Single-Atom Mn Active Site in a Triol-Stabilized β-Anderson Manganohexamolybdate for Enhanced Catalytic Activity towards Adipic Acid Production
Abstract
:1. Introduction
2. Results and Discussion
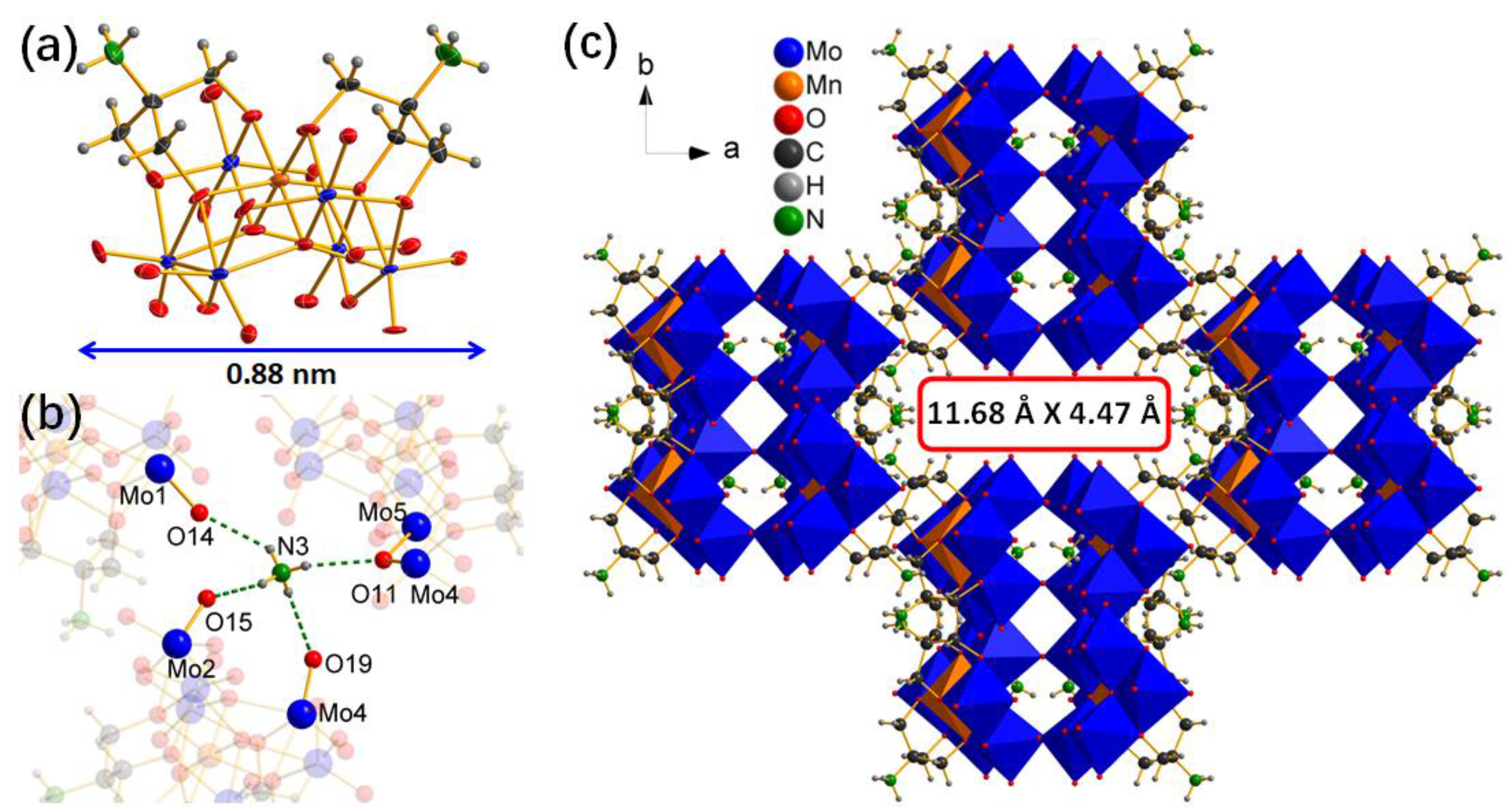
2.1. The Structure Characterizations of Catalyst 1
2.2. Cyclohexanone Oxidation Reaction
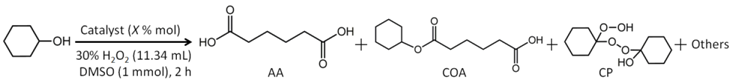
2.3. Cyclohexanol Oxidation Reaction
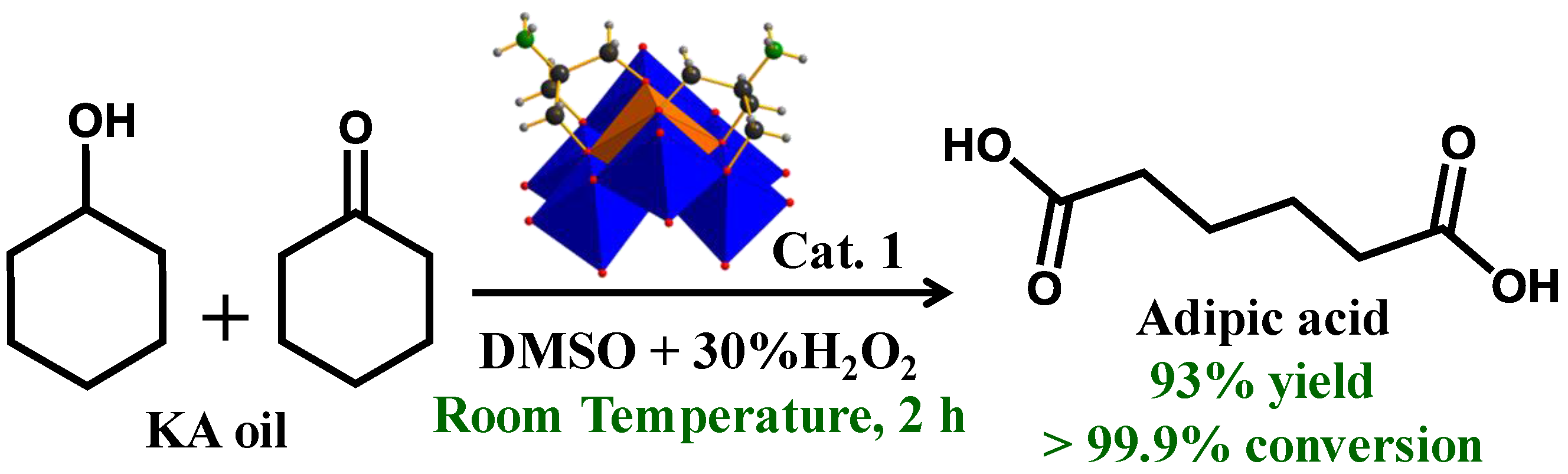
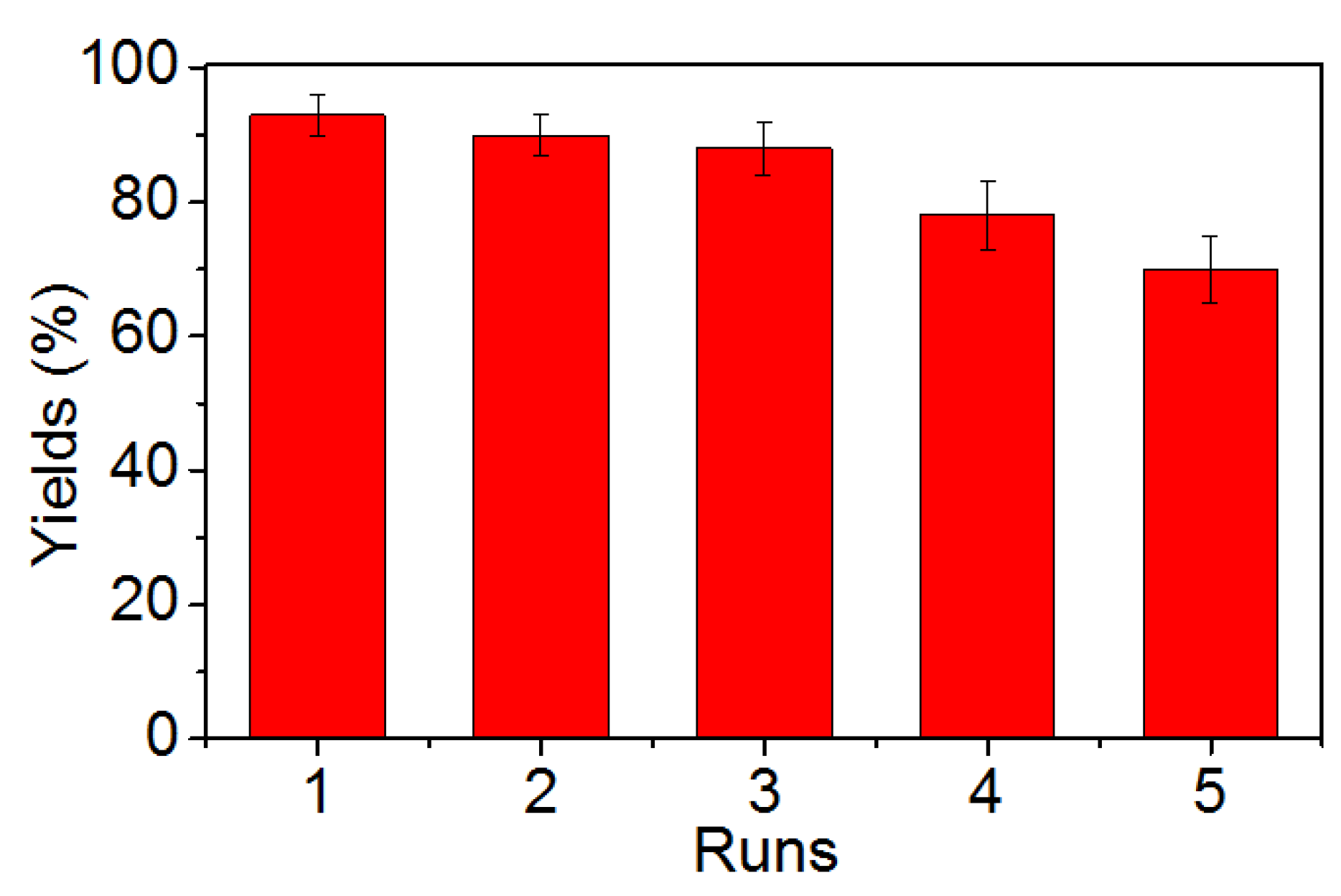
2.4. KA Oil Oxidation Reaction and Catalyst Recyclability
3. Materials and Methods
3.1. General Methods and Materials
3.2. The Synthesis of (NH4)3[Mn(OH)6Mo6O18] and [TBA]3[Mn(OH)6Mo6O18]
3.3. The Synthesis of Compound 1
3.4. Cyclohexanone, Cyclohexanol, and KA Oil Oxidation
3.5. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davis, D.D.; Kemp, D.R. Adipic Acid. In Kirk–Othmer Encyclopedia of Chemical Technology, 4th ed.; Kroscwitz, J.I., Howe-Grant, M., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1991; Volume 1, pp. 466–493. ISBN 9780471238966. [Google Scholar]
- Wen, Y.; Wang, X.; Wei, H.; Li, B.; Jin, P.; Li, L. A large-scale continuous-flow process for the production of adipic acid via catalytic oxidation of cyclohexene with H2O2. Green Chem. 2012, 14, 2868–2875. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef]
- Adipic Acid (ADPA), 2018 World Market Outlook and Forecast up to 2027; Merchant Research and Consulting: Birmingham, UK, 2018.
