Catalytic Conversion of Model Tars over Carbon-Supported Ni and Fe
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
2.1.1. Compositional and Textural Properties
2.1.2. Crystal Phases, Cluster Sizes and Thermal Resistance
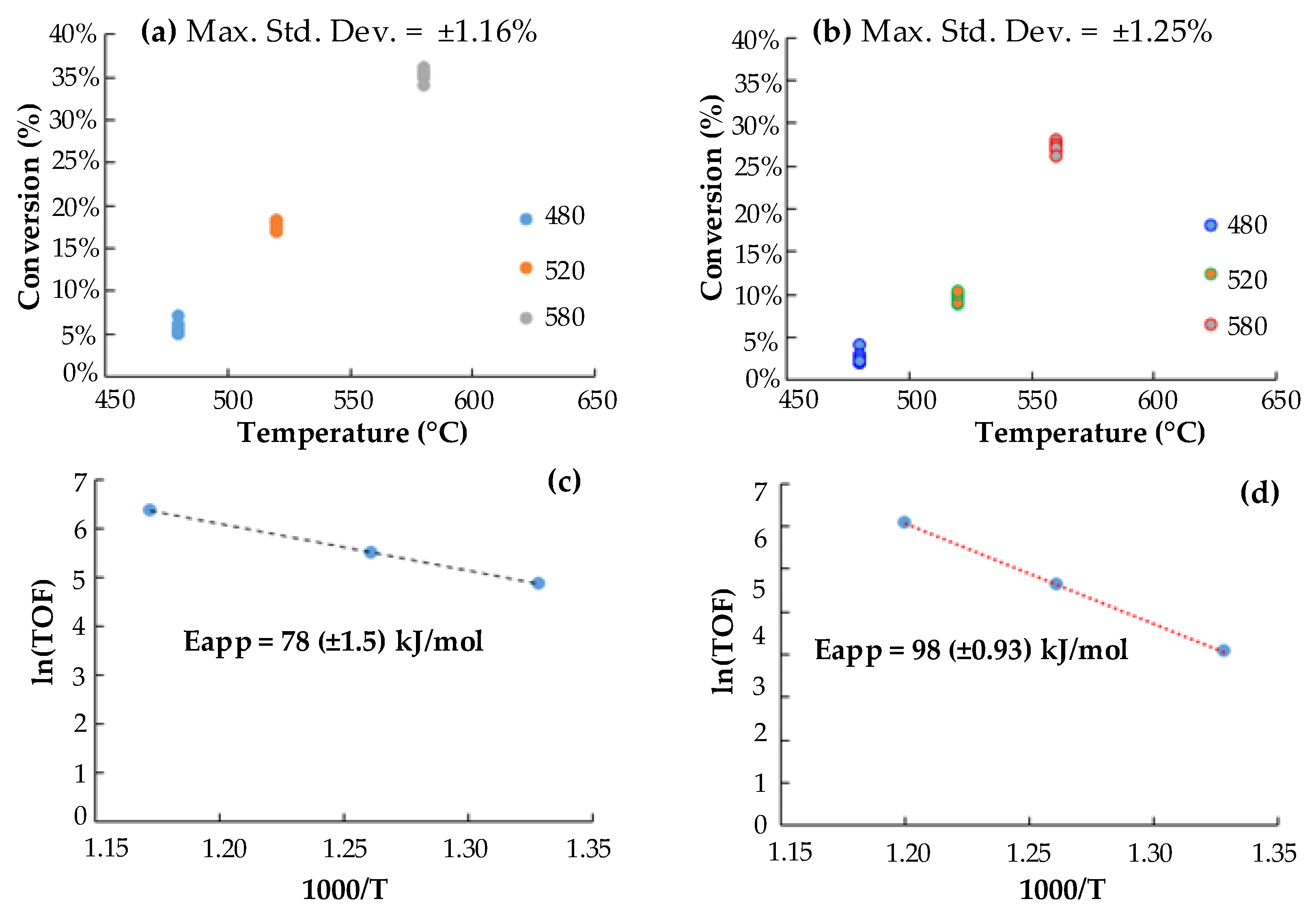
2.2. Preliminary Activity Tests. Tar (Toluene) Conversion at Lab-Scale
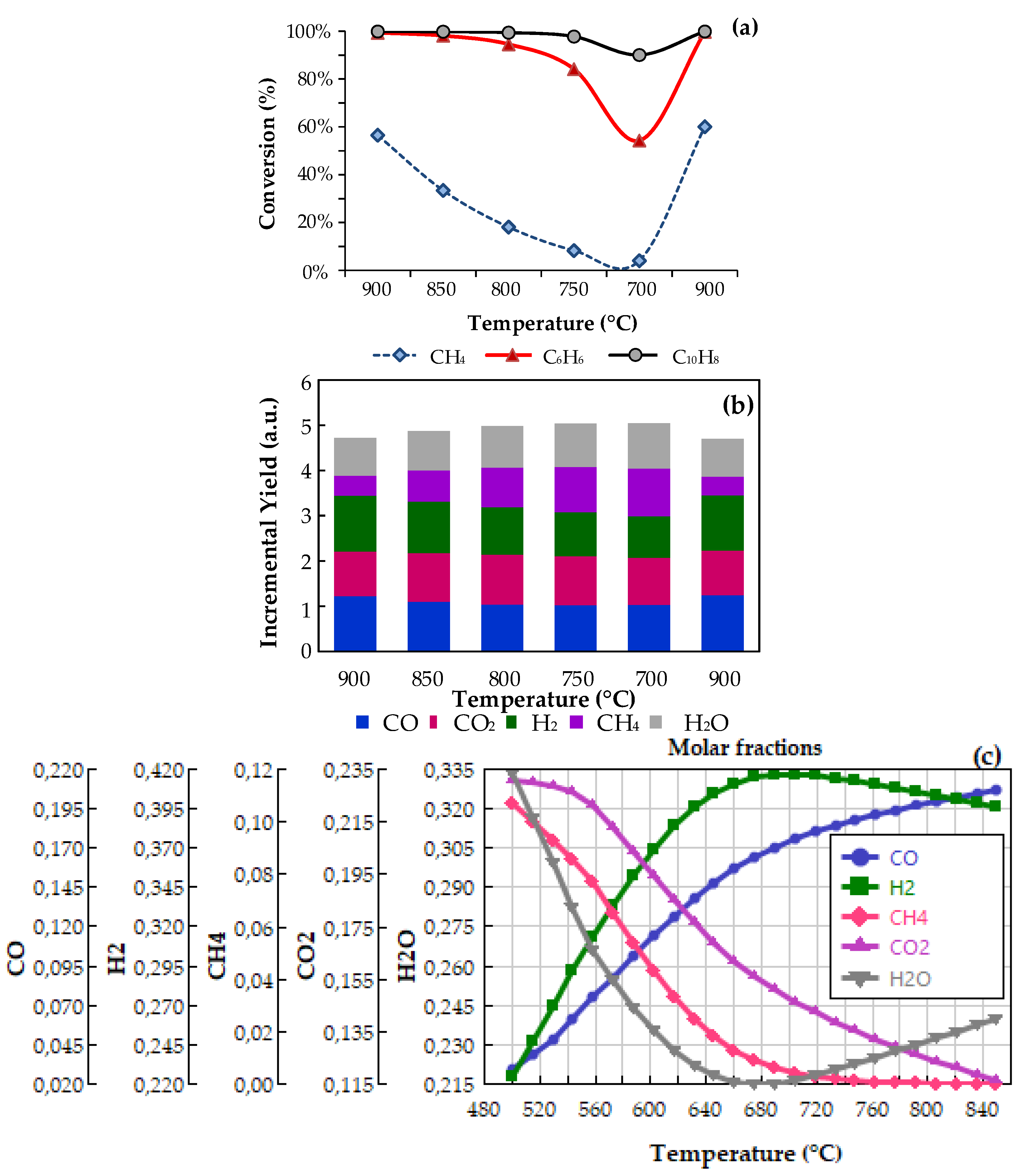
2.3. Bench-Scale Activity Tests
- RI: Benzene steam reforming: C6H6 + 6 H2O → 6 CO + 9 H2
- RII: Naphthalene steam reforming: C10H8 + 10 H2O → 10 CO + 14 H2
- RIII: Methane steam reforming: CH4 + H2O → CO + 3 H2
- RIV: Methane dry reforming: CH4 + CO2 → 2 CO + 2 H2
- RV: Boudouard: C + CO2 → 2 CO
- RVI: Water gas: C + H2O → CO + H2
3. Discussion
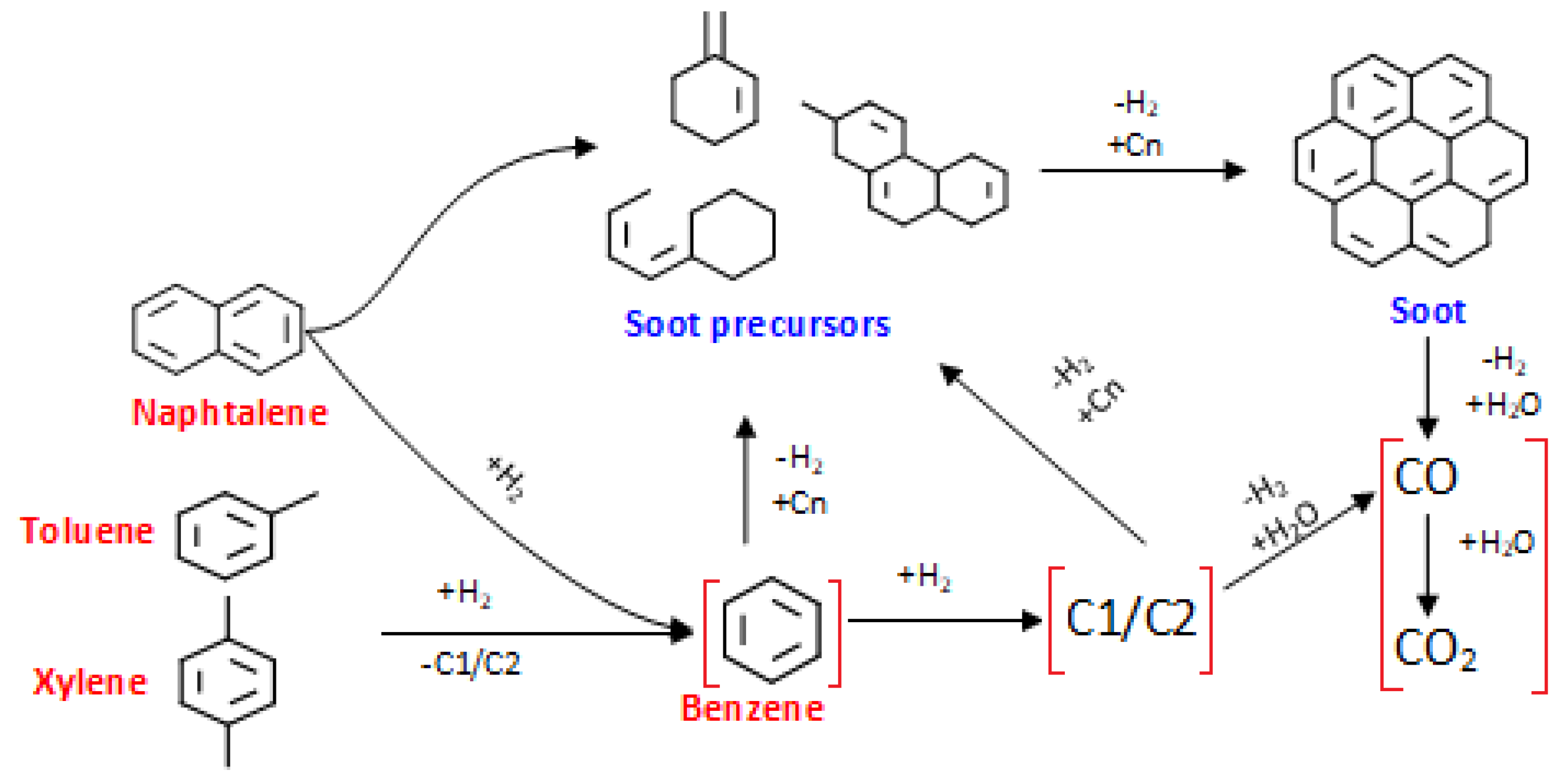
3.1. Competing Reaction Schemes: Soot Formation versus Cracking and Reforming
3.2. Kinetic Implications
3.3. Auto-Reduction and Catalysts Activity
- nH2 + MemOn → mMe + nH2O: Hydrogen reduction
- nC + MemOn → mMe + nCO: Carbothermal reduction
3.4. Future Work on Gas Cleaning
4. Materials and Methods
4.1. Support and Catalysts Preparation
4.2. Materials Characterization
4.2.1. Textural Properties Measurement
4.2.2. X-Ray Diffraction
4.2.3. Transmission Electron Microscopy (TEM)
4.2.4. Thermogravimetric Performance (TGA)
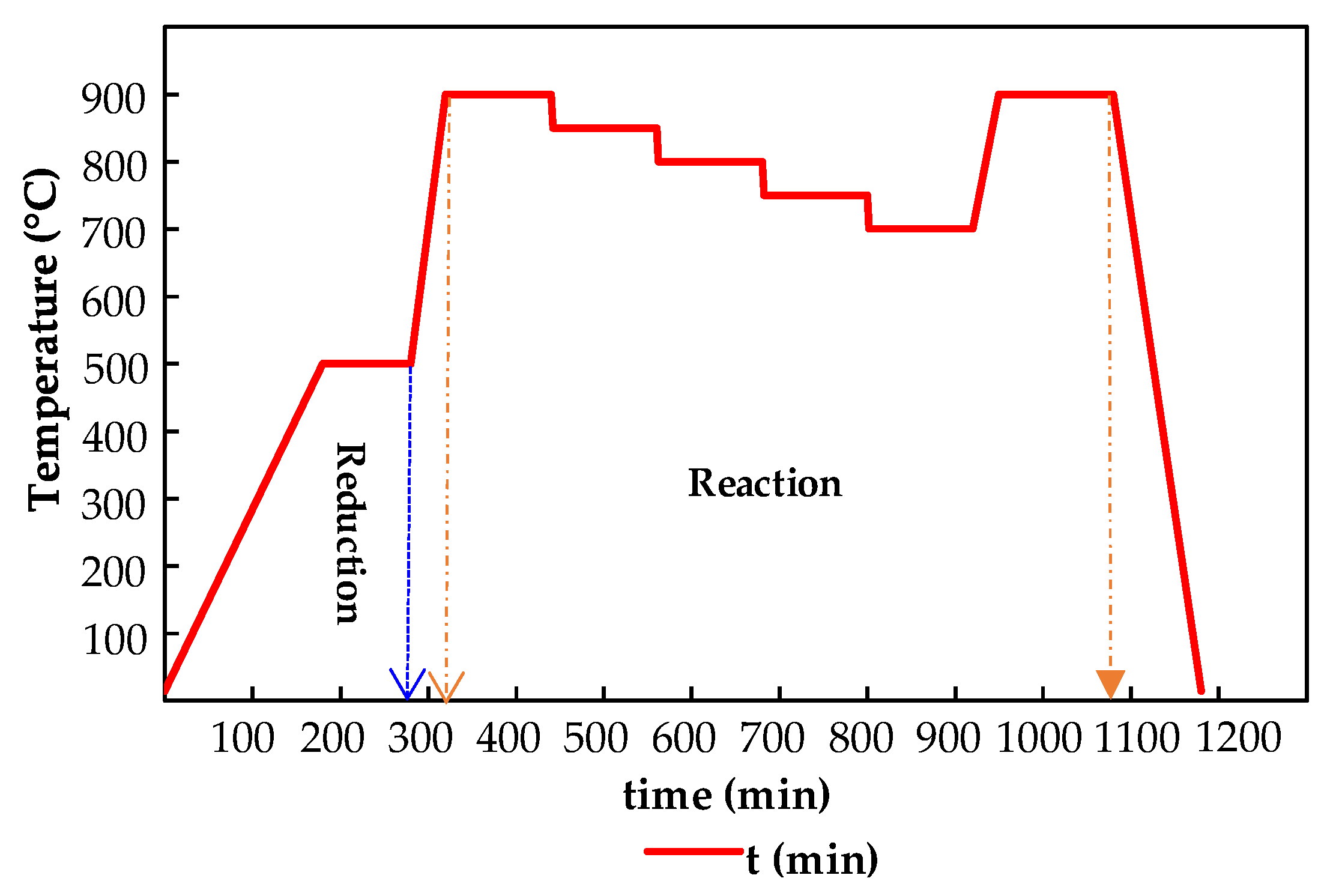
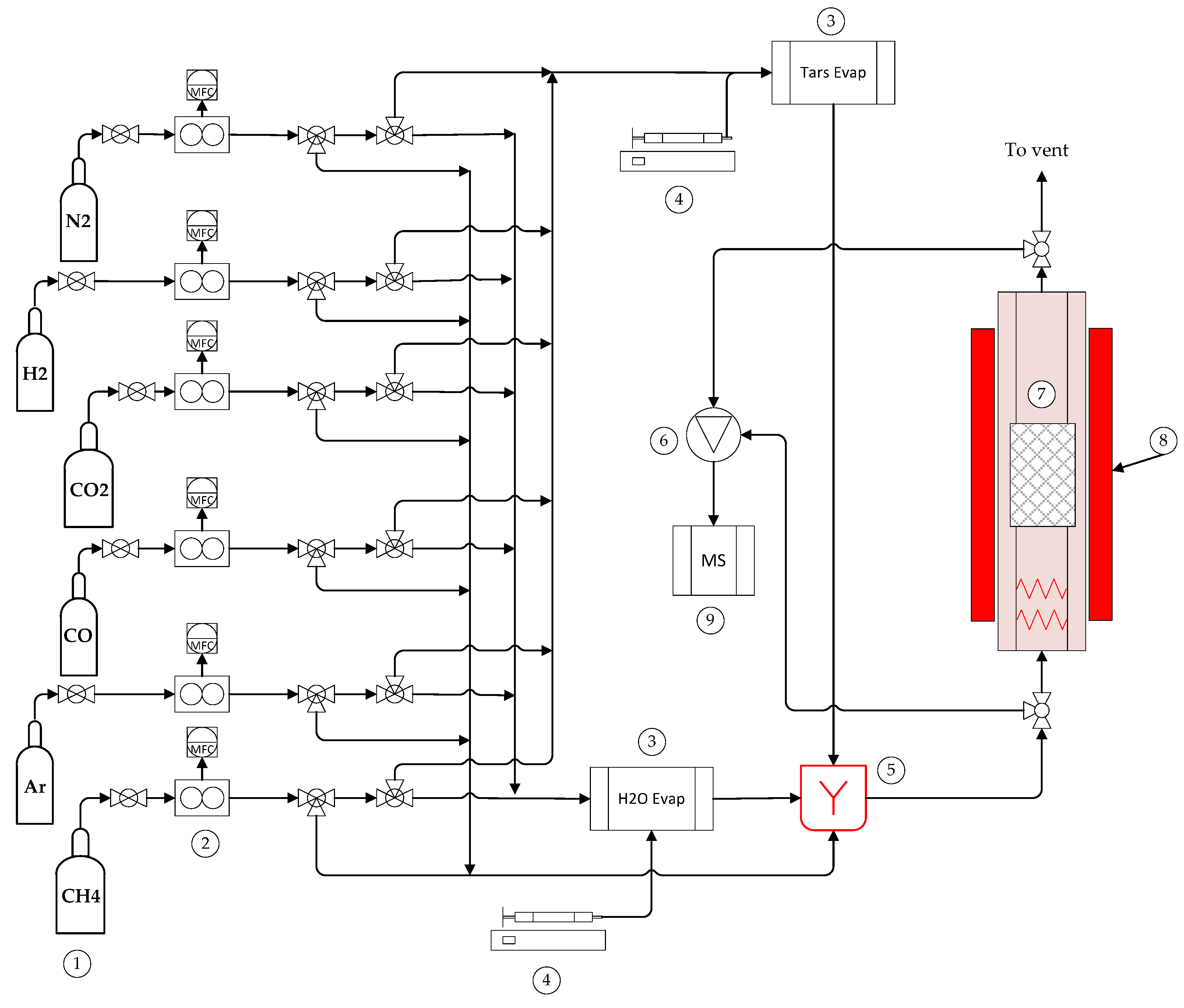
4.3. Installation of Kinetic Assays
4.3.1. Experimental Setups: Toluene Decomposition
4.3.2. Reforming Catalytic Test Rig
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction. Practical design and Theory, 2nd ed.; Elsevier Ltd.: New York, NY, USA, 2013; ISBN 9780123964885. [Google Scholar]
