Recent Progress in Asymmetric Catalysis and Chromatographic Separation by Chiral Metal–Organic Frameworks
Abstract
:1. Introduction
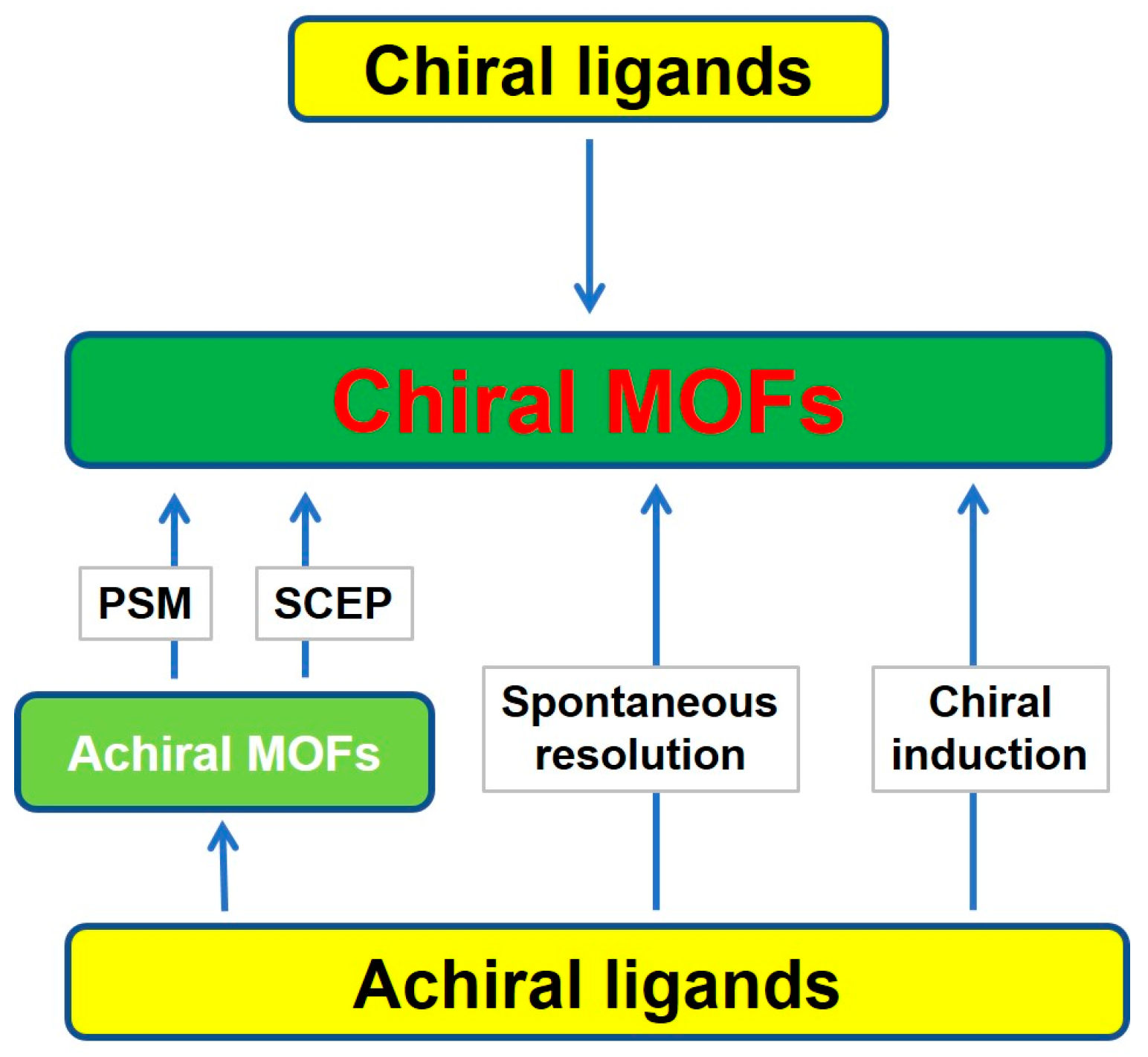
2. Synthetic Strategies of Chiral MOFs
2.1. Straightforward Method
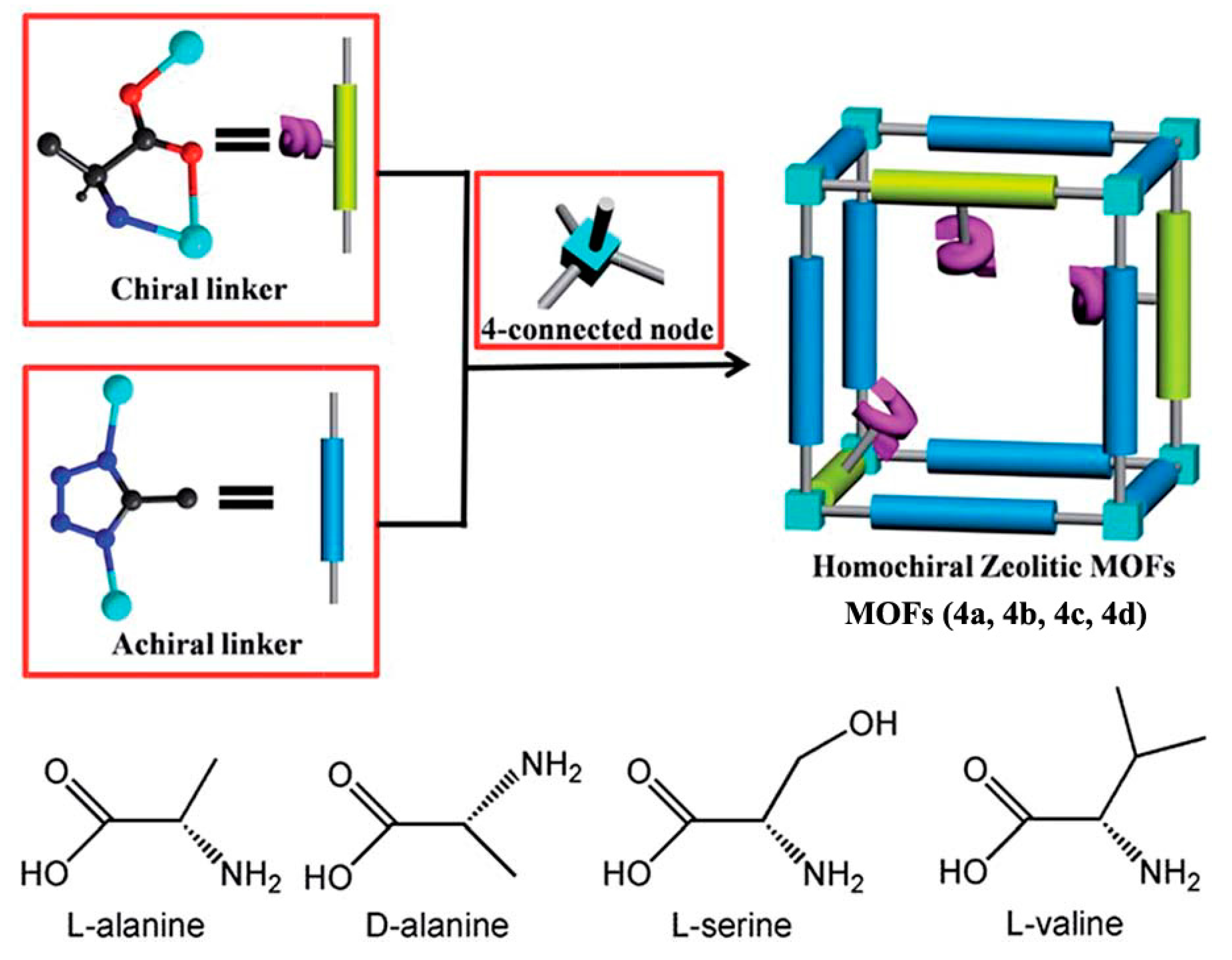
2.1.1. Chiral MOFs Prepared from Chiral Ligands
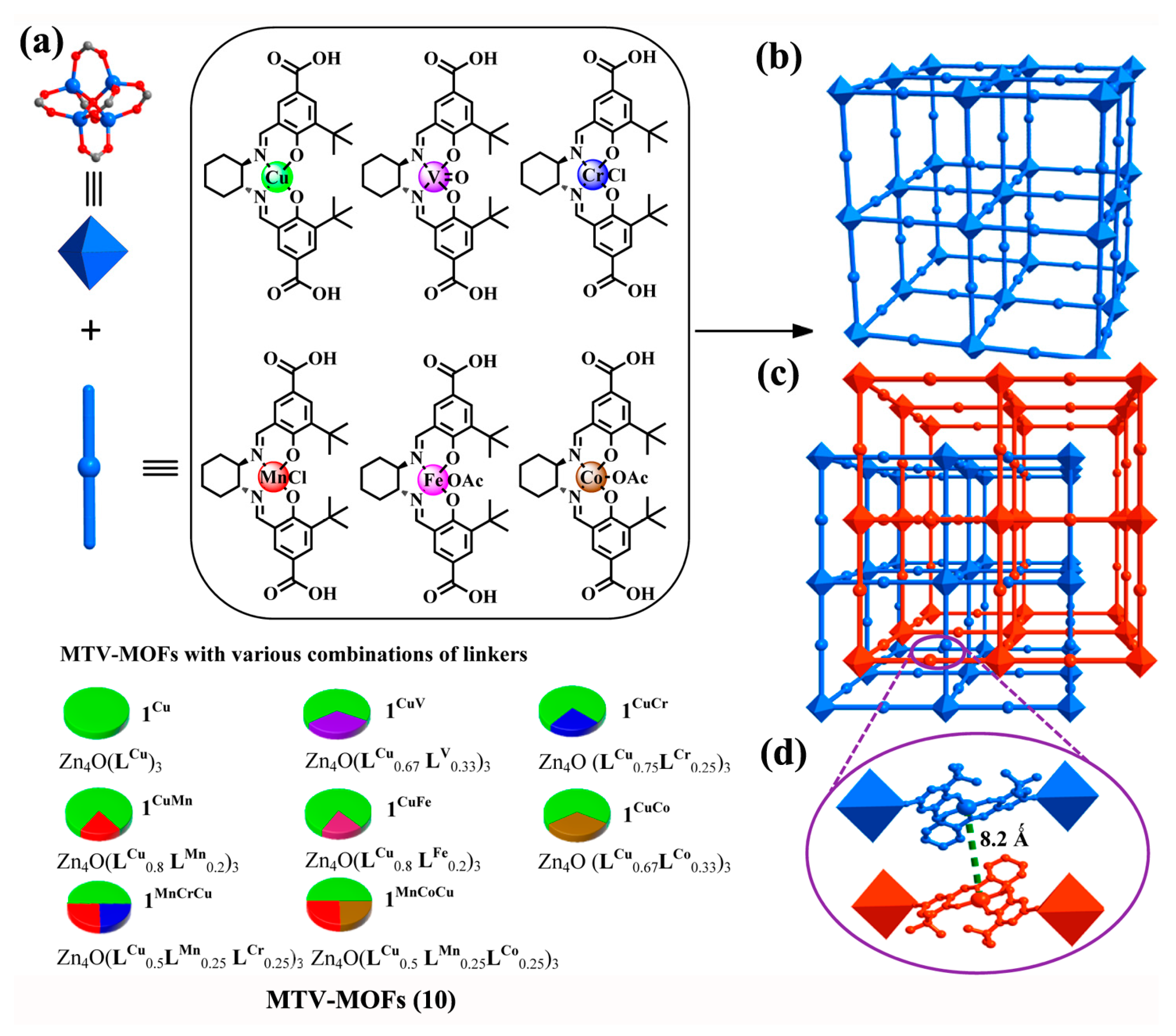
2.1.2. Chiral MOFs from Chiral Salen Ligands
2.2. Indirect Method
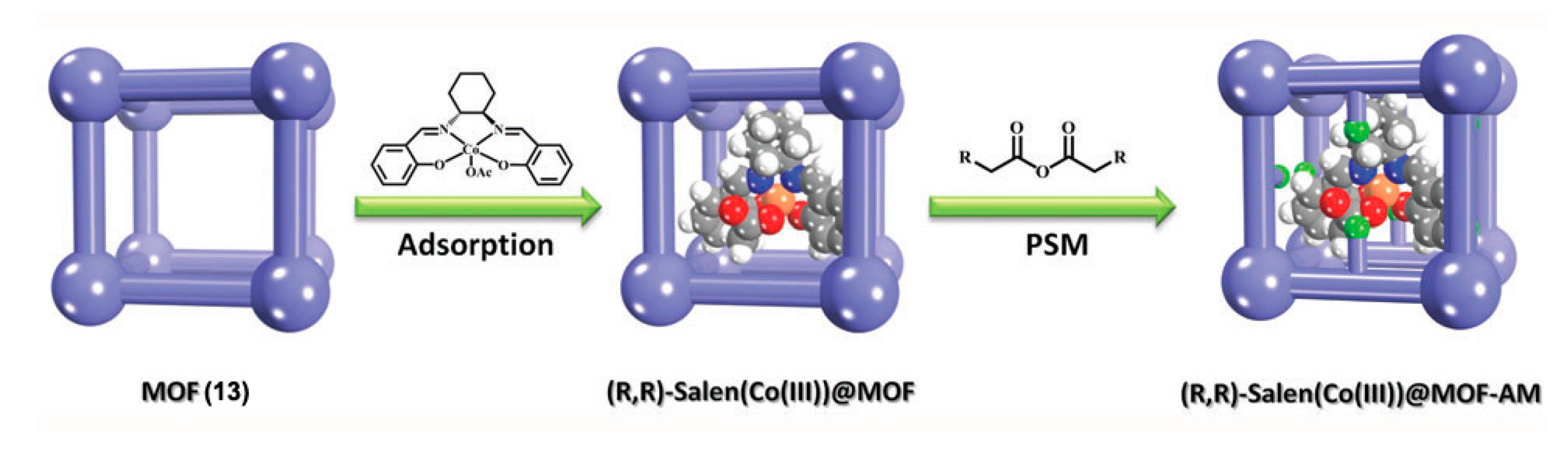
2.2.1. Post-Synthetic Modification (PSM)
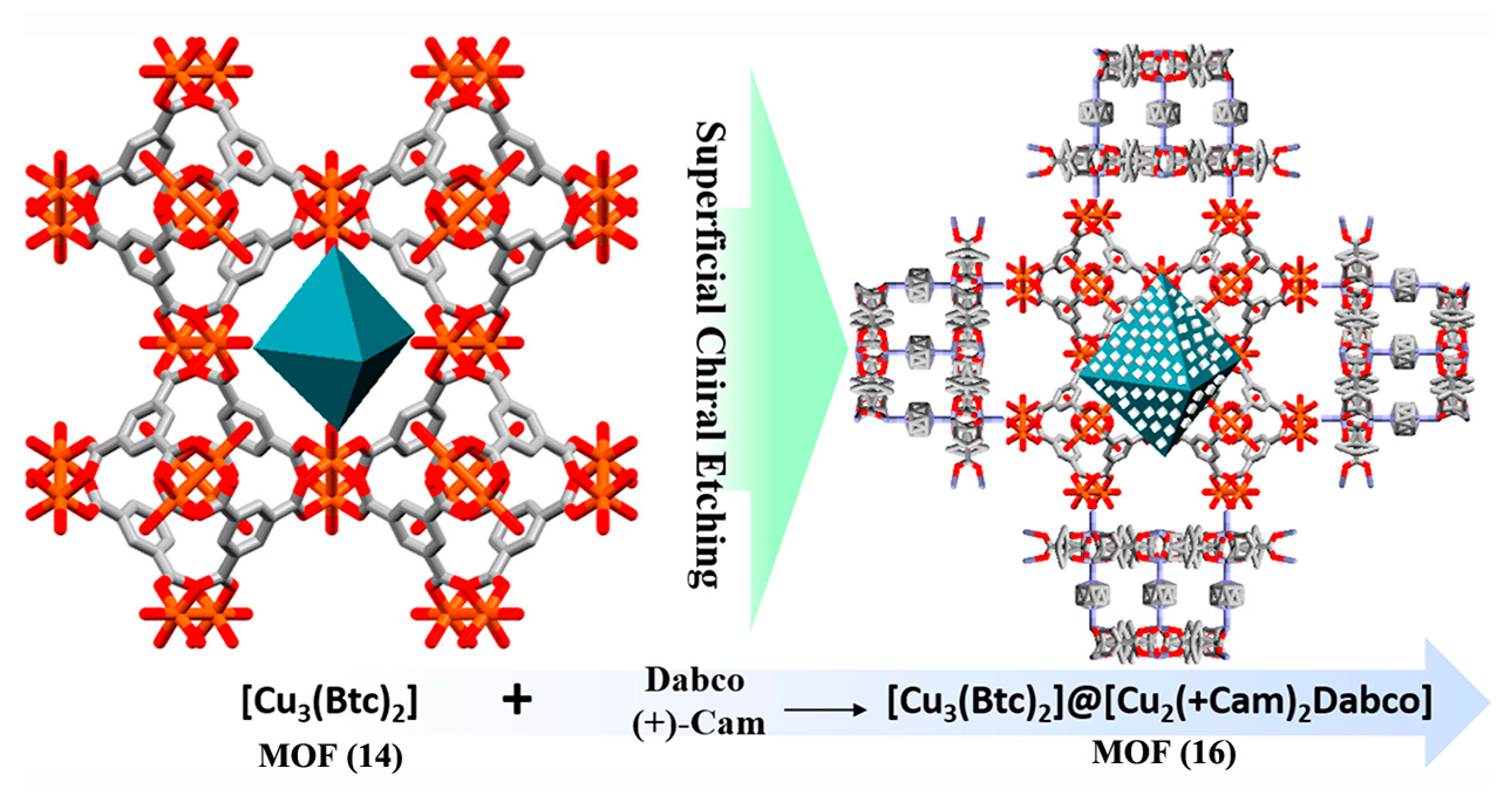
2.2.2. Superficial Chiral Etching Process
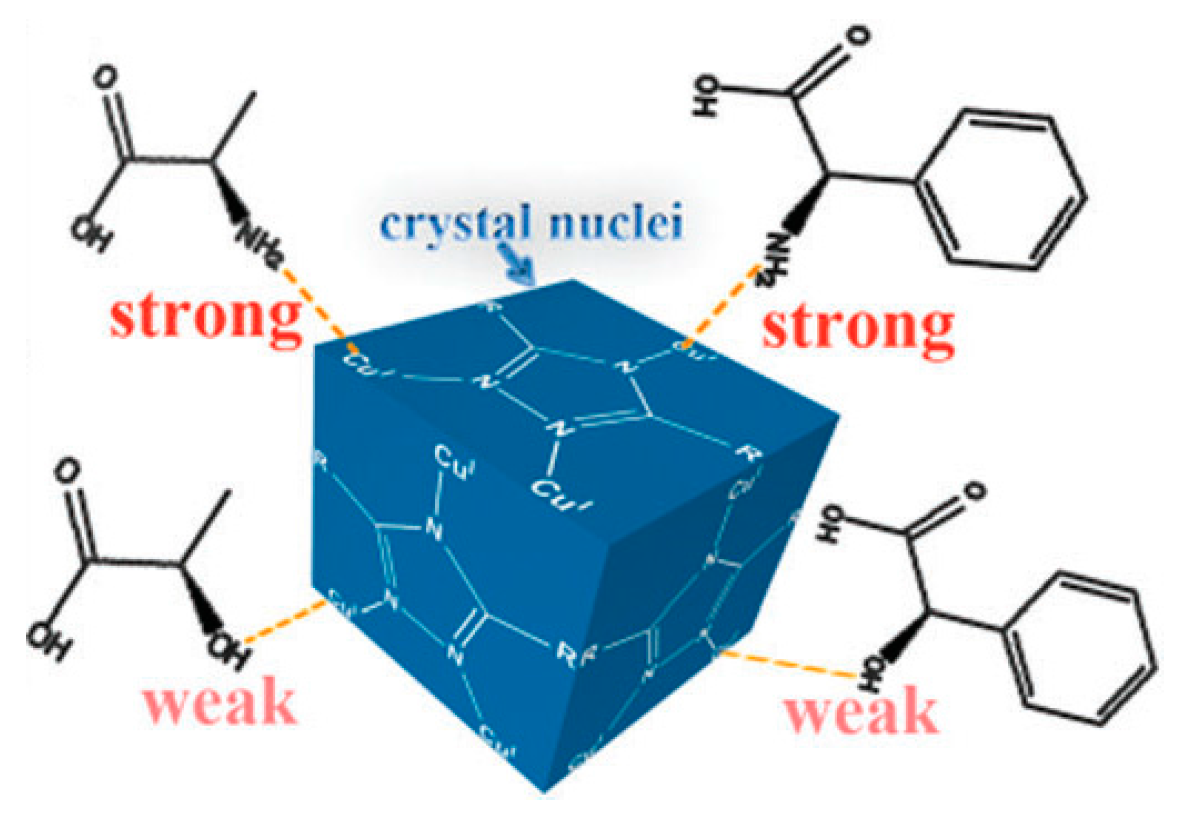
2.2.3. Spontaneous Resolution
2.2.4. Chiral Induction
3. Applications of Chiral MOFs
3.1. Asymmetric Catalysis
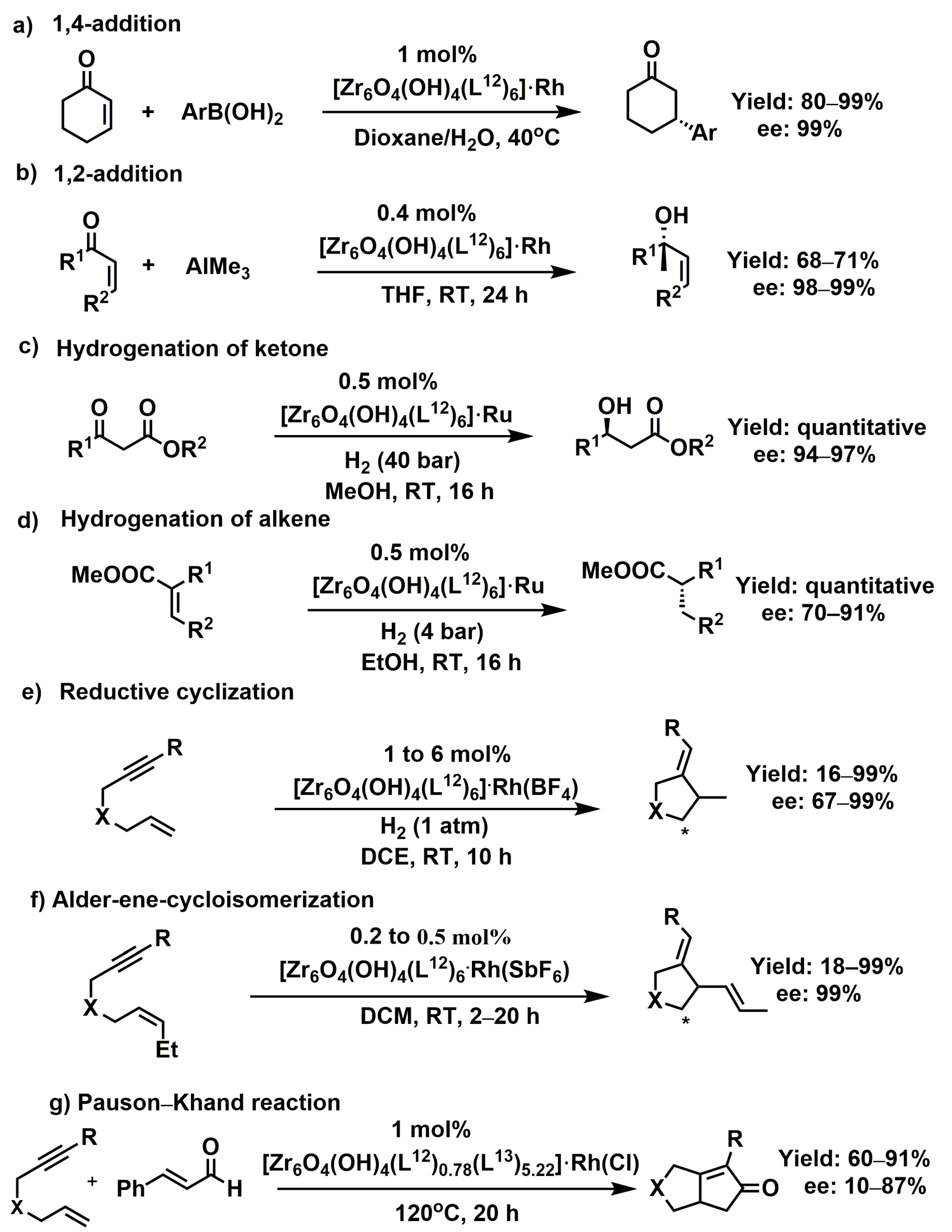
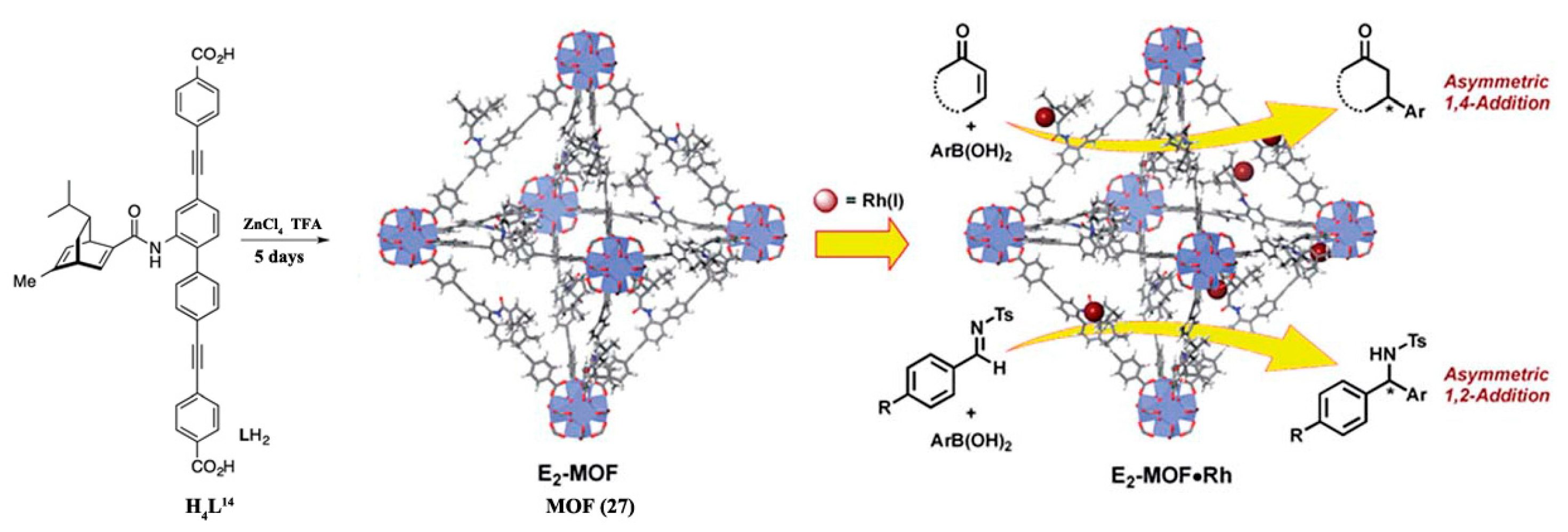
3.1.1. 1,4-, 1,2-, and Cyclo-Addition Reactions
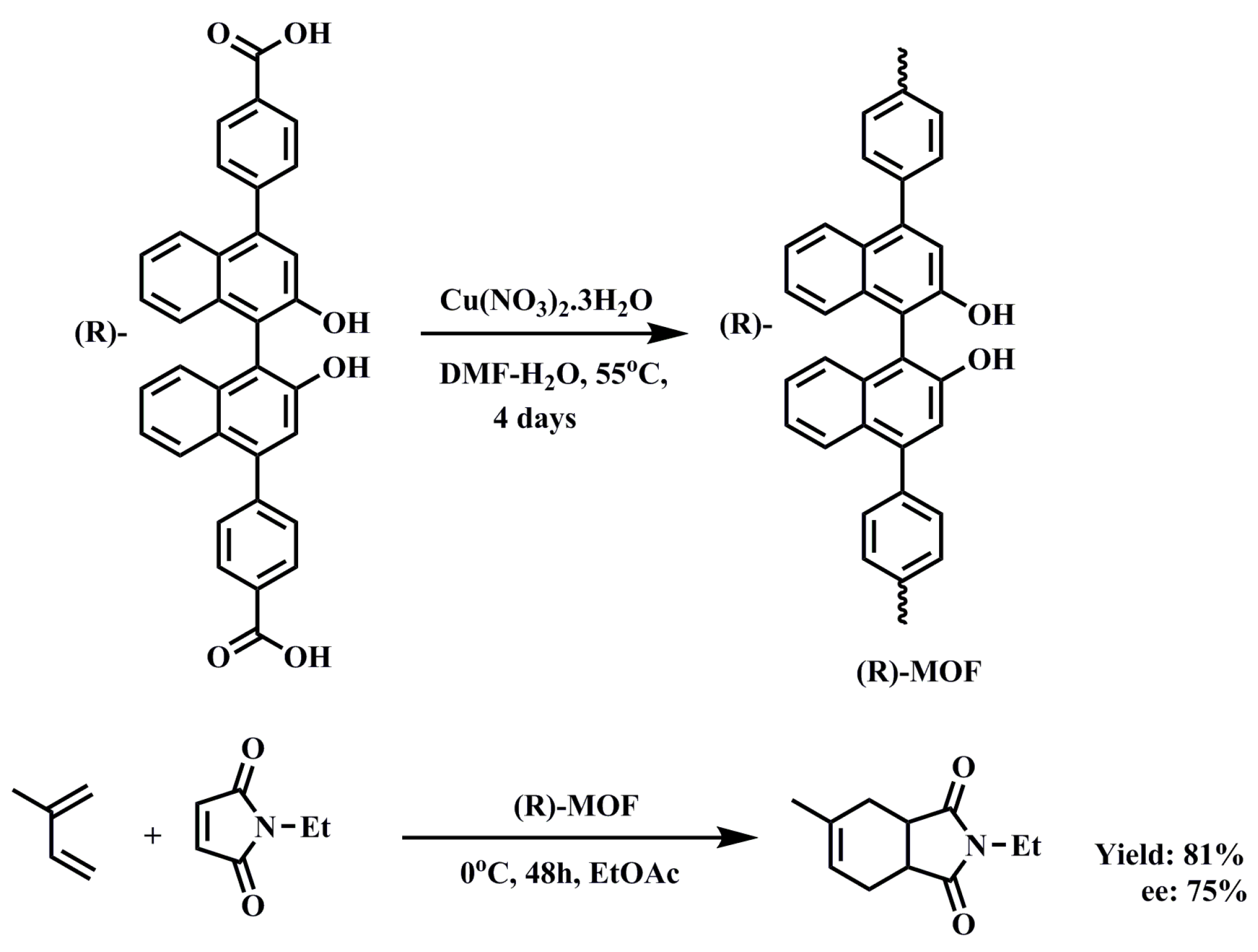
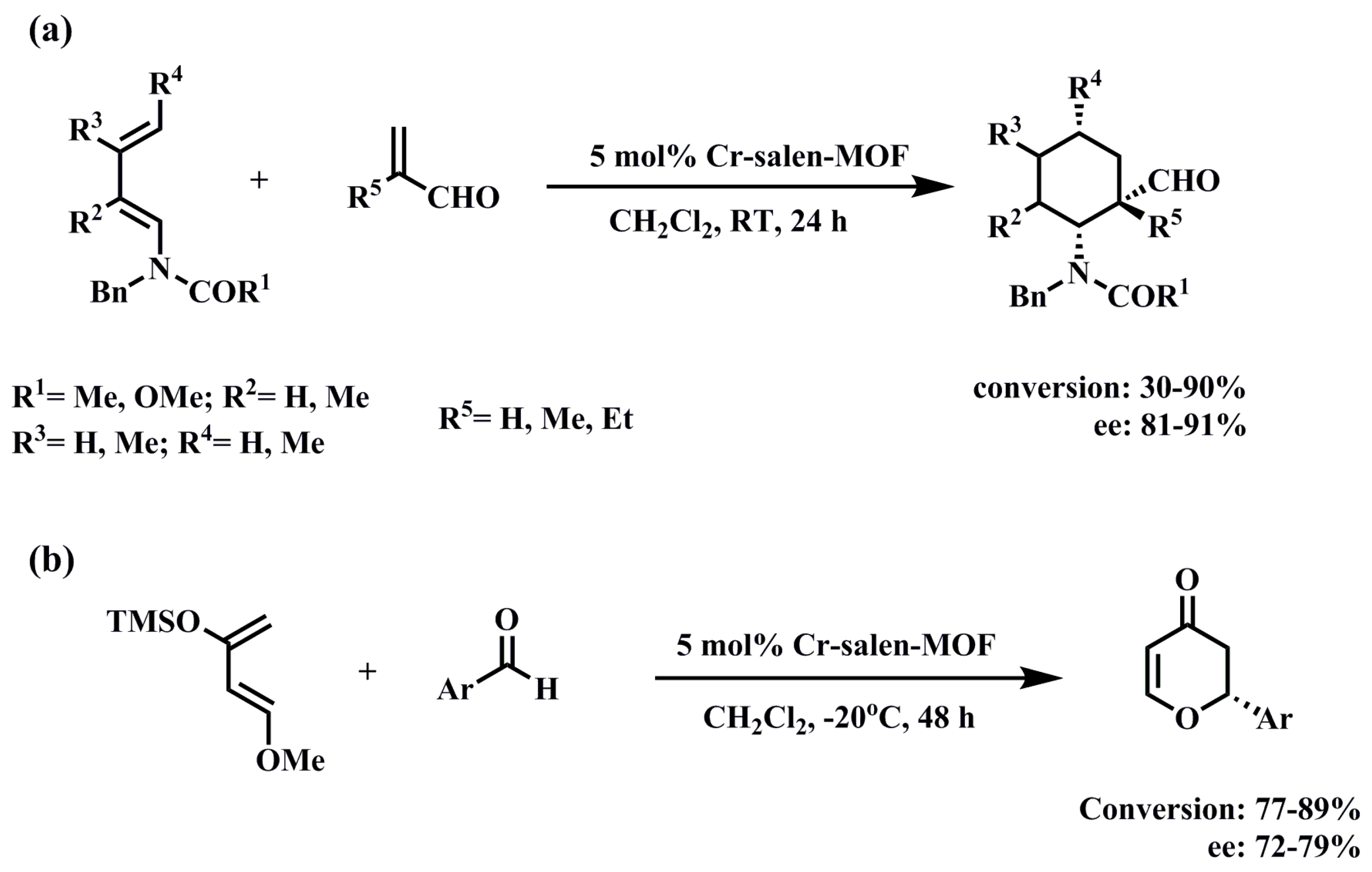
3.1.2. Diels–Alder Reaction
3.1.3. Cyanosilylation of Aldehydes
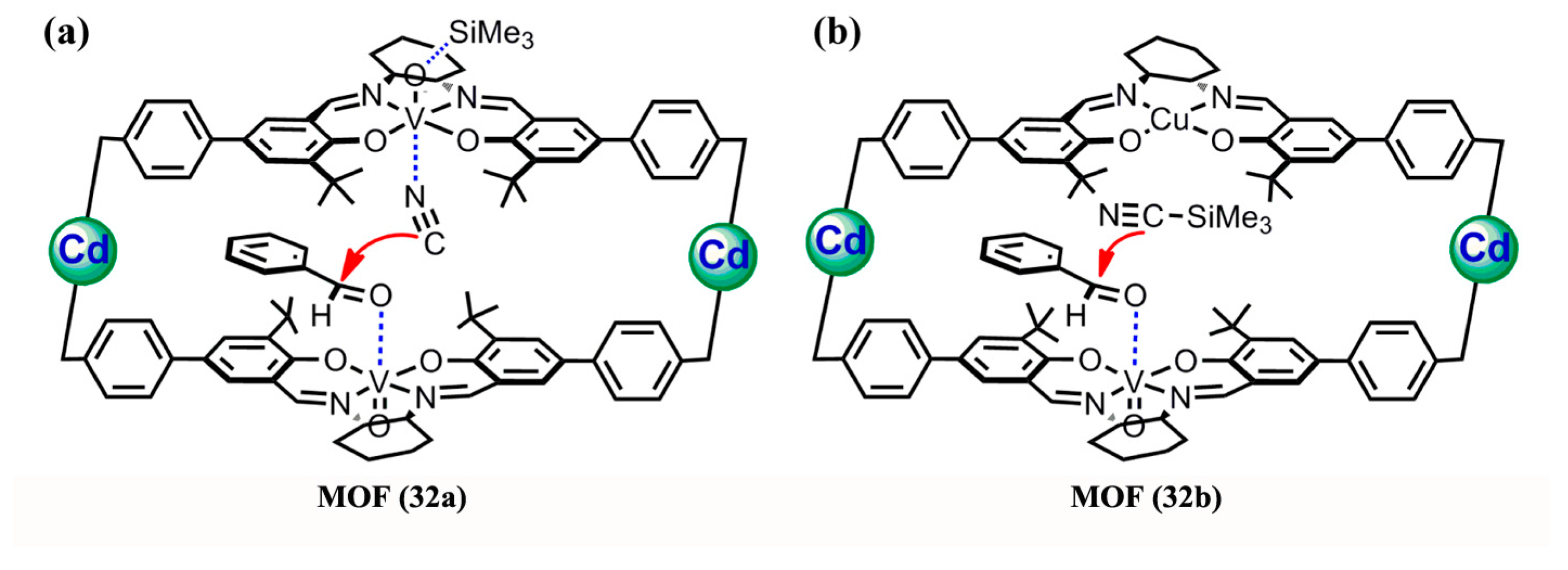
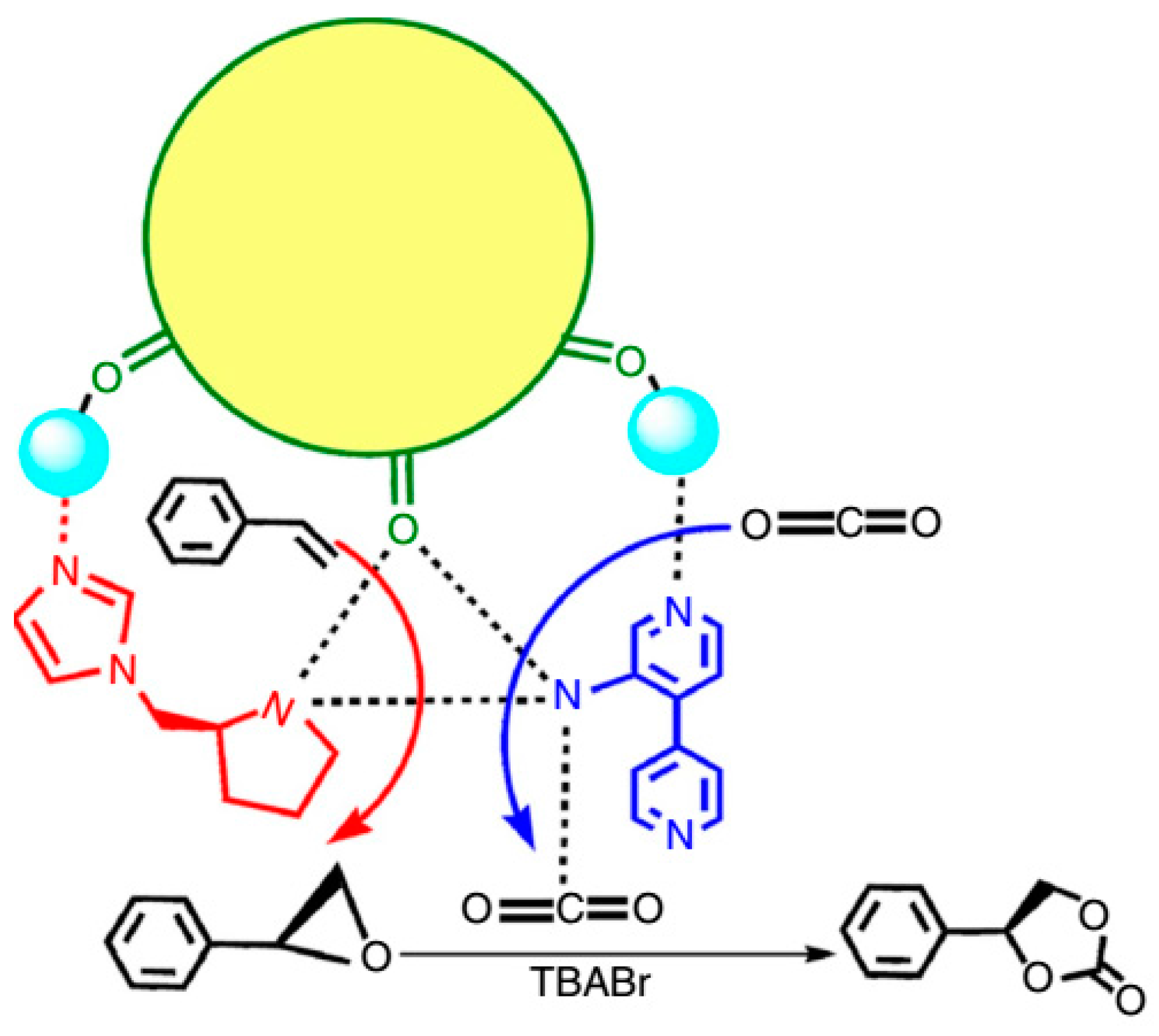
3.1.4. Epoxidation of Alkenes and Cleavage of Epoxide Ring
3.1.5. Aldol Condensation
3.1.6. Imine Reduction
3.2. Enantioselective Separation
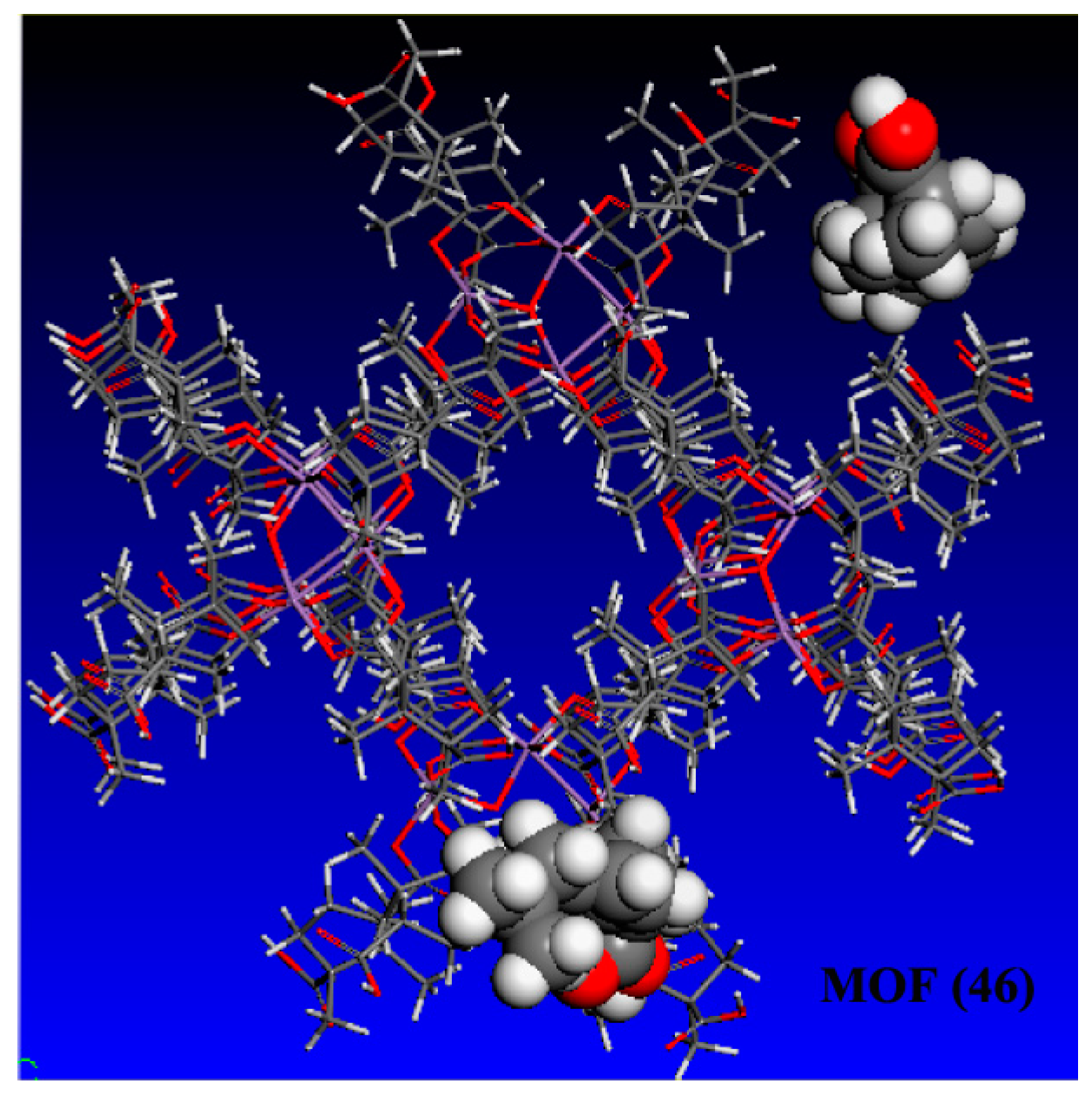
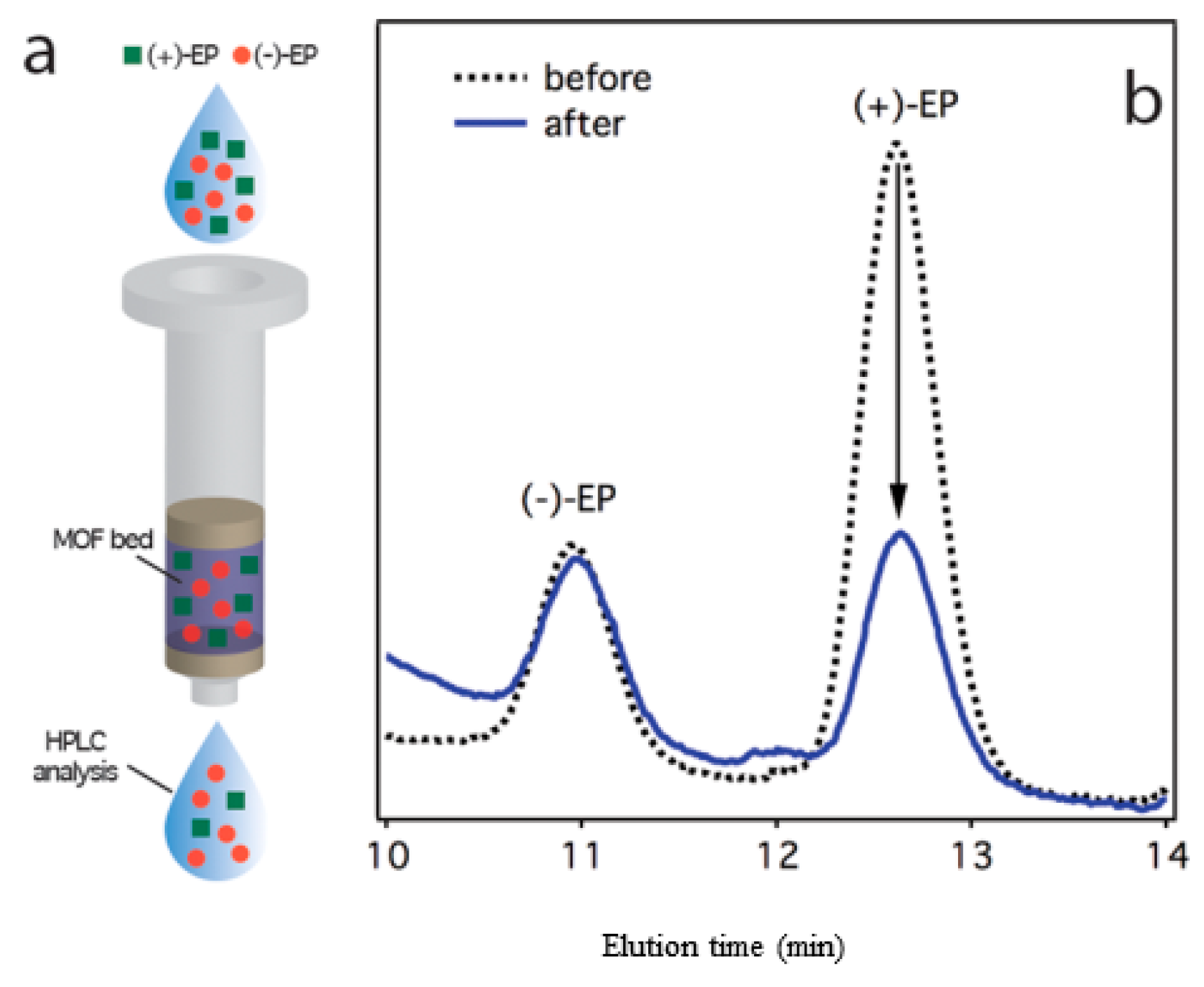
3.2.1. High-performance Liquid Chromatographic (HPLC) Analysis
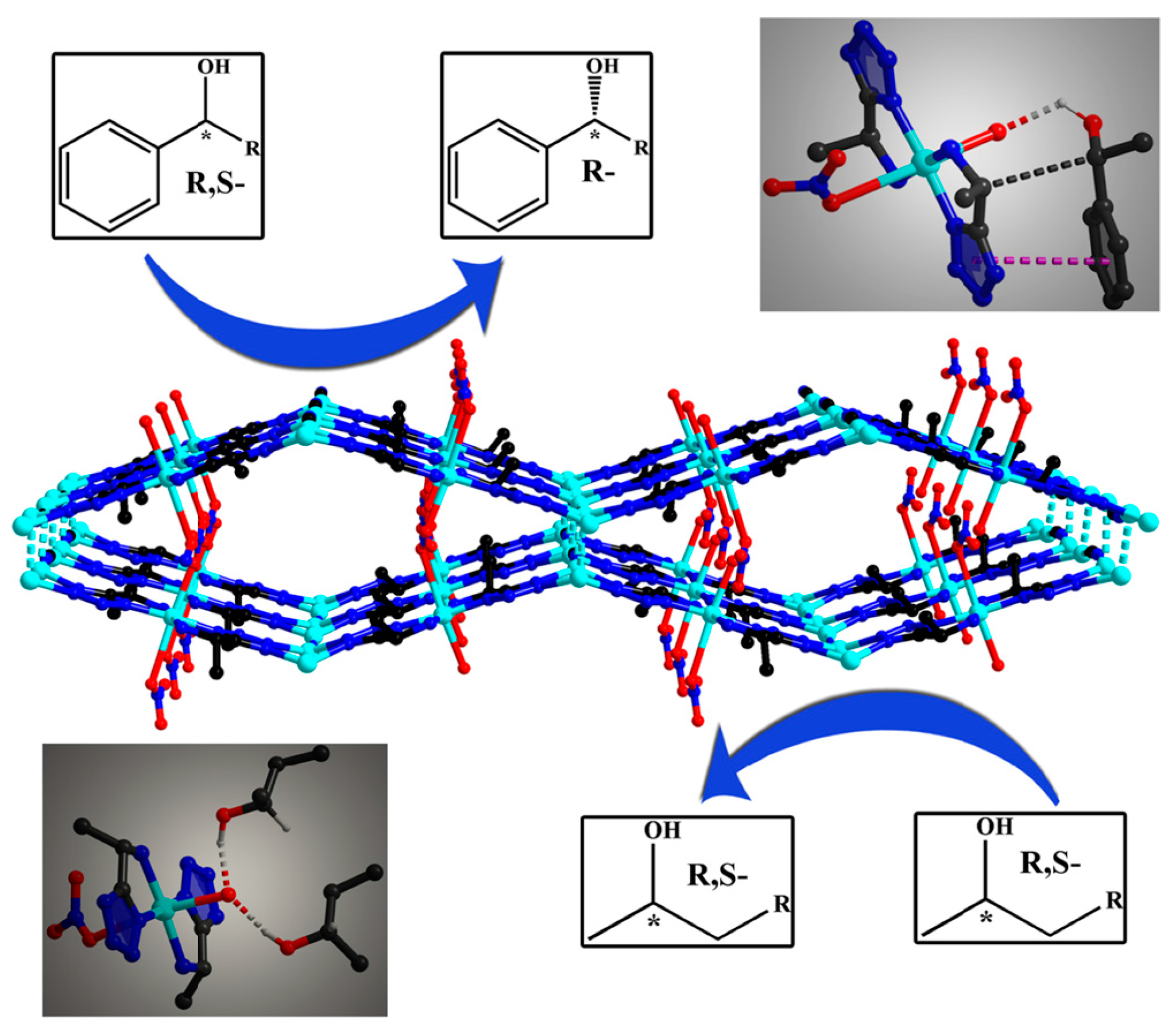
3.2.2. Gas Chromatographic (GC) Analysis
4. Conclusions and Prospects
Acknowledgments
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal–Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.R.; Uemura, T.; Kitagawa, S. Inorganic nanoparticles in porous coordination polymers. Chem. Soc. Rev. 2016, 45, 3828–3845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.-C. Tuning the Topology and Functionality of Metal−Organic Frameworks by Ligand Design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal–Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Li, J.; Xu, Q. Immobilizing Metal Nanoparticles to Metal–Organic Frameworks with Size and Location Control for Optimizing Catalytic Performance. J. Am. Chem. Soc. 2013, 135, 10210–10213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–Organic Frameworks Meet Metal Nanoparticles: Synergistic Effect for Enhanced Catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Zhou, W.; Xu, J.Q.; Chen, B. Porous Metal–Organic Frameworks: Promising Materials for Methane Storage. Chem 2016, 1, 557–580. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; De Vos, D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, C.; Wang, X.; Bai, Y.; Liu, H. Applications of homochiral metal‒organic frameworks in enantioselective adsorption and chromatography separation. Electrophoresis 2014, 35, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.-L.; Xia, Y.; Wang, G.-X.; Feng, Y.-L. A robust luminescent Ba (II) metal–organic framework based on pyridine carboxylate ligand for sensing of small molecules. Inorg. Chem. Commun. 2015, 53, 42–45. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Tian, D.; Gao, Q.; Sun, H.-W.; Xu, J.; Bu, X.-H. The international journal for high quality, original research in inorganic and organometallic chemistry. Dalton Trans. 2016, 45, 845. [Google Scholar]