- Castellan, A.; Bart, J.C.J.; Cavallaro, S. Industrial production and use of adipic acid. Catal. Today 1991, 9, 237–254. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Fu, Y.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Lu, G. Catalytic Performance of MgO-Supported Co Catalyst for the Liquid Phase Oxidation of Cyclohexane with Molecular Oxygen. Catalysts 2017, 7, 155. [Google Scholar] [CrossRef]
- Cavani, F.; Teles, J.H. Sustainability in Catalytic Oxidation: An Alternative Approach or a Structural Evolution? ChemSusChem 2009, 2, 508–534. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.P.; Kharitonov, A.S.; Piryutko, L.V. Phenol oxidation by nitrous oxide: The role of zeolite catalyst acidity. Catal. Ind. 2015, 7, 275–281. [Google Scholar] [CrossRef]
- Zhang, R.; Hua, C.; Wang, B.; Jiang, Y. N2O Decomposition over Cu–Zn/γ–Al2O3 Catalysts. Catalysts 2016, 6, 200. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-Free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(ii) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Joo, J.C.; Khusnutdinova, A.N.; Flick, R.; Kim, T.; Bornscheuer, U.T.; Yakunin, A.F.; Mahadevan, R. Alkene hydrogenation activity of enoate reductases for an environmentally benign biosynthesis of adipic acid. Chem. Sci. 2017, 8, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.C.; Sagadevan, A. One-pot room-temperature conversion of cyclohexane to adipic acid by ozone and UV light. Science 2014, 346, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Aoki, M.; Noyori, R. A “Green” Route to Adipic Acid: Direct Oxidation of Cyclohexenes with 30 Percent Hydrogen Peroxide. Science 1998, 281, 1646–1647. [Google Scholar] [CrossRef]
- Dai, J.; Zhong, W.; Yi, W.; Liu, M.; Mao, L.; Xu, Q.; Yin, D. Bifunctional H2WO4/TS-1 catalysts for direct conversion of cyclohexane to adipic acid: Active sites and reaction steps. Appl. Catal. B Environ. 2016, 192, 325–341. [Google Scholar] [CrossRef]
- Zou, G.; Zhong, W.; Xu, Q.; Xiao, J.; Liu, C.; Li, Y.; Mao, L.; Kirk, S.; Yin, D. Oxidation of cyclohexane to adipic acid catalyzed by Mn-doped titanosilicate with hollow structure. Catal. Commun. 2015, 58, 46–52. [Google Scholar] [CrossRef]
- Acharyya, S.S.; Ghosh, S.; Bal, R. Nanoclusters of Cu(ii) supported on nanocrystalline W(vi) oxide: A potential catalyst for single-step conversion of cyclohexane to adipic acid. Green Chem. 2015, 17, 3490–3499. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Mahmoodi Hashemi, M. One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nano-rods in a Bronsted acidic ionic liquid. RSC Adv. 2015, 5, 31298–31302. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Mahmoodi Hashemi, M. Simple and green oxidation of cyclohexene to adipic acid with an efficient and durable silica-functionalized ammonium tungstate catalyst. Catal. Commun. 2014, 43, 169–172. [Google Scholar] [CrossRef]