- Brown, R.C. Thermochemical Processing of Biomass; John Wiley & Sons, Ltd.: Chichester, UK, 2011; ISBN 9781119990840. [Google Scholar]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Zwart, R.W.R. Gas cleaning downstream biomass gasification Status Report 2009. ECN SenterNovem 2009. Available online: https://www.ecn.nl/publications/E/2009/ECN-E-08-078 (accessed on 20 November 2017).
- Asadullah, M. Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Brandin, J.; Tunér, M.; Odenbrand, I.; Lund, V. Swedish Energy Agency Report Small Scale Gasification: Gas Engine CHP for Biofuels; Linnaeus University: Växjö, Sweden, 2011. [Google Scholar]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- Richardson, Y.; Blin, J.; Julbe, A. A short overview on purification and conditioning of syngas produced by biomass gasification: Catalytic strategies, process intensification and new concepts. Prog. Energy Combust. Sci. 2012, 38, 765–781. [Google Scholar] [CrossRef]
- Xu, C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent advances in catalysts for hot-gas removal of tar and NH3 from biomass gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- Min, Z.; Zhang, S.; Yimsiri, P.; Wang, Y.; Asadullah, M.; Li, C.Z. Catalytic reforming of tar during gasification. Part IV. Changes in the structure of char in the char-supported iron catalyst during reforming. Fuel 2013, 106, 858–863. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Ma, D.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B Environ. 2014, 152–153, 140–151. [Google Scholar] [CrossRef]
- Courson, C.; Udron, L.; Swierczy, D.; Petit, C.; Kiennemann, A. Hydrogen production from biomass gasification on nickel catalysts: Tests for dry reforming of methane. Catal. Today 2002, 76, 75–86. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, W.; Ji, W. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl. Energy 2011, 88, 1656–1663. [Google Scholar] [CrossRef]
