Methyl Chloride Synthesis over Metal Chlorides-Modified Mesoporous Alumina Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Results of Mesoporous Alumina
2.1.1. Characterization by Nitrogen Physisorption
2.1.2. X-ray Powder Diffraction (XRD)
2.1.3. Quantification of Lewis Acid Sites by FTIR
2.2. Catalytic Performance
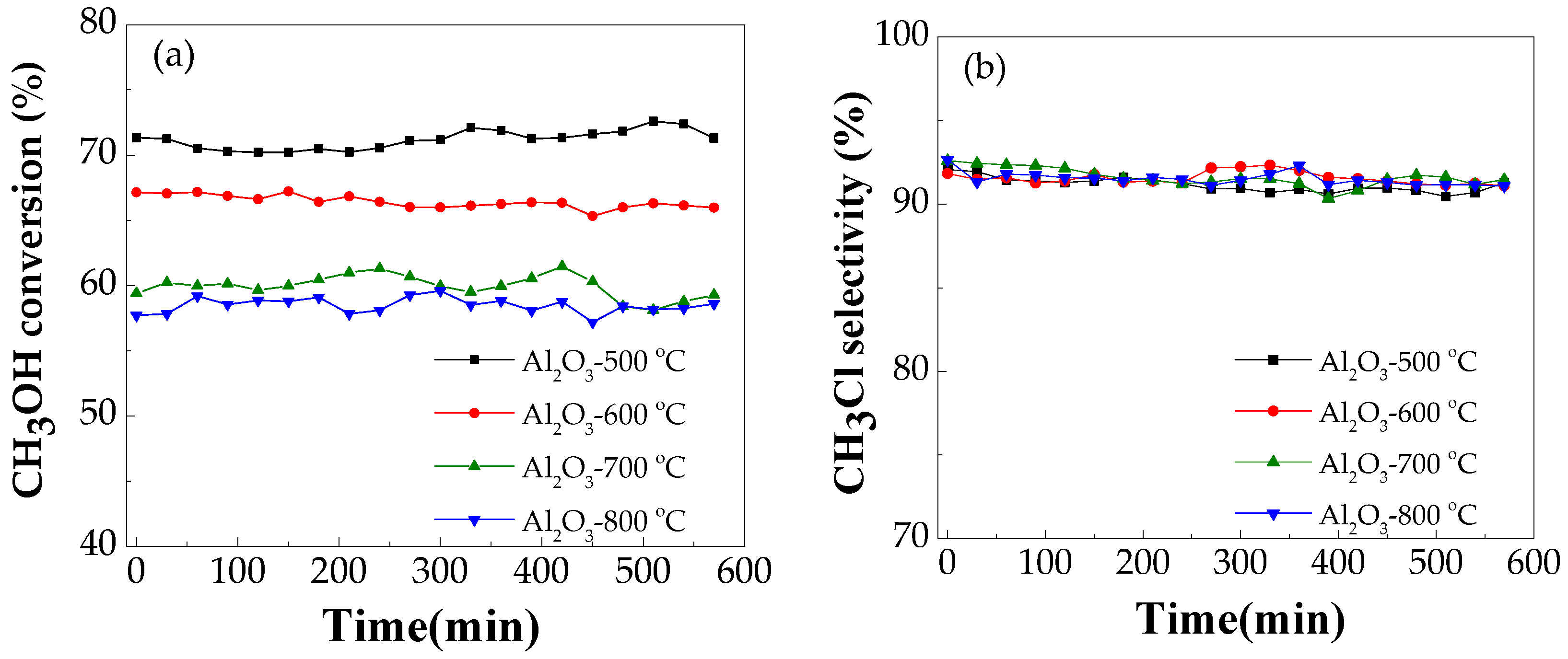
2.2.1. Effect of Calcination Temperature on Catalytic Performance
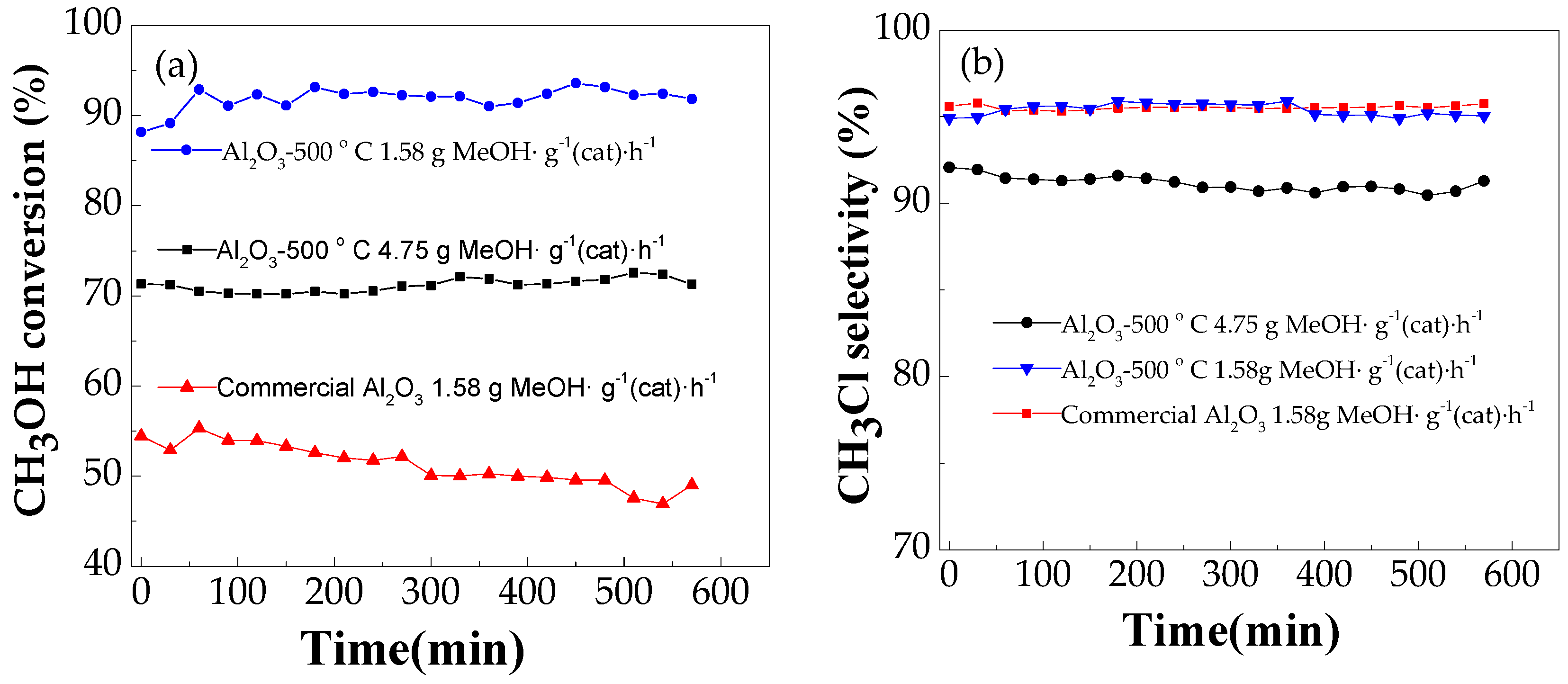
2.2.2. Effect of Methanol Space Velocity on Catalytic Performance
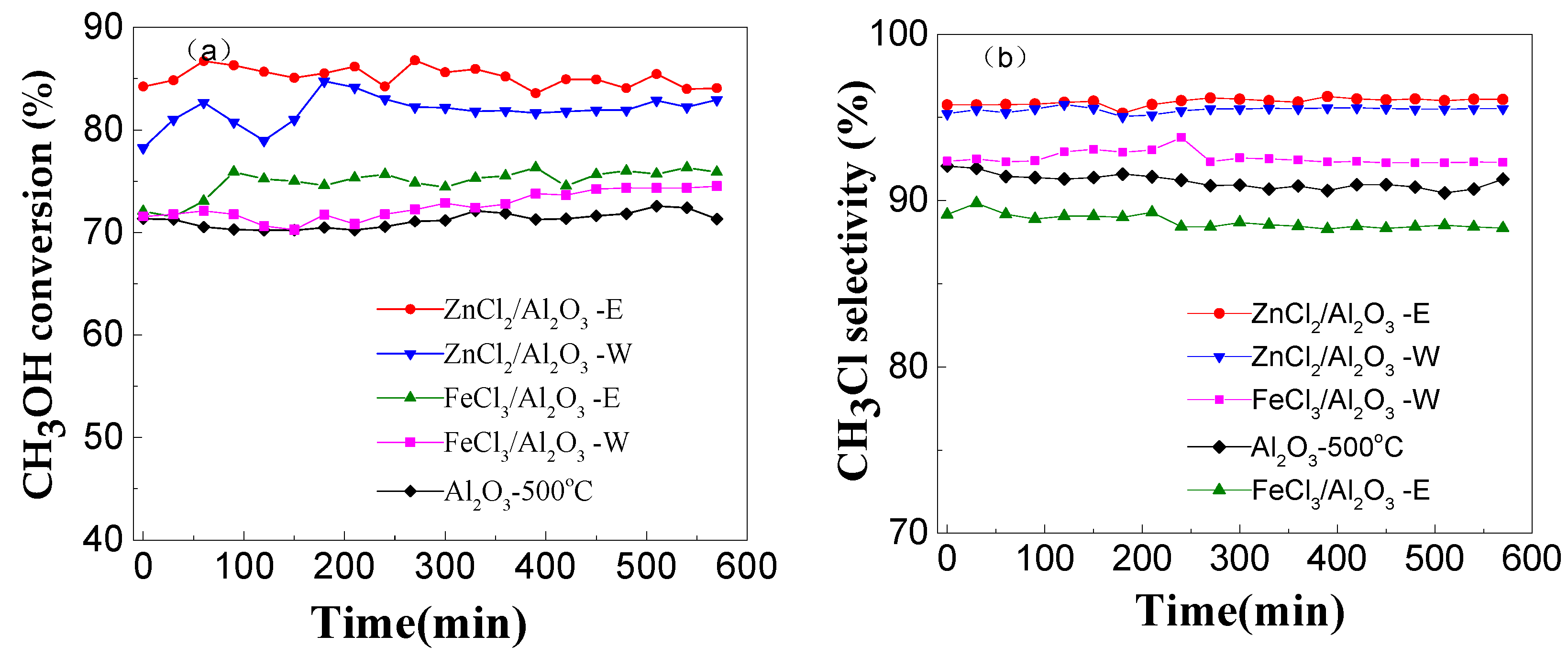
2.2.3. Effect of Modification on Catalytic Performance
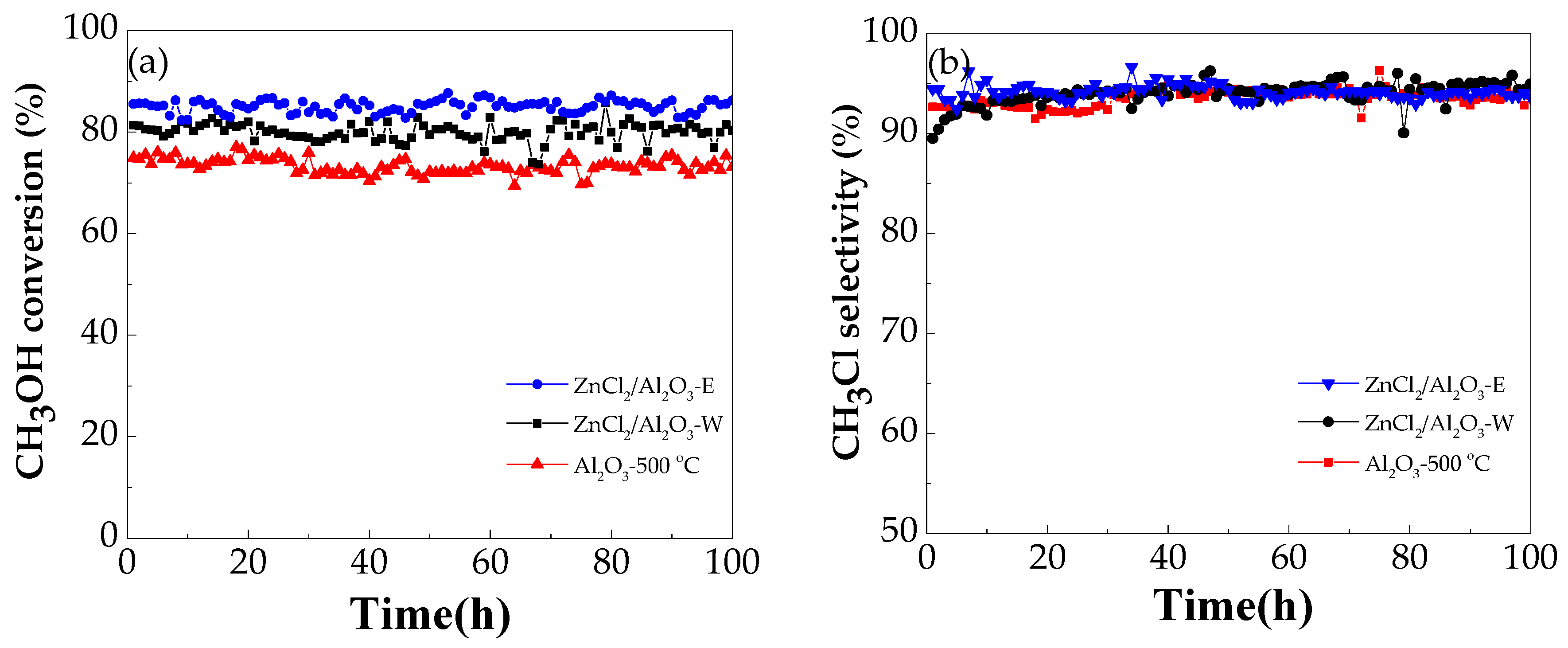
2.2.4. Catalyst Stability
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. Nitrogen-Physisorption
3.2.2. X-ray Diffraction