- Ghosh, S.; Acharyya, S.S.; Adak, S.; Konathala, L.N.S.; Sasaki, T.; Bal, R. Selective oxidation of cyclohexene to adipic acid over silver supported tungsten oxide nanostructured catalysts. Green Chem. 2014, 16, 2826–2834. [Google Scholar] [CrossRef]
- Ghiaci, M.; Hosseini, S.M.; Shahzeydi, A.; Martínez-Huerta, M.V. Oxidation of cyclohexanol to adipic acid with molecular oxygen catalyzed by ZnO nanoparticles immobilized on hydroxyapatite. RSC Adv. 2016, 6, 78487–78495. [Google Scholar] [CrossRef]
- Zou, G.; Zhong, W.; Mao, L.; Xu, Q.; Xiao, J.; Yin, D.; Xiao, Z.; Kirk, S.R.; Shu, T. A non-nitric acid method of adipic acid synthesis: Organic solvent- and promoter-free oxidation of cyclohexanone with oxygen over hollow-structured Mn/TS-1 catalysts. Green Chem. 2015, 17, 1884–1892. [Google Scholar] [CrossRef]
- Tahar, A.; Benadji, S.; Mazari, T.; Dermeche, L.; Marchal-Roch, C.; Rabia, C. Preparation, Characterization and Reactivity of Keggin Type Phosphomolybdates, H3−2xNixPMo12O40 and (NH4)3−2x NixPMo12O40, for Adipic Acid Synthesis. Catal. Lett. 2015, 145, 569–575. [Google Scholar] [CrossRef]
- Benadji, S.; Mazari, T.; Dermeche, L.; Salhi, N.; Cadot, E.; Rabia, C. Clean Alternative for Adipic Acid Synthesis Via Liquid-Phase Oxidation of Cyclohexanone and Cyclohexanol Over H3−2xCoxPMo12O40 Catalysts with Hydrogen Peroxide. Catal. Lett. 2013, 143, 749–755. [Google Scholar] [CrossRef]
- Chavan, S.A.; Srinivas, D.; Ratnasamy, P. Oxidation of Cyclohexane, Cyclohexanone, and Cyclohexanol to Adipic Acid by a Non-HNO3 Route over Co/Mn Cluster Complexes. J. Catal. 2002, 212, 39–45. [Google Scholar] [CrossRef]
- Béziat, J.C.; Besson, M.; Gallezot, P. Liquid phase oxidation of cyclohexanol to adipic acid with molecular oxygen on metal catalysts. Appl. Catal. A Gen. 1996, 135, L7–L11. [Google Scholar] [CrossRef]
- Usui, Y.; Sato, K. A green method of adipic acid synthesis: Organic solvent- and halide-free oxidation of cycloalkanones with 30% hydrogen peroxide. Green Chem. 2003, 5, 373–375. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, Z.; Wang, K.; Chen, J. Clean synthesis of adipic acid by direct oxidation of cyclohexene with H2O2 over peroxytungstate-organic complex catalysts. Green Chem. 1999, 1, 275–276. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Li, G.; Wei, Y. Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coord. Chem. Rev. 2017. [Google Scholar] [CrossRef]
- Li, T.; Miras, H.; Song, Y.-F. Polyoxometalate (POM)-Layered Double Hydroxides (LDH) Composite Materials: Design and Catalytic Applications. Catalysts 2017, 7, 260. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; He, X.; Ma, A.; Le, L.; Lin, S. Facile Electrodeposition of Flower-Like PMo12-Pt/rGO Composite with Enhanced Electrocatalytic Activity towards Methanol Oxidation. Catalysts 2015, 5, 1275. [Google Scholar] [CrossRef]