- Wanderley, M.M.; Wang, C.; Wu, C.-D.; Lin, W. A Chiral Porous Metal‒Organic Framework for Highly Sensitive and Enantioselective Fluorescence Sensing of Amino Alcohols. J. Am. Chem. Soc. 2012, 134, 9050–9053. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Duerinck, T.; Denayer, J.F.M. Metal‒organic frameworks as stationary phases for chiral chromatographic and membrane separations. Chem. Eng. Sci. 2015, 124, 179–187. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.-Q.; Zhong, X.-H.; Wu, L.-H.; Liu, G.-D.; Sheng, N. Nontoxic and renewable metal–organic framework based on [α]-cyclodextrin with efficient drug delivery. RSC Adv. 2016, 6, 82977–82983. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Thomas, K.G.; Liz-Marzan, L.M. Nanoscale chirality in metal and semiconductor nanoparticles. Chem. Commun. 2016, 52, 12555–12569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Duan, X.; Yao, S.; Wang, Z.; Lin, Z.; Li, Y.-G.; Long, L.-S.; Wang, E.-B.; Lin, W. Cation-mediated optical resolution and anticancer activity of chiral polyoxometalates built from entirely achiral building blocks. Chem. Sci. 2016, 7, 4220–4229. [Google Scholar] [CrossRef]
- Burneo Saavedra, I.P. Metal‒Organic Frameworks Made of Amino Acids and Adenine: Chirality and Hydrochromism. Available online: http://hdl.handle.net/10803/457357 (accessed on 18 March 2018).
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral Metal–Organic Frameworks for Asymmetric Heterogeneous Catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wang, C.; Lin, W. A chiral metal–organic framework for sequential asymmetric catalysis. Chem. Commun. 2011, 47, 8256–8258. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, M.; Lin, W. Asymmetric Catalysis with Chiral Porous Metal‒Organic Frameworks: Critical Issues. J. Phys. Chem. Lett. 2011, 2, 1701–1709. [Google Scholar] [CrossRef]
- Leus, K.; Liu, Y.Y.; Voort, P.V.D. Metal‒Organic Frameworks as Selective or Chiral Oxidation Catalysts. Catal. Rev. Sci. Eng. 2014, 56, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Shinde, S.; Pan, J.; Ma, Y.; Yan, Y.; Pan, G. Interface-induced growth of boronate-based metal‒organic framework membrane on porous carbon substrate for aqueous phase molecular recognition. Chem. Eng. J. 2017, 324, 216–227. [Google Scholar] [CrossRef]
- Mendiratta, S.; Lee, C.-H.; Lee, S.-Y.; Kao, Y.-C.; Chang, B.-C.; Lo, Y.-H.; Lu, K.-L. Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal–Organic Framework. Molecules 2015, 20, 8941–8951. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Xu, H.; Zang, S.-Q.; Mak, T.C.W. A viologen-functionalized chiral Eu-MOF as a platform for multifunctional switchable material. Chem. Commun. 2016, 52, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Liu, Q.; Liu, L.-H.; Ding, L.; Chen, Y.-P. Two 3D nonlinear optical and luminescent lanthanide–organic frameworks with multidirectional helical intersecting channels. New J. Chem. 2017, 41, 6736–6741. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, M.; Duan, A.-H.; Zhang, J.-H.; Yang, R.; Yuan, L.-M. Homochiral metal–organic framework used as a stationary phase for high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, Y.; Guang, J.; Weng, L.; Zhao, J.C.-G.; Xiang, S.; Chen, B. A Homochiral Microporous Hydrogen-Bonded Organic Framework for Highly Enantioselective Separation of Secondary Alcohols. J. Am. Chem. Soc. 2014, 136, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Cavazzini, A.; Pasti, L.; Massi, A.; Marchetti, N.; Dondi, F. Recent applications in chiral high performance liquid chromatography: A review. Anal. Chim. Acta 2011, 706, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, X.; Bai, Y.; Liu, H. Applications of nanomaterials in enantioseparation and related techniques. Trends Anal. Chem. 2012, 39, 195–206. [Google Scholar] [CrossRef]