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.4. X-ray Photoelectron Spectroscopy
3.2.5. UV
3.2.6. ICP
3.3. Catalytic Performance Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thyagarajan, M.S.; Kumar, R.; Kuloor, N.R. Hydrochlorination of methanol to methyl chloride in fixed catalyst beds. Ind. Eng. Chem. Proc. Des. Dev. 1966, 5, 209–213. [Google Scholar] [CrossRef]
- Podkolzin, S.G.; Stangland, E.E.; Jones, M.E.; Peringer, E.; Lercher, J.A. Methyl chloride production from methane over lanthanum-based catalysts. J. Am. Chem. Soc. 2007, 129, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Grenouillet, P.; Bornette, F.; Bellefon, C. Switching from water to ionic liquids for the production of methylchloride: Catalysis and reactor issues. Chem. Eng. J. 2009, 145, 441–445. [Google Scholar] [CrossRef]
- Becerra, A.M.; Castro Luna, A.E.; Ardissone, D.E.; Ponzi, M.I. Kinetics of the catalytic hydrochlorination of methanol to methyl chloride. Ind. Eng. Chem. Res. 1992, 31, 1045–1050. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Kumar, N.; Reinsdorf, A.; Eränen, K.; Wärnå, J.; Murzin, D.Y.; Salmi, T. Methyl chloride synthesis over Al2O3 catalyst coated microstructured reactor-Thermodynamics, kinetics and mass transfer. Chem. Eng. Sci. 2013, 95, 232–245. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Peurla, M.; Narendra Kumar, N.; Eränen, K.; Murzin, D.Y.; Salmi, T. Preparation of selective ZnCl2/alumina catalysts for methyl chloride synthesis: Influence of pH, precursor and zinc loading. Appl. Catal. A Gen. 2015, 490, 117–127. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Kumar, N.; Zhang, B.; Eranen, K.; Murzin, D.Y.; Salmi, T. Preparation and characterization of alumina-based microreactors for application in methyl chloride synthesis. Ind. Eng. Chem. Res. 2012, 51, 4545–4555. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Kumar, N.; Shchukarev, A.; Eränen, K.; Mikkola, J.-P.; Murzin, D.Y.; Salmi, T. Preparation and characterization of neat and ZnCl2 modified zeolites and alumina for methyl chloride synthesis. Appl. Catal. A Gen. 2013, 468, 120–134. [Google Scholar] [CrossRef]
- Pillai, S.K.; Hamoudi, S.; Belkacemi, K. Functionalized value-added products via metathesis of methyloleate over methyltrioxorhenium supported on ZnCl2-promoted mesoporous alumina. Fuel 2013, 110, 32–39. [Google Scholar] [CrossRef]
- Aoun, M.; Chater, M. Préparation et caractérisation des catalyseurs Rh/Al2O3 et Rh/ZnO-Al2O3. C. R. Chim. 2007, 10, 644–651. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K. Thermal activation of a clayzic catalyst useful for Friedel-Crafts reactions: HCl evolved with creation of active sites in different thermal treatments to ZnCl2/Mont-K10. Catal. Lett. 2002, 81, 163–168. [Google Scholar] [CrossRef]
- Conte, M.; Davies, T.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Selective formation of chloroethane by the hydrochlorination of ethane using zinc catalysts. J. Catal. 2007, 252, 23–29. [Google Scholar] [CrossRef]
- Ma, J.H.; Yang, H.Q.; Li, L.; Xie, X.L.; Liu, B.; Zhang, L.H.; Zhang, L.N. Synthesis of aligned ZnO submicron rod arrays by heating zinc foil covered with ZnCl2 solution. Acta Chim. Sin. 2009, 67, 1515–1522. [Google Scholar]