- Nassos, S.; Elm Svensson, E.; Boutonnet, M.; Järås, S.G. The influence of Ni load and support material on catalysts for the selective catalytic oxidation of ammonia in gasified biomass. Appl. Catal. B Environ. 2007, 74, 92–102. [Google Scholar] [CrossRef]
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas. Fuel Process. Technol. 2015, 132, 39–48. [Google Scholar] [CrossRef]
- Chianese, S.; Fail, S.; Binder, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Blasi, A.; Musmarra, D. Experimental investigations of hydrogen production from CO catalytic conversion of tar rich syngas by biomass gasification. Catal. Today 2016, 277, 182–191. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, S.; Chen, Z.D.; Liu, C.; Wang, Y.G. Tar reforming using char as catalyst during pyrolysis and gasification of Shengli brown coal. J. Anal. Appl. Pyrolysis 2014, 105, 269–275. [Google Scholar] [CrossRef]
- Asadullah, M.; Miyazawa, T.; Ito, S.; Kunimori, K.; Koyama, S.; Tomishige, K. A comparison of Rh/CeO2/SiO2 catalysts with steam reforming catalysts, dolomite and inert materials as bed materials in low throughput fluidized bed gasification systems. Biomass Bioenergy 2004, 26, 269–279. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Dou, B.; Gao, J.; Sha, X.; Baek, S.W. Catalytic cracking of tar component from high-temperature fuel gas. Appl. Therm. Eng. 2003, 23, 2229–2239. [Google Scholar] [CrossRef]