- Lin, W. Asymmetric Catalysis with Chiral Porous Metal–Organic Frameworks. Top. Catal. 2010, 53, 869–875. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-G.; Zhan, C.; Zhang, J.; Bu, X. Chiral chemistry of metal–camphorate frameworks. Chem. Soc. Rev. 2016, 45, 3122–3144. [Google Scholar] [CrossRef] [PubMed]
- Nickerl, G.; Henschel, A.; Grünker, R.; Gedrich, K.; Kaskel, S. Chiral Metal–Organic Frameworks and Their Application in Asymmetric Catalysis and Stereoselective Separation. Chem. Ing. Tech. 2011, 83, 90–103. [Google Scholar] [CrossRef]
- Bisht, K.K.; Parmar, B.; Rachuri, Y.; Kathalikattil, A.C.; Suresh, E. Progress in the synthetic and functional aspects of chiral metal–organic frameworks. CrystEngComm 2015, 17, 5341–5356. [Google Scholar] [CrossRef]
- Han, Z.; Shi, W.; Cheng, P. Synthetic strategies for chiral metal–organic frameworks. Chin. Chem. Lett. 2017. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal–organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982. [Google Scholar] [CrossRef] [PubMed]
- Ingleson, M.J.; Barrio, J.P.; Bacsa, J.; Dickinson, C.; Park, H.; Rosseinsky, M.J. Generation of a solid Bronsted acid site in a chiral framework. Chem. Commun. 2008, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Rebilly, J.-N.; Bacsa, J.; Rosseinsky, M.J. 1 D Tubular and 2 D Metal–Organic Frameworks Based on a Flexible Amino Acid Derived Organic Spacer. Chem. Asian J. 2009, 4, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Sartor, M.; Stein, T.; Hoffmann, F.; Fröba, M. A New Set of Isoreticular, Homochiral Metal–Organic Frameworks with ucp Topology. Chem. Mater. 2016, 28, 519–528. [Google Scholar] [CrossRef]
- Zhu, Q.; Sheng, T.; Fu, R.; Tan, C.; Hu, S.; Wu, X. Two luminescent enantiomorphic 3D metal–organic frameworks with 3D homochiral double helices. Chem. Commun. 2010, 46, 9001–9003. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, H.; Miao, H.; Wang, J.; Xu, Y. Synthesis, structures and properties of two new chiral rare earth–organic frameworks constructed by l/d-tartaric acid. J. Solid State Chem. 2015, 229, 208–212. [Google Scholar] [CrossRef]
- Ren, W.X.; Jian, L.Z.; Wei, G.; Yan, L.; Zhan, L.B.; Yong, C. A Novel TADDOL-based Chiral Metal–organic Framework: Synthesis, Structure and Photoluminescence Study. Chin. J. Struct. Chem. 2016, 35, 1399–1405. [Google Scholar]
- Evans, O.R.; Ngo, H.L.; Lin, W. Chiral Porous Solids Based on Lamellar Lanthanide Phosphonates. J. Am. Chem. Soc. 2001, 123, 10395–10396. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Falkowski, J.M.; Abney, C.; Lin, W. A series of isoreticular chiral metal–organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem. 2010, 2, 838. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.D.; Hu, A.; Zhang, L.; Lin, W. A Homochiral Porous Metal−Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Qi, B.; Ren, W.; He, C.; Niu, J.; Duan, C. Polyoxometalate-based homochiral metal–organic frameworks for tandem asymmetric transformation of cyclic carbonates from olefins. Nat. Commun. 2015, 6, 10007. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Ye, S.; Li, X.; Ma, Y.; Zhang, C.; Tang, B. A new type of surface-enhanced Raman scattering sensor for the enantioselective recognition of d/l-cysteine and d/l-asparagine based on a helically arranged Ag NPs@homochiral MOF. Chem. Commun. 2016, 52, 5432–5435. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Wang, F.; Gu, Z.-G.; Zhang, J. Synthesis of homochiral zeolitic metal–organic frameworks with amino acid and tetrazolates for chiral recognition. RSC Adv. 2017, 7, 4872–4875. [Google Scholar] [CrossRef]
