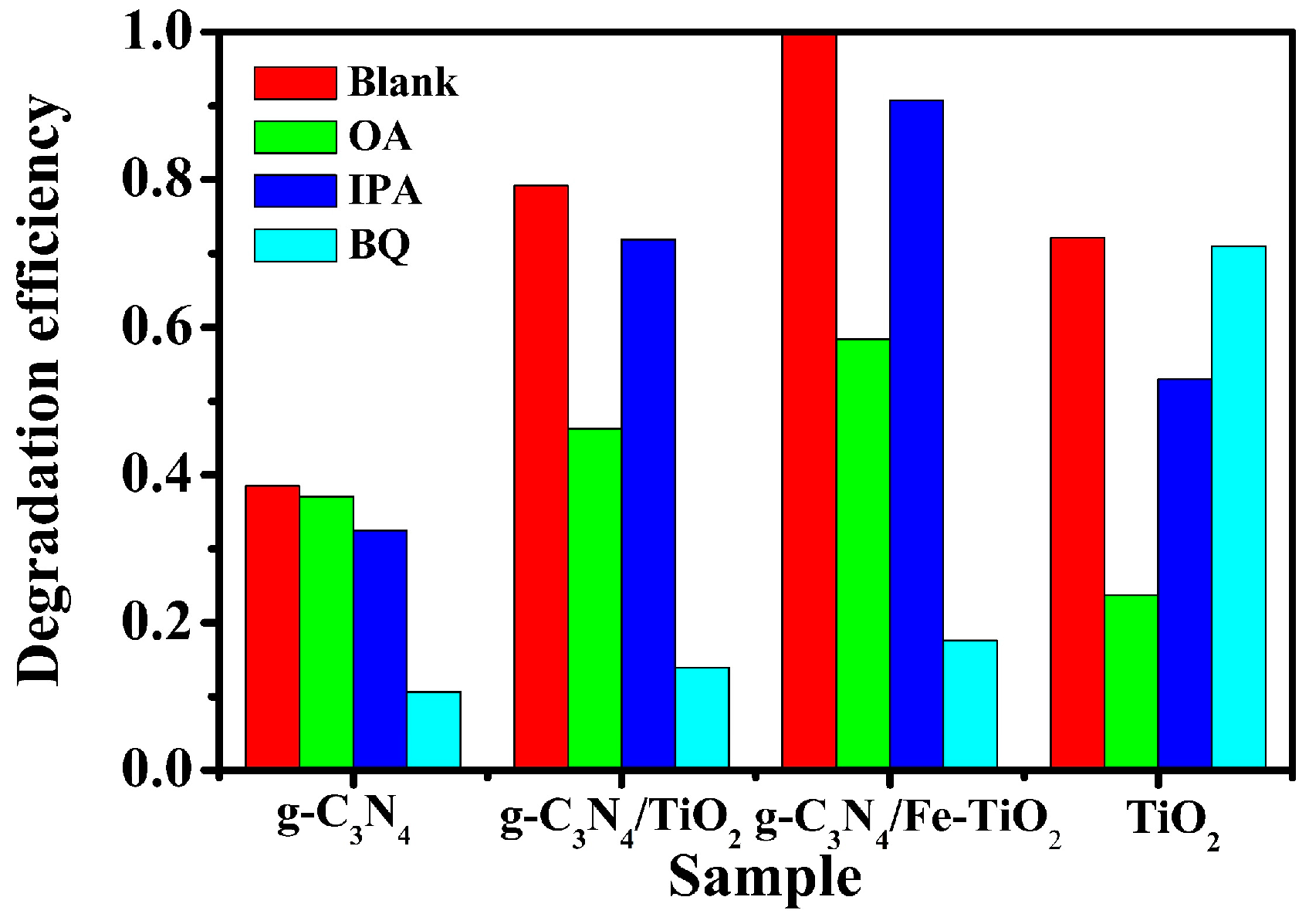
2.3. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemical and Material
3.2. Catalyst Preparation
3.3. Characterization
3.4. Photocatalytic Activity Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Q.; Zhu, Z.; Zhang, J. Preparation and properties of vanadium-doped TiO2 photocatalysts. J Phys. D Appl. Phys. 2010, 43, 035301. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Ji, Z.; Henderson, B.L.; Xia, T.; Li, L.; Zink, J.I.; Nel, A.E.; Madler, L. Role of Fe doping in tuning the band gap of TiO2 for the photo-oxidation-induced cytotoxicity paradigm. J. Am. Chem. Soc. 2011, 133, 11270–11278. [Google Scholar] [CrossRef] [PubMed]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Ohno, T.; Murakami, N.; Tsubota, T.; Nishimura, H. Development of metal cation compound-loaded S-doped TiO2 photocatalysts having a rutile phase under visible light. Appl. Catal. A Gen. 2008, 349, 70–75. [Google Scholar] [CrossRef]
- Samiolo, L.; Valigi, M.; Gazzoli, D.; Amadelli, R. Photo-electro catalytic oxidation of aromatic alcohols on visible light-absorbing nitrogen-doped TiO2. Electrochim. Acta 2010, 55, 7788–7795. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Seh, Z.W.; Liu, S.; Low, M.; Zhang, S.Y.; Liu, Z.; Mlayah, A.; Han, M.Y. Janus Au-TiO2 photocatalysts with strong localization of plasmonic near-fields for efficient visible-light hydrogen generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Liang, C.; Liu, Q.; Zhu, G. Heterojunction of facet coupled g-C3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible LED light irradiation. Appl. Catal. B Environ. 2014, 156–157, 331–340. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tatsuma, T. Oxidative Energy Storage Ability of a TiO2-Ni(OH)2 Bilayer Photocatalyst. Langmuir 2005, 21, 12357–12361. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Yao, L.; Zhang, G.; Dionysiou, D.D.; Li, J.; Du, X. One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(VI). Appl. Catal. B Environ. 2014, 144, 730–738. [Google Scholar] [CrossRef]
- Dai, X.; Xie, M.; Meng, S.; Fu, X.; Chen, S. Coupled systems for selective oxidation of aromatic alcohols to aldehydes and reduction of nitrobenzene into aniline using CdS/g-C3N4 photocatalyst under visible light irradiation. Appl. Catal. B Environ. 2014, 158–159, 382–390. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Efficient Complete Oxidation of Acetaldehyde into CO2 over CuBi2O4/WO3 CompositePhotocatalyst under Visible and UV Light Irradiation. J. Phys. Chem. C 2007, 111, 7574–7577. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Guo, Y.; Zhao, Y.; Yuan, X.; Guo, Y. Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2014, 271, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, G.; Wang, W.; Yang, X.; Zhang, W.F. Photophysical and enhanced daylight photocatalytic properties of N-doped TiO2/g-C3N4 composites. J. Phys. Chem. Solids 2011, 72, 1319–1324. [Google Scholar] [CrossRef]