- Simell, P.; Kurkela, E.; Stahlberg, P.; Hepola, J. Catalytic hot gas cleaning of gasification gas. Catal. Today 1996, 27, 55–62. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Doyle, A.; Ross, J.R. Investigation of nickel supported catalysts for the upgrading of brown peat derived gasification products. Bioresour. Technol. 2001, 80, 111–116. [Google Scholar] [CrossRef]
- Virginie, M.; Courson, C.; Niznansky, D.; Chaoui, N.; Kiennemann, A. Characterization and reactivity in toluene reforming of a Fe/olivine catalyst designed for gas cleanup in biomass gasification. Appl. Catal. B Environ. 2010, 101, 90–100. [Google Scholar] [CrossRef]
- Tamhankar, S.S.; Tsuchiya, K.; Riggs, J.B. Catalytic cracking of benzene on iron oxide-silica: Activity and reaction mechanism. Appl. Catal. B Environ. 1985, 16, 103–108. [Google Scholar] [CrossRef]
- Azhar Uddin, M.; Tsuda, H.; Wu, S.; Sasaoka, E. Catalytic decomposition of biomass tars with iron oxide catalysts. Fuel 2008, 87, 451–459. [Google Scholar] [CrossRef]
- Nemanova, V.; Nordgreen, T.; Engvall, K.; Sjöström, K. Biomass gasification in an atmospheric fluidised bed: Tar reduction with experimental iron-based granules from Höganäs AB, Sweden. Catal. Today 2011, 176, 253–257. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Hu, M.; Laghari, M.; Cui, B.; Xiao, B.; Zhang, B.; Guo, D. Catalytic cracking of biomass tar over char supported nickel catalyst. Energy 2018, 145, 228–237. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Huber, G.W.; Boudart, M. Handbook of Heterogeneous Catalysis; Vancouver Coastal Health: Vancouver, BC, Canada, 1997. [Google Scholar]