- Gupta, A.K.; De, D.; Katoch, R.; Garg, A.; Bharadwaj, P.K. Synthesis of a NbO Type Homochiral Cu(II) Metal–Organic Framework: Ferroelectric Behavior and Heterogeneous Catalysis of Three-Component Coupling and Pechmann Reactions. Inorg. Chem. 2017, 56, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Trickett, C.A.; Alahmadi, S.B.; Alshammari, A.S.; Yaghi, O.M. Calcium l-Lactate Frameworks as Naturally Degradable Carriers for Pesticides. J. Am. Chem. Soc. 2017, 139, 8118–8121. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.; Audebrand, N.; Poriel, C.; Canivet, J.; Calvez, G.; Roisnel, T.; Dorcet, V.; Roussel, P. A series of chiral metal–organic frameworks based on fluorene di- and tetra-carboxylates: Syntheses, crystal structures and luminescence properties. CrystEngComm 2017, 19, 2042–2056. [Google Scholar] [CrossRef]
- Kutzscher, C.; Janssen-Muller, D.; Notzon, A.; Stoeck, U.; Bon, V.; Senkovska, I.; Kaskel, S.; Glorius, F. Synthesis of the homochiral metal–organic framework DUT-129 based on a chiral dicarboxylate linker with 6 stereocenters. CrystEngComm 2017, 19, 2494–2499. [Google Scholar] [CrossRef]
- Cho, S.-H.; Ma, B.; Nguyen, S.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. A metal–organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem. Commun. 2006, 2563–2565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Yuan, G.; Xia, Q.; Yuan, C.; Cui, Y. Chiral Cu(salen)-Based Metal–Organic Framework for Heterogeneously Catalyzed Aziridination and Amination of Olefins. Inorg. Chem. 2016, 55, 12500–12503. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Ren, Y.; Jiang, H. A Ni(salen)-Based Metal–Organic Framework: Synthesis, Structure, and Catalytic Performance for CO2 Cycloaddition with Epoxides. Eur. J. Inorg. Chem. 2017, 4982–4989. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Qi, C.; Jiang, H. A chiral salen-based MOF catalytic material with high thermal, aqueous and chemical stabilities. Dalton Trans. 2017, 46, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, Z.; Tan, C.; Liu, Y.; Gong, W.; Cui, Y. Multivariate Metal–Organic Frameworks as Multifunctional Heterogeneous Asymmetric Catalysts for Sequential Reactions. J. Am. Chem. Soc. 2017, 139, 8259–8266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Tandem Modification of Metal–Organic Frameworks by a Postsynthetic Approach. Angew. Chem. Int. Ed. 2008, 47, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Cohen, S.M. Engineering a Metal–Organic Framework Catalyst by Using Postsynthetic Modification. Angew. Chem. Int. Ed. 2009, 48, 7424–7427. [Google Scholar] [CrossRef] [PubMed]