- Yu, H.; Ru, S.; Dai, G.; Zhai, Y.; Lin, H.; Han, S.; Wei, Y. An Efficient Iron(III)-Catalyzed Aerobic Oxidation of Aldehydes in Water for the Green Preparation of Carboxylic Acids. Angew. Chem. Int. Ed. 2017, 56, 3867–3871. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Xu, B.; Xu, X.; Song, L.; Wang, X. Surfactant encapsulated palladium-polyoxometalates: Controlled assembly and their application as single-atom catalysts. Chem. Sci. 2016, 7, 1011–1015. [Google Scholar] [CrossRef]
- Al-Oweini, R.; Sartorel, A.; Bassil, B.S.; Natali, M.; Berardi, S.; Scandola, F.; Kortz, U.; Bonchio, M. Photocatalytic Water Oxidation by a Mixed-Valent MnIII3MnIVO3 Manganese Oxo Core that Mimics the Natural Oxygen-Evolving Center. Angew. Chem. Int. Ed. 2014, 53, 11182–11185. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yin, P.; Zhang, J.; Hao, J.; Xiao, Z.; Wei, Y. Single-side organically functionalized Anderson-type polyoxometalates. Eur. J. Chem. 2011, 17, 12002–12005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Hao, J.; Wei, Y. β-{Cr[RC(CH2O)3]2Mo6O18}3−: The first organically-functionalized β isomer of Anderson-type polyoxometalates. Inorg. Chem. Front. 2017, 4, 1215–1218. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Zhang, J.; She, S.; Hao, J.; Wei, Y. A direct anchoring of Anderson-type polyoxometalates in aqueous media with tripodal ligands especially containing the carboxyl group. Dalton Trans. 2014, 43, 2722–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Q.; Zeng, M.; Huang, Y.; Zhang, J.; Hao, J.; Wei, Y. The proton-controlled synthesis of unprecedented diol functionalized Anderson-type POMs. Chem. Commun. 2016, 52, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Huang, Y.; Zhang, J.; Hao, J.; Wei, Y. Unprecedented [small chi] isomers of single-side triol-functionalized Anderson polyoxometalates and their proton-controlled isomer transformation. Chem. Commun. 2015, 51, 9097–9100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, J.; Wang, P.; Ding, B.; Huang, Y.; Zhao, Z.; Zhang, J.; Wei, Y. Step-by-Step Strategy from Achiral Precursors to Polyoxometalates-Based Chiral Organic–Inorganic Hybrids. Inorg. Chem. 2015, 54, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Zhang, J.; She, S.; Huang, Y.; Wei, Y. Spontaneous resolution of polyoxometalate-based inorganic-organic hybrids driven by solvent and common ion. Dalton Trans. 2014, 43, 17296–17302. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz-Monge, J.; Trautwein, G.; Garcia-Garcia, A. Influence of peroxometallic intermediaries present on polyoxometalates nanoparticles surface on the adipic acid synthesis. J. Mol. Catal. A Chem. 2014, 394, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Nomiya, K.; Takahashi, T.; Shirai, T.; Miwa, M. Anderson-type heteropolyanions of molybdenum(VI) and tungsten(VI). Polyhedron 1987, 6, 213–218. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Huebschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]

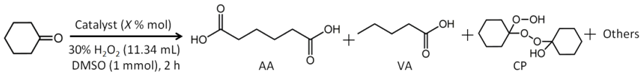
| Entry | Cat. | mol % Cat. | Conv. % 2 | TOF h−1 | Yield % AA | Selectivity % 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| AA | VA | CP | Others | ||||||