- Arteaga-Pérez, L.E.; Gòmez-Capiro, O.; Delgado, A.; Alejandro, S.; Jímenez, R. Elucidating the role of ammonia-based salts on the preparation of cellulose-derived carbon aerogels. Chem. Eng. Sci. 2017, 161, 80–91. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 0080444636. [Google Scholar]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Girgis, B.S.; Temerk, Y.M.; Gadelrab, M.M.; Abdullah, I.D. X-ray diffraction patterns of activated carbons prepared under various conditions. Carbon Lett. 2007, 8, 95–100. [Google Scholar] [CrossRef]
- Short, M.; Walker, P. Measurement of interlayer spacings and crystal sizes in turbostratic carbons. Carbon 1963, 1, 3–9. [Google Scholar] [CrossRef]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni-Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J. Ethylene conversion on activated carbon-supported NiMo catalysts: Effect of the support. Appl. Catal. A Gen. 2003, 241, 25–38. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, J.M.; Aznar, M.P. Improving the modelling of the kinetics of the catalytic tar elimination in biomass gasification. Ind. Eng. Chem. Res. 2002, 313–332. [Google Scholar] [CrossRef]
- Vannice, A. Kinetics of Catalytic Reactions, 1st ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Korus, A.; Samson, A.; Szlęk, A.; Katelbach-Woźniak, A.; Sładek, S. Pyrolytic toluene conversion to benzene and coke over activated carbon in a fixed-bed reactor. Fuel 2017, 207, 283–292. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Kumar, A.; Bellmer, D.D.; Huhnke, R.L. Synthesis and evaluation of biochar-derived catalysts for removal of toluene (model tar) from biomass-generated producer gas. Renew. Energy 2014, 66, 346–353. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, B.S.; Lee, J.W. Breakthrough data analysis of adsorption of toluene vapor in a fixed-bed of granular. Sep. Sci. Technol. 2007, 42, 2221–2233. [Google Scholar] [CrossRef]