- Garibay, S.J.; Wang, Z.; Tanabe, K.K.; Cohen, S.M. Postsynthetic Modification: A Versatile Approach toward Multifunctional Metal–Organic Frameworks. Inorg. Chem. 2009, 48, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, J.; Legrand, A.; Quadrelli, E.A.; Canivet, J.; Farrusseng, D. Enantiopure Peptide-Functionalized Metal–Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 9409–9416. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Luo, R.; Li, M.; Wen, M.; Li, Y.; Chen, C.; Zhang, N. Salen(Co(III)) imprisoned within pores of a metal–organic framework by post-synthetic modification and its asymmetric catalysis for CO2 fixation at room temperature. Chem. Commun. 2017, 53, 10930–10933. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, T.; Wang, Y.; Liu, S.; Tong, J.; Liu, B. Superficial Chiral Etching on Achiral Metal–Organic Framework for Enantioselective Sorption. ACS Appl. Mater. Interfaces 2017, 9, 32264–32269. [Google Scholar] [CrossRef] [PubMed]
- Ezuhara, T.; Endo, K.; Aoyama, Y. Helical Coordination Polymers from Achiral Components in Crystals. Homochiral Crystallization, Homochiral Helix Winding in the Solid State, and Chirality Control by Seeding. J. Am. Chem. Soc. 1999, 121, 3279–3283. [Google Scholar] [CrossRef]
- Li, Z.; Du, L.; Zhou, J.; Li, L.; Hu, Y.; Qiao, Y.; Xie, M.; Zhao, Q. A chiral porous cobalt-organic framework based on reinforced sinusoidal-like SBUs involving in situ-generated formate. New J. Chem. 2013, 37, 2473–2478. [Google Scholar] [CrossRef]
- Durá, G.; Carrión, M.C.; Jalón, F.A.; Rodríguez, A.M.; Manzano, B.R. Self-Assembly of Silver(I) and Ditopic Heteroscorpionate Ligands. Spontaneous Chiral Resolution in Helices and Sequence Isomerism in Coordination Polymers. Cryst. Growth Des. 2013, 13, 3275–3282. [Google Scholar] [CrossRef]
- Liu, W.; Bao, X.; Mao, L.-L.; Tucek, J.; Zboril, R.; Liu, J.-L.; Guo, F.-S.; Ni, Z.-P.; Tong, M.-L. A chiral spin crossover metal–organic framework. Chem. Commun. 2014, 50, 4059–4061. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, A.-L.; Wang, K.; Yi, X.-C.; Gao, E.-Q. Chiral or achiral: Four isomeric Cd(II) coordination polymers based on phenylenediacrylate ligands. CrystEngComm 2015, 17, 1389–1397. [Google Scholar] [CrossRef]
- Mei, H.-X.; Zhang, T.; Wang, D.-F.; Huang, R.-B.; Zheng, L.-S. A Zn-oxalate helix linked by a water helix: Spontaneous chiral resolution of a Zn helical coordination polymer. New J. Chem. 2015, 39, 2075–2080. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Li, X.; Guo, X. Solvent-Mediated Transformation from Achiral to Chiral Nickel(II) Metal–Organic Frameworks and Reassembly in Solution. Chem. Eur. J. 2015, 21, 16593–16600. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; De, D.; Tomar, K.; Bharadwaj, P.K. Chiral Cadmium(II) Metal–Organic Framework from an Achiral Ligand by Spontaneous Resolution: An Efficient Heterogeneous Catalyst for the Strecker Reaction of Ketones. Inorg. Chem. 2017, 56, 13629–13633. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Slawin, A.M.Z.; Morris, R.E. Chiral Induction in the Ionothermal Synthesis of a 3-D Coordination Polymer. J. Am. Chem. Soc. 2007, 129, 4880–4881. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Bu, X. Induction of chiral porous solids containing only achiral building blocks. Nat. Chem. 2010, 2, 353. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; He, C.; Dong, D.; Yang, L.; Duan, C. Homochiral Crystallization of Metal–Organic Silver Frameworks: Asymmetric [3+2] Cycloaddition of an Azomethine Ylide. Angew. Chem. Int. Ed. 2012, 51, 10127–10131. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.K.; Suresh, E. Spontaneous Resolution to Absolute Chiral Induction: Pseudo-Kagomé Type Homochiral Zn(II)/Co(II) Coordination Polymers with Achiral Precursors. J. Am. Chem. Soc. 2013, 135, 15690–15693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Li, D.; Guo, D.; Zhang, H.; Shi, W.; Cheng, P.; Wojtas, L.; Zaworotko, M.J. Synthesis of a Chiral Crystal Form of MOF-5, CMOF-5, by Chiral Induction. J. Am. Chem. Soc. 2015, 137, 15406–15409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Weng, Z.; Wang, Y.; Chen, L.; Sheng, D.; Liu, Y.; Diwu, J.; Chai, Z.; Albrecht-Schmitt, T.E.; Wang, S. Centrosymmetric and chiral porous thorium organic frameworks exhibiting uncommon thorium coordination environments. Dalton Trans. 2015, 44, 20867–20873. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-Q.; Chen, D.-Q.; Ji, Z.; Tang, J.; Wang, X.-L.; Zang, H.-Y.; Su, Z.-M. Control of bulk homochirality and proton conductivity in isostructural chiral metal–organic frameworks. Chem. Commun. 2017, 53, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-T.; Cai, Z.-W.; Ye, Q.-Y.; Weng, C.-H.; Huang, X.-H.; Hu, X.-L.; Huang, C.-C.; Zhuang, N.-F. Enantioselective Synthesis of a Chiral Coordination Polymer with Circularly Polarized Visible Laser. Angew. Chem. Int. Ed. 2014, 53, 12860–12864. [Google Scholar] [CrossRef] [PubMed]
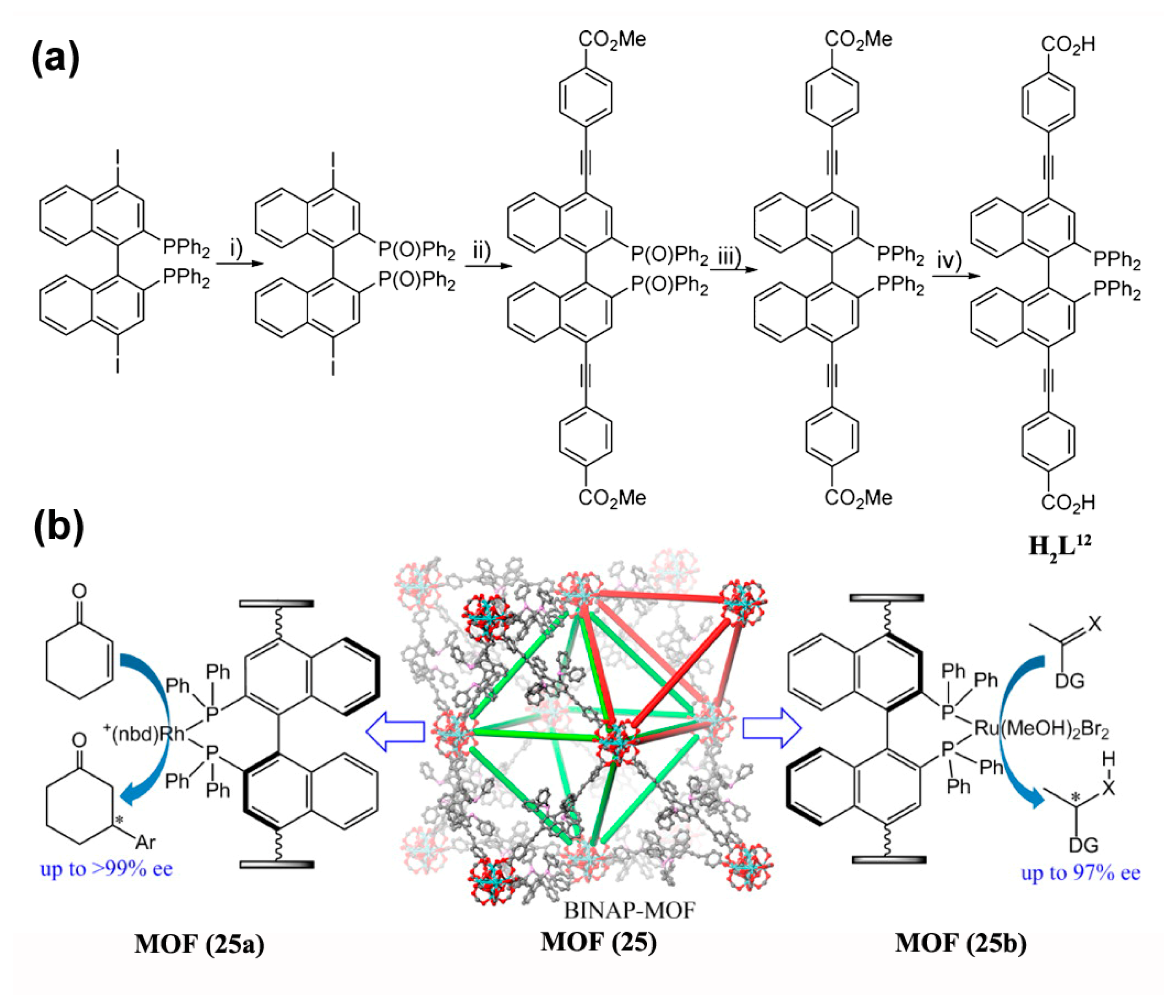
- Falkowski, J.M.; Sawano, T.; Zhang, T.; Tsun, G.; Chen, Y.; Lockard, J.V.; Lin, W. Privileged Phosphine-Based Metal–Organic Frameworks for Broad-Scope Asymmetric Catalysis. J. Am. Chem. Soc. 2014, 136, 5213–5216. [Google Scholar] [CrossRef] [PubMed]
- Sawano, T.; Thacker, N.C.; Lin, Z.; McIsaac, A.R.; Lin, W. Robust, Chiral, and Porous BINAP-Based Metal–Organic Frameworks for Highly Enantioselective Cyclization Reactions. J. Am. Chem. Soc. 2015, 137, 12241–12248. [Google Scholar] [CrossRef] [PubMed]
- Sawano, T.; Ji, P.; McIsaac, A.R.; Lin, Z.; Abney, C.W.; Lin, W. The first chiral diene-based metal–organic frameworks for highly enantioselective carbon-carbon bond formation reactions. Chem. Sci. 2015, 6, 7163–7168. [Google Scholar] [CrossRef]
- Lee, M.; Shin, S.M.; Jeong, N.; Thallapally, P.K. Chiral environment of catalytic sites in the chiral metal–organic frameworks. Dalton Trans. 2015, 44, 9349–9352. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nagase, S.; Anami, T.; Wierzbicki, M.; Urbanczyk-Lipkowska, Z. Enantioselective Diels-Alder reaction in the confined space of homochiral metal–organic frameworks. RSC Adv. 2016, 6, 111436–111439. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Li, Z.; Gong, W.; Cui, Y. A Cr(salen)-based metal–organic framework as a versatile catalyst for efficient asymmetric transformations. Chem. Commun. 2016, 52, 13167–13170. [Google Scholar] [CrossRef] [PubMed]
- Mo, K.; Yang, Y.; Cui, Y. A Homochiral Metal–Organic Framework as an Effective Asymmetric Catalyst for Cyanohydrin Synthesis. J. Am. Chem. Soc. 2014, 136, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xia, Q.; Chen, X.; Liu, Y.; Du, X.; Cui, Y. Chiral Metal–Organic Framework as a Platform for Cooperative Catalysis in Asymmetric Cyanosilylation of Aldehydes. ACS Catal. 2016, 6, 7590–7596. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Qi, C.; Jiang, H. The first porphyrin-salen based chiral metal–organic framework for asymmetric cyanosilylation of aldehydes. Chem. Commun. 2017, 53, 8223–8226. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Xia, Q.; Cui, Y. Chiral binary metal–organic frameworks for asymmetric sequential reactions. Chem. Commun. 2017, 53, 12313–12316. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Zhang, Z.; Bao, Z.; Xing, H.; Yang, Q.; Ren, Q. MIL-101(Cr) as a synergistic catalyst for the reduction of imines with trichlorosilane. Mol. Catal. 2018, 445, 163–169. [Google Scholar] [CrossRef]