| 1 | Blank | 0 | 1.6 | - | 0 | - | - | >99.9 | - |
| 2 | 1 | 0.02 | >99.9 | 14775 | 97.1 | 97.1 | 2.9 | - | - |
| 3 | 1 | 0.01 | 98.5 | 14775 | 95.9 | 97.4 | 2.6 | - | - |
| 4 | 1 | 0.04 | >99.9 | 14775 | 96.8 | 96.8 | 3 | - | 0.2 |
| 5 | 1 | 0.08 | >99.9 | 14775 | 96.5 | 96.5 | 2.5 | - | 1 |
| 6 | 1 | 0.1 | >99.9 | 14775 | 96.4 | 96.4 | 2.2 | - | 1.4 |
| 7 4 | 1 | 0.02 | 46.8 | 14775 | 45.7 | 97.6 | 2.4 | - | - |
| 8 5 | 1 | 0.02 | >99.9 | 14775 | 97.2 | 97.2 | 2.8 | - | - |
| 9 | Na2MoO4 | 0.02 | 24.7 | 3705 | 28.9 | 11.7 | - | 88.3 | - |
| 10 | (NH4)6Mo7O24 | 0.02 | 35.9 | 5385 | 52.1 | 14.5 | - | 85.5 | - |
| 11 | Mn(CH3COO)2 | 0.02 | 2.5 | 375 | 1.59 | 63.5 | 23.7 | - | 12.8 |
| 12 | [NH4]3·α-[Mn(OH)6Mo6O18] | 0.02 | 67.4 | 10110 | 46.4 | 68.9 | - | 31.1 | - |
| Entry | Cat. | mol % Cat. | Conv. % 2 | TOF h−1 | Yield % AA | Selectivity % 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| AA | COA | CP | Others | ||||||
| 1 | Blank | 0 | 0.5 | - | 0 | - | - | >99.9 | |
| 2 | 1 | 0.02 | >99.9 | 13257 | 85.3 | 85.3 | 8.3 | 6.4 | - |
| 3 | 1 | 0.01 | 98.2 | 13257 | 84.9 | 86.5 | 7.9 | 5.6 | - |
| 4 | 1 | 0.04 | >99.9 | 13257 | 85.8 | 85.8 | 6.4 | 7.8 | - |
| 5 | 1 | 0.08 | >99.9 | 13257 | 85.9 | 85.9 | 5.8 | 8.3 | - |
| 6 | 1 | 0.1 | >99.9 | 13257 | 85.4 | 85.4 | 5.2 | 9.4 | - |
| 7 4 | 1 | 0.02 | 0.1 | 13.5 | 0 | - | - | - | >99.9 |
| 8 5 | 1 | 0.02 | 45.8 | 13257 | 40.3 | 88.1 | 2.9 | 3.7 | 5.3 |
| 9 6 | 1 | 0.02 | 21.4 | 2889 | 13.7 | 64 | - | 29.2 | 6.8 |
| 10 7 | 1 | 0.02 | 85.7 | 11569.5 | 40 | 87.5 | 4.8 | 5.6 | 2.1 |
| 11 8 | 1 | 0.02 | 46.7 | 13257 | 26.2 | 56.2 | 2.8 | 1.5 | 39.59 |
| 12 | Na2MoO4 | 0.02 | 12.2 | 1647 | 1.15 | 9.4 | 4.1 | 86.5 | - |
| 13 | (NH4)6Mo7O24 | 0.02 | 18.4 | 2484 | 2.32 | 12.6 | 4.5 | 82.9 | - |
| 14 | Mn(CH3COO)2 | 0.02 | 3.1 | 418.5 | 1.65 | 53.3 | 23.7 | - | 14 |
| 15 | [NH4]3·α-[Mn(OH)6Mo6O18] | 0.02 | 53.7 | 7249.5 | 41.6 | 77.4 | 12.3 | 10.3 | - |
| Substrate (one/ol) | 0/10 | 2/8 | 4/6 | 6/4 | 2/1 | 8/2 | 10/0 |
| Yield % | 84 | 88 | 90 | 92 | 93 | 95 | 98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Huang, Y.; Ding, B.; Wang, P.; Geng, X.; Zhang, J.; Wei, Y. Single-Atom Mn Active Site in a Triol-Stabilized β-Anderson Manganohexamolybdate for Enhanced Catalytic Activity towards Adipic Acid Production. Catalysts 2018, 8, 121. https://doi.org/10.3390/catal8030121
Luo J, Huang Y, Ding B, Wang P, Geng X, Zhang J, Wei Y. Single-Atom Mn Active Site in a Triol-Stabilized β-Anderson Manganohexamolybdate for Enhanced Catalytic Activity towards Adipic Acid Production. Catalysts. 2018; 8(3):121. https://doi.org/10.3390/catal8030121
Chicago/Turabian StyleLuo, Jianhui, Yichao Huang, Bin Ding, Pingmei Wang, Xiangfei Geng, Jiangwei Zhang, and Yongge Wei. 2018. "Single-Atom Mn Active Site in a Triol-Stabilized β-Anderson Manganohexamolybdate for Enhanced Catalytic Activity towards Adipic Acid Production" Catalysts 8, no. 3: 121. https://doi.org/10.3390/catal8030121