- Levenspiel, O. Experimental search deactivating for a simple porous rate equation particles to describe catalyst. J. Catal. 1972, 25, 265–272. [Google Scholar] [CrossRef]
- Robinson, A.M.; Hensley, J.E.; Will Medlin, J. Bifunctional catalysts for upgrading of biomass-derived oxygenates: A review. ACS Catal. 2016, 6, 5026–5043. [Google Scholar] [CrossRef]
- Juntgen, H.; van Heek, K.H. (Translated by Belov and Assoc., Denver, CO, APTIC-TR-0776). Fortschr. Chem. Forsch. 1970, 13, 601. [Google Scholar]
- Gao, N.; Wang, X.; Li, A.; Wu, C.; Yin, Z. Hydrogen production from catalytic steam reforming of benzene as tar model compound of biomass gasi fi cation. Fuel Process. Technol. 2016, 148, 380–387. [Google Scholar]
- Park, H.J.; Park, S.H.; Sohn, J.M.; Park, J.; Jeon, J.K.; Kim, S.S.; Park, Y.K. Steam reforming of biomass gasification tar using benzene as a model compound over various Ni supported metal oxide catalysts. Bioresour. Technol. 2010, 101, S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Jess, A. Mechanisms and kinetics of thermal reactions of aromatic hydrocarbons from pyrolysis of solid fuels. Fuel 1996, 75, 1441–1448. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Artetxe, M.; Alvarez, J.; Nahil, M.A.; Olazar, M.; Williams, P.T. Steam reforming of different biomass tar model compounds over Ni/Al2O3 catalysts. Energy Convers. Manag. 2017, 136, 119–126. [Google Scholar] [CrossRef]
- Wang, L.; Schütz, C.; Salazar-Alvarez, G.; Titirici, M.-M. Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv. 2014, 4, 17549. [Google Scholar] [CrossRef]
- Meng, Y.; Young, T.M.; Liu, P.; Contescu, C.I.; Huang, B.; Wang, S. Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 2014, 22, 435–447. [Google Scholar] [CrossRef]