- Ran, R.; You, L.; Di, B.; Hao, W.; Su, M.; Yan, F.; Huang, L. A novel chiral mesoporous binaphthyl-silicas: Preparation, characterization, and application in HPLC. J. Sep. Sci. 2012, 35, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Nuzhdin, A.L.; Dybtsev, D.N.; Bryliakov, K.P.; Talsi, E.P.; Fedin, V.P. Enantioselective Chromatographic Resolution and One-Pot Synthesis of Enantiomerically Pure Sulfoxides over a Homochiral Zn-Organic Framework. J. Am. Chem. Soc. 2007, 129, 12958–12959. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gong, T.; Zhang, K.; Lin, X.; Liu, Y.; Jiang, J.; Cui, Y. Engineering chiral porous metal–organic frameworks for enantioselective adsorption and separation. Nat. Commun. 2014, 5, 4406. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Ma, Y.; Su, H.; Zhang, J.; Dong, Y.-B.; Tang, B. High-Performance Liquid Chromatographic Enantioseparation of Racemic Drugs Based on Homochiral Metal–Organic Framework. Anal. Chem. 2014, 86, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, J.-H.; Zhang, Y.; Wang, B.-J.; Xie, S.-M.; Yuan, L.-M. Chromatographic study on the high performance separation ability of a homochiral [Cu2(d-Cam)2(4,4′-bpy)]n based-column by using racemates and positional isomers as test probes. J. Chromatogr. A 2014, 1325, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kutzscher, C.; Hoffmann, H.C.; Krause, S.; Stoeck, U.; Senkovska, I.; Brunner, E.; Kaskel, S. Proline Functionalization of the Mesoporous Metal–Organic Framework DUT-32. Inorg. Chem. 2015, 54, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Muraoka, T.; Otubo, Y.; Takahashi, H.; Ohnishi, A. HPLC enantioseparation on a homochiral MOF-silica composite as a novel chiral stationary phase. RSC Adv. 2016, 6, 21293–21301. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zhang, J.; Kong, J.; Yuan, L. A 3D Homochiral MOF [Cd2(d-cam)3]·2Hdma·4dma for HPLC Chromatographic Enantioseparation. Chirality 2016, 28, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Nong, R.-Y.; Xie, S.-M.; Wang, B.-J.; Ai, P.; Yuan, L.-M. Homochiral metal–organic frameworks based on amino acid ligands for HPLC separation of enantiomers. Electrophoresis 2017, 38, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Hartlieb, K.J.; Holcroft, J.M.; Moghadam, P.Z.; Vermeulen, N.A.; Algaradah, M.M.; Nassar, M.S.; Botros, Y.Y.; Snurr, R.Q.; Stoddart, J.F. CD-MOF: A Versatile Separation Medium. J. Am. Chem. Soc. 2016, 138, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Gong, W.; Han, X.; Liu, Y.; Cui, Y. Chiral DHIP-Based Metal–Organic Frameworks for Enantioselective Recognition and Separation. Inorg. Chem. 2016, 55, 7229–7232. [Google Scholar] [CrossRef] [PubMed]