- Pany, S.; Parida, K.M. A facile in situ approach to fabricate N,S-TiO2/g-C3N4 nanocomposite with excellent activity for visible light induced water splitting for hydrogen evolution. Phys. Chem. Chem. Phys. 2015, 17, 8070–8077. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Murakami, N.; Ye, C.; Tsubota, T.; Ohno, T. Development of highly efficient sulfur-doped TiO2 photocatalysts hybridized with graphitic carbon nitride. Appl. Catal. B Environ. 2013, 142–143, 362–367. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2013, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Murugananthan, M.; Zhang, Y. Synthesis of Z-scheme g-C3N4-Ti3+/TiO2 material: An efficient visible light photoelectrocatalyst for degradation of phenol. Phys. Chem. Chem. Phys. 2015, 17, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dai, G.; Huang, B. Fabrication and Characterization of Visible-Light-Driven Plasmonic Photocatalyst Ag/AgCl/TiO2 Nanotube Arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Murugananthan, M.; Zhang, Y. Development of novel α-Fe2O3/NiTiO3 heterojunction nanofibers material with enhanced visible-light photocatalytic performance. J. Alloy Comp. 2015, 630, 110–116. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Surendar, T.; Baruah, A.; Shanker, V. Synthesis of a novel and stable g-C3N4–Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. A 2013, 1, 5333–5340. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, D.; Zhou, H.; Tian, Y.; Yanagisawa, O. Turbostratic carbon nitride prepared by pyrolysis of melamine. J. Mater. Sci. 2005, 40, 2645–2647. [Google Scholar] [CrossRef]
- Islam, M.J.; Reddy, D.A.; Choi, J.; Kim, T.K. Surface oxygen vacancy assisted electron transfer and shuttling for enhanced photocatalytic activity of a Z-scheme CeO2–AgI nanocomposite. RSC Adv. 2016, 6, 19341–19350. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Bajnóczi, É.G.; Balázs, N.; Mogyorósi, K.; Srankó, D.F.; Pap, Z.; Ambrus, Z.; Canton, S.E.; Norén, K.; Kuzmann, E.; Vértes, A.; et al. The influence of the local structure of Fe(III) on the photocatalytic activity of doped TiO2 photocatalysts—An EXAFS, XPS and Mössbauer spectroscopic study. Appl. Catal. B Environ. 2011, 103, 232–239. [Google Scholar] [CrossRef]
- Egerton, T.A.; Harris, E.; John Lawson, E.; Mile, B.; Rowlands, C.C. An EPR study of diffusion of iron into rutile. Phys. Chem. Chem. Phys. 2001, 3, 497–504. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.; Madras, G. Structure and Photocatalytic Activity of Ti1−xMxO2±δ(M = W, V, Ce, Zr, Fe, and Cu) Synthesized by Solution Combustion Method. J. Phys. Chem. B 2004, 108, 20204–20212. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Mitani, Y.; Nosaka, Y. Photocatalytic Reaction Mechanism of Fe(III)-Grafted TiO2 Studied by Means of ESR Spectroscopy and Chemiluminescence Photometry. J. Phys. Chem. C 2012, 116, 14900–14907. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Lopez, T.; Gómez, R.; Moreno, A.; Fierro, J.; Martínez-Arias, A. Catalytic Combustion of Methane on Fe-TiO2 Catalysts Prepared by Sol-Gel Method. J. Sol-Gel Sci. Technol. 2003, 27, 205–214. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Gupta, A.K.; Sekhar, P.S.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Fe-doped TiO2 powders for solar light response and photocatalytic applications. Process. Appl. Ceram. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Yalçın, Y.; Kılıç, M.; Çınar, Z. Fe3+-doped TiO2: A combined experimental and computational approach to the evaluation of visible light activity. Appl. Catal. B Environ. 2010, 99, 469–477. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Dhara, S.; Imakita, K.; Fujii, M. Oxygen vacancy-mediated enhanced ferromagnetism in undoped and Fe-doped TiO2 nanoribbons. J Phys. D Appl. Phys. 2014, 47, 235304. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J Photoch. Photobio. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Islam, M.J.; Seo, B.; Joo, S.H.; Kim, T.K. Green synthesis of the reduced graphene oxide–CuI quasi-shell–core nanocomposite: A highly efficient and stable solar-light-induced catalyst for organic dye degradation in water. Appl. Surf. Sci. 2015, 358, 159–167. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, X.; Huang, K.; Zhou, B.; Cai, W.; Shangguan, W. Visible-light responsive photocatalytic fuel cell based on WO3/W photoanode and Cu2O/Cu photocathode for simultaneous wastewater treatment and electricity generation. Environ. Sci. Technol. 2012, 46, 11451–114588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Murugananthan, M.; Zhang, Y. Degradation of p-Nitrophenol by thermally activated persulfate in soil system. Chem. Eng. J. 2016, 283, 1357–1365. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Meng, S.; Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B Environ. 2014, 150–151, 564–573. [Google Scholar] [CrossRef]
- Yang, J.; Liao, W.; Liu, Y.; Murugananthan, M.; Zhang, Y. Degradation of Rhodamine B using a Visible-light driven Photocatalytic Fuel Cell. Electrochim. Acta 2014, 144, 7–15. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S. Flat-Band Potential of a Semiconductor: Using the Mott-Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Kontos, A.I.; Likodimos, V.; Stergiopoulos, T.; Tsoukleris, D.S.; Falaras, P.; Rabias, I.; Papavassiliou, G.; Kim, D.; Kunze, J.; Schmuki, P. Self-Organized Anodic TiO2 Nanotube Arrays Functionalized by Iron Oxide Nanoparticles. Chem. Mater. 2009, 21, 662–672. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid. Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zheng, Q.; van de Krol, R. Creating Oxygen Vacancies as a Novel Strategy to Form Tetrahedrally Coordinated Ti4+ in Fe/TiO2 Nanoparticles. J. Phys. Chem. C 2012, 116, 7219–7226. [Google Scholar] [CrossRef]
- Wu, Q.; van de Krol, R. Selective photoreduction of nitric oxide to nitrogen by nanostructured TiO2 photocatalysts: Role of oxygen vacancies and iron dopant. J. Am. Chem. Soc. 2012, 134, 9369–9375. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zhang, Y.; Zhang, M.; Murugananthan, M.; Yoshihara, S. Photoelectrocatalytic degradation of microcystin-LR using Ag/AgCl/TiO2 nanotube arrays electrode under visible light irradiation. Chem. Eng. J. 2013, 231, 455–463. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Murugananthan, M.; Gu, J.; Zhang, Y. Fabrication of a Z-Scheme g-C3N4/Fe-TiO2 Photocatalytic Composite with Enhanced Photocatalytic Activity under Visible Light Irradiation. Catalysts 2018, 8, 112. https://doi.org/10.3390/catal8030112
Zhu Z, Murugananthan M, Gu J, Zhang Y. Fabrication of a Z-Scheme g-C3N4/Fe-TiO2 Photocatalytic Composite with Enhanced Photocatalytic Activity under Visible Light Irradiation. Catalysts. 2018; 8(3):112. https://doi.org/10.3390/catal8030112
Chicago/Turabian StyleZhu, Zedong, Muthu Murugananthan, Jie Gu, and Yanrong Zhang. 2018. "Fabrication of a Z-Scheme g-C3N4/Fe-TiO2 Photocatalytic Composite with Enhanced Photocatalytic Activity under Visible Light Irradiation" Catalysts 8, no. 3: 112. https://doi.org/10.3390/catal8030112