- Karacan, I.; Soy, T. Enhancement of oxidative stabilization of viscose rayon fibers impregnated with ammonium sulfate prior to carbonization and activation steps. J. Appl. Polym. Sci. 2013, 128, 1239–1249. [Google Scholar] [CrossRef]
- Charles, G.W.; Susott, R.A. Effects of Ammonium Phosphate and Sulfate on the Pyrolysis and Combustion of Cellulose. 1971. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201300717137 (accessed on 5 October 2016).
- Grams, J.; Niewiadomski, M.; Ruppert, A.M.; Kwapiński, W. Influence of Ni catalyst support on the product distribution of cellulose fast pyrolysis vapors upgrading. J. Anal. Appl. Pyrolysis 2015, 113, 557–563. [Google Scholar] [CrossRef]
- Li, D.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Metal catalysts for steam reforming of tar derived from the gasification of lignocellulosic biomass. Bioresour. Technol. 2015, 178, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, D.; Hu, Y.; Sun, P.; Xia, J.; Huang, H. Effect of preparation method on the structure and catalytic property of activated carbon supported nickel oxide catalysts. Carbon 2010, 48, 4547–4555. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Xu, C.; Kong, D.; Tsubouchi, N. Decomposition of ammonia with iron and calcium catalysts supported on coal chars. Fuel 2004, 83, 685–692. [Google Scholar] [CrossRef]
- Hoekstra, J.; Beale, A.M.; Soulimani, F.; Versluijs-helder, M.; Van De Kleut, D.; Koelewijn, J.M.; Geus, J.W.; Jenneskens, L.W. The effect of iron catalyzed graphitization on the textural properties of carbonized cellulose: Magnetically separable graphitic carbon bodies for catalysis and remediation. Carbon 2016, 107, 248–260. [Google Scholar] [CrossRef]