- Hailili, R.; Wang, L.; Qv, J.; Yao, R.; Zhang, X.-M.; Liu, H. Planar Mn4O Cluster Homochiral Metal–Organic Framework for HPLC Separation of Pharmaceutically Important (±)-Ibuprofen Racemate. Inorg. Chem. 2015, 54, 3713–3715. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sánchez, J.; Argente-García, A.I.; Moliner-Martínez, Y.; Roca-Sanjuán, D.; Antypov, D.; Campíns-Falcó, P.; Rosseinsky, M.J.; Martí-Gastaldo, C. Peptide Metal–Organic Frameworks for Enantioselective Separation of Chiral Drugs. J. Am. Chem. Soc. 2017, 139, 4294–4297. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-M.; Zhang, Z.-J.; Wang, Z.-Y.; Yuan, L.-M. Chiral Metal–Organic Frameworks for High-Resolution Gas Chromatographic Separations. J. Am. Chem. Soc. 2011, 133, 11892–11895. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, S.-M.; Ai, P.; Zhang, J.-H.; Zhang, M.; Yuan, L.-M. Metal–Organic Framework Co(D-Cam)1/2(bdc)1/2(tmdpy) for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in Gas Chromatography. ChemPlusChem 2014, 79, 1103–1108. [Google Scholar] [CrossRef]
- Yang, J.-R.; Xie, S.-M.; Liu, H.; Zhang, J.-H.; Yuan, L.-M. Metal–Organic Framework InH(d-C10H14O4)2 for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in GC. Chromatographia 2015, 78, 557–564. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, M.; Xie, S.; Yuan, L. Homochiral metal–organic framework [Zn2(d-Cam)2(4,4′-bpy)]n for high-resolution gas chromatographic separations. Acta Chromatogr. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Yang, J.-R.; Xie, S.-M.; Zhang, J.-H.; Chen, L.; Nong, R.-Y.; Yuan, L.-M. Metal–Organic Framework [Cd(LTP)2]n for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in GC. J. Chromatogr. Sci. 2016, 54, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Yao, R.-X.; Zhang, X.-M. Fourfold-Interpenetrated MOF [Ni(pybz)2] as Coating Material in Gas Chromatographic Capillary Column for Separation. Inorg. Chem. 2017, 56, 8912–8919. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Liu, L.-Y.; Zhang, J. Interpenetrated Three-Dimensional Copper–Iodine Cluster-Based Framework with Enantiopure Porphyrin-like Templates. Inorg. Chem. 2016, 55, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.I.; Shengming, X.I.E.; Junhui, Z.; Ling, C.; Pengjing, Z.; Liming, Y. A Gas Chromatographic Stationary of Homochiral Metal-peptide Framework Material and Its Applications. Chem. Res. Chin. Univ. 2017, 33, 24–30. [Google Scholar]
- Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Multivariate Chiral Covalent Organic Frameworks with Controlled Crystallinity and Stability for Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8277–8285. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xia, Q.; Huang, J.; Liu, Y.; Tan, C.; Cui, Y. Chiral Covalent Organic Frameworks with High Chemical Stability for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8693–8697. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-J.; Lü, J.; Li, L.; Li, H.-F.; Cao, R. Defect porous organic frameworks (dPOFs) as a platform for chiral organocatalysis. J. Catal. 2017, 355, 131–138. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharjee, S.; Khan, M.I.; Li, X.; Zhu, Q.-L.; Wu, X.-T. Recent Progress in Asymmetric Catalysis and Chromatographic Separation by Chiral Metal–Organic Frameworks. Catalysts 2018, 8, 120. https://doi.org/10.3390/catal8030120
Bhattacharjee S, Khan MI, Li X, Zhu Q-L, Wu X-T. Recent Progress in Asymmetric Catalysis and Chromatographic Separation by Chiral Metal–Organic Frameworks. Catalysts. 2018; 8(3):120. https://doi.org/10.3390/catal8030120
Chicago/Turabian StyleBhattacharjee, Suchandra, Muhammad Imran Khan, Xiaofang Li, Qi-Long Zhu, and Xin-Tao Wu. 2018. "Recent Progress in Asymmetric Catalysis and Chromatographic Separation by Chiral Metal–Organic Frameworks" Catalysts 8, no. 3: 120. https://doi.org/10.3390/catal8030120