- De Lange, M.F.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Adsorptive characterization of porous solids: Error analysis guides the way. Microporous Mesoporous Mater. 2014, 200, 199–215. [Google Scholar] [CrossRef]
- Fernández, C.; Miranda, N.; García, X.; Eloy, P.; Ruiz, P.; Gordon, A.; Jiménez, R. Insights into dynamic surface processes occurring in Rh supported on Zr-grafted γ-Al2O3 during dry reforming of methane. Appl. Catal. B Environ. 2014, 156, 202–212. [Google Scholar] [CrossRef]
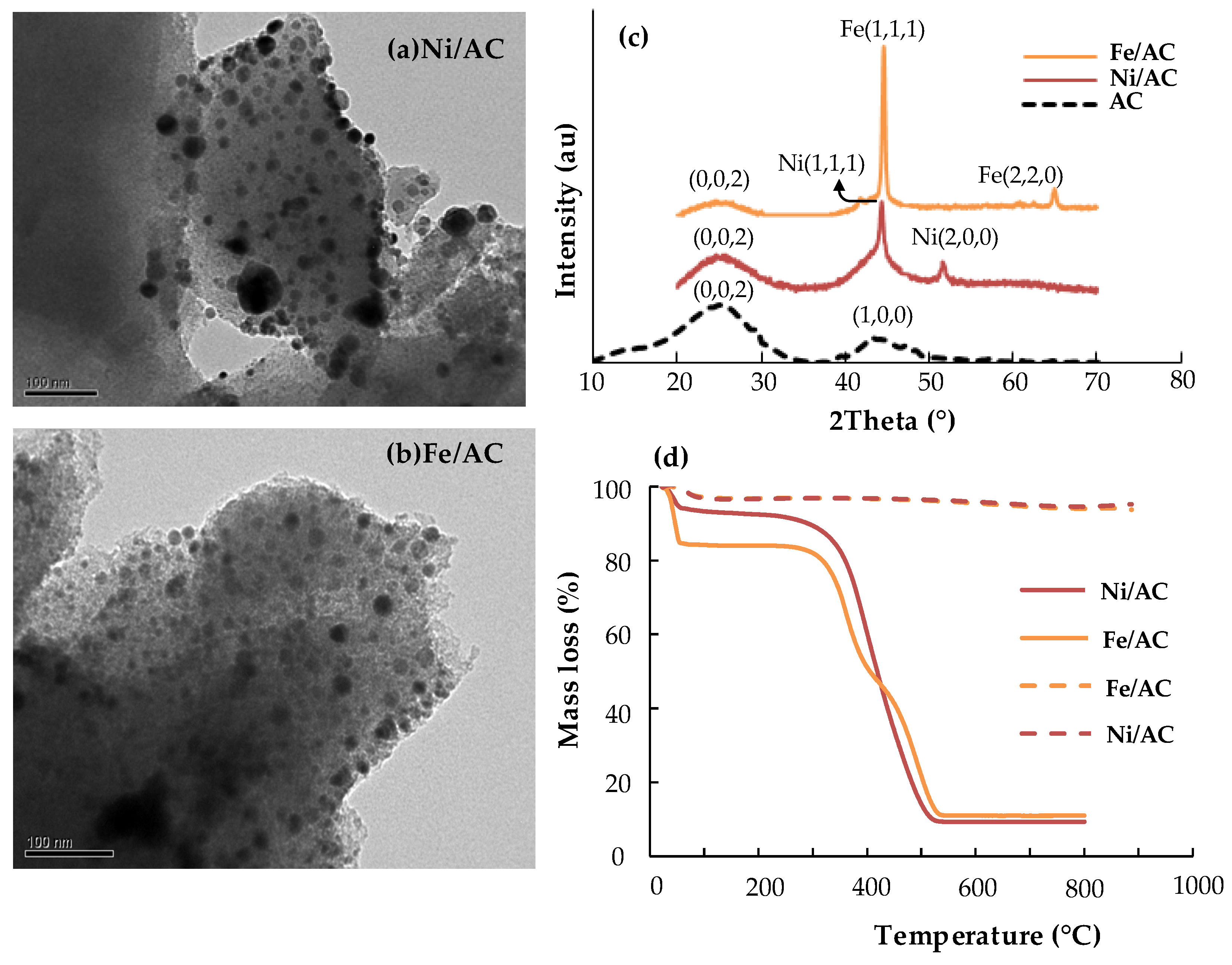

| Catalyst | Pore Volume Vp (cm3/g) | Avg. Pore Diameter Dp (nm) | Surface Area SBET (m2/g) | Metal Loading (% wt/wt) | Crystallite Dimensions (Å) |
|---|---|---|---|---|---|
| Ni/AC | 0.28 | 5.8–5.7 | 684 | 5.1 | - |
| Fe/AC | 0.30 | 4.3–4.2 | 678 | 5.3 | - |
| AC | 0.37 | 4.0–6.0 | 743 | - | La = 28; Lc = 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arteaga-Pérez, L.E.; Delgado, A.M.; Flores, M.; Olivera, P.; Matschuk, K.; Hamel, C.; Schulzke, T.; Jiménez, R. Catalytic Conversion of Model Tars over Carbon-Supported Ni and Fe. Catalysts 2018, 8, 119. https://doi.org/10.3390/catal8030119
Arteaga-Pérez LE, Delgado AM, Flores M, Olivera P, Matschuk K, Hamel C, Schulzke T, Jiménez R. Catalytic Conversion of Model Tars over Carbon-Supported Ni and Fe. Catalysts. 2018; 8(3):119. https://doi.org/10.3390/catal8030119
Chicago/Turabian StyleArteaga-Pérez, Luis E., Aaron M. Delgado, Mauricio Flores, Patricia Olivera, Kimberley Matschuk, Christian Hamel, Tim Schulzke, and Romel Jiménez. 2018. "Catalytic Conversion of Model Tars over Carbon-Supported Ni and Fe" Catalysts 8, no. 3: 119. https://doi.org/10.3390/catal8030119