- Ma, Y.H.; Wang, Q.H.; Wang, X.N.; Sun, X.H.; Wang, X.Q. A comprehensive study on activated carbon prepared from spent shiitake substrate via pyrolysis with ZnCl2. J. Porous Mater. 2015, 22, 157–169. [Google Scholar] [CrossRef]
- Zhang, D.; Azakami, T.; Yazawa, A. Utilization of alcohols for the dehydration of magnesium chloride. Can. Metall. Q. 2013, 31, 189–194. [Google Scholar] [CrossRef]
- Ballinger, T.H.; Yates, J.T. IR spectroscopic detection of Lewis acid sites on Al2O3 using adsorbed CO Correlation with Al-OH group removal. Langmuir 1991, 7, 3041–3045. [Google Scholar] [CrossRef]
- Xu, B.J.; Long, J.; Yan, Z.F.; Zhu, Y.X.; Tian, H.P. Synthesis of mesoporous alumina using sucrose as the template. Ind. Catal. 2010, 18, 31–35. [Google Scholar]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
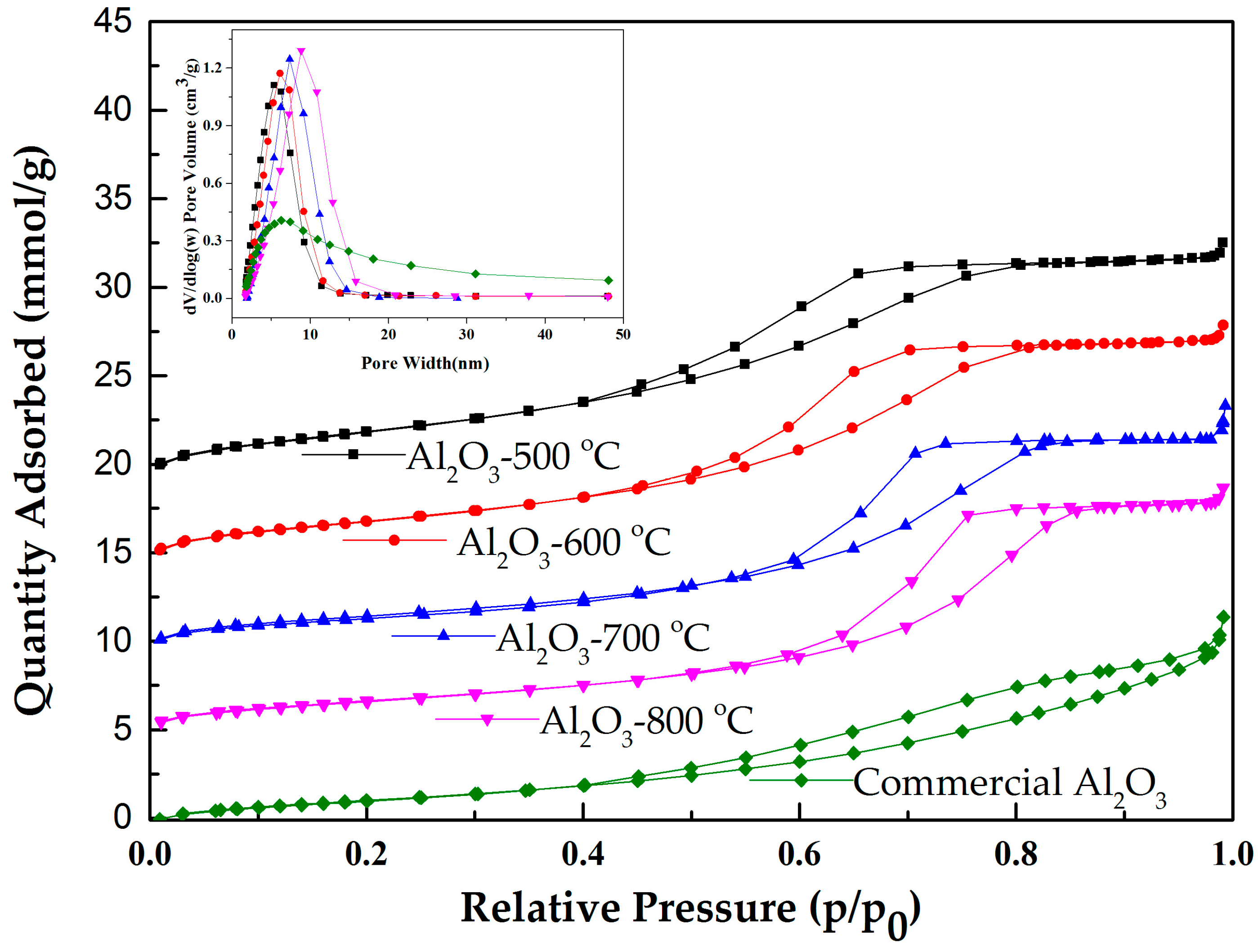
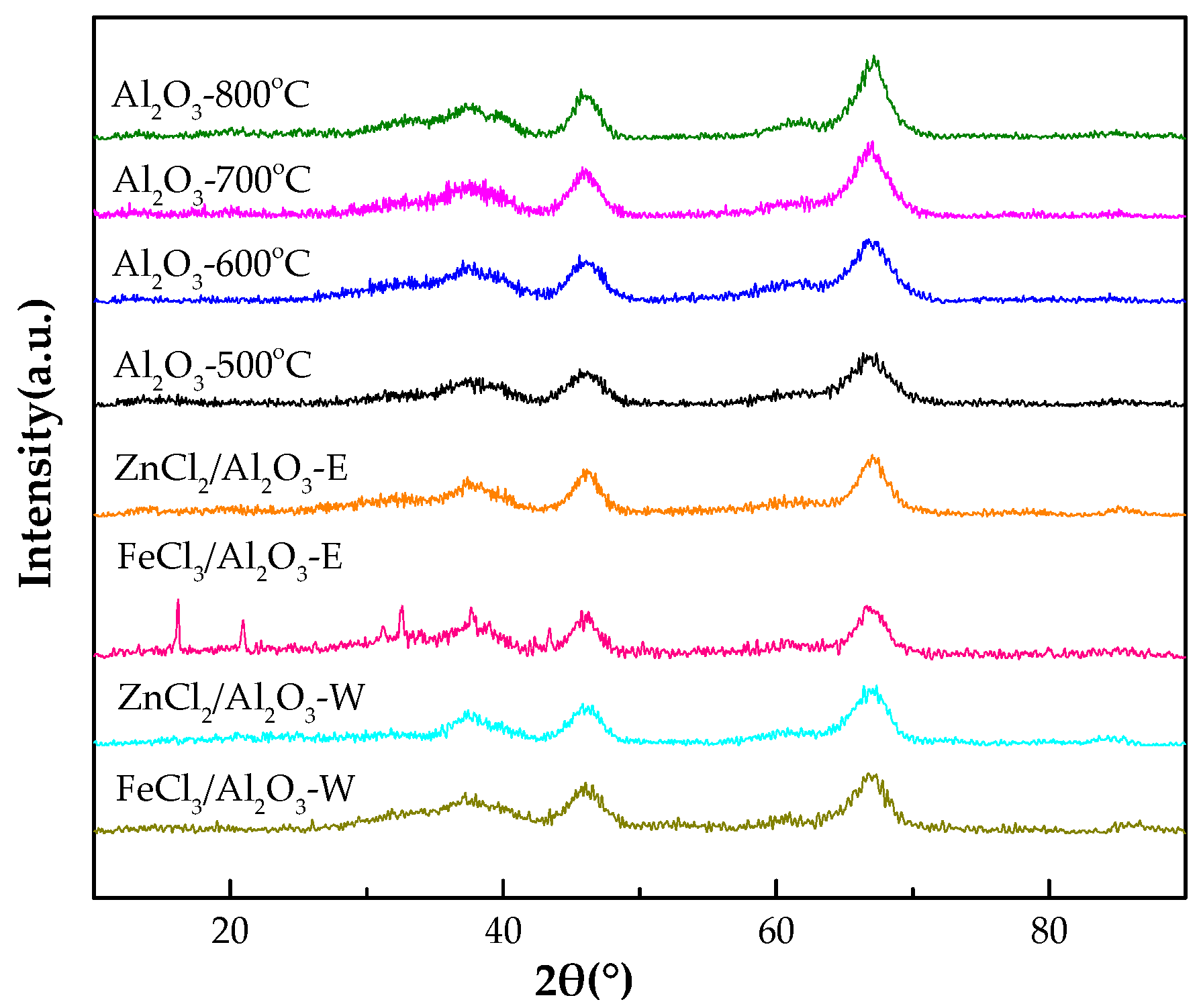
| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Pore Width (nm) |
|---|---|---|---|
| Al2O3-500 °C | 343 | 0.49 | 5.7 |
| Al2O3-600 °C | 308 | 0.51 | 6.6 |
| Al2O3-700 °C | 246 | 0.46 | 7.4 |
| Al2O3-800 °C | 216 | 0.48 | 8.9 |
| Commercial alumina | 188 | 0.36 | 7.6 |
| ZnCl2/Al2O3-E | 273 | 0.45 | 5.3 |
| FeCl3/Al2O3-E | 282 | 0.45 | 5.4 |
| ZnCl2/Al2O3-W | 256 | 0.44 | 5.8 |
| FeCl3/Al2O3-W | 268 | 0.43 | 5.9 |
| Sample | Lewis Acid Sites (μmol/g) | |||
|---|---|---|---|---|
| Total | Weak | Medium | Strong | |
| Al2O3-500 °C | 54.4 | 23.7 | 18.3 | 12.4 |
| Al2O3-600 °C | 36.3 | 21.3 | 8.1 | 6.9 |
| Al2O3-700 °C | 26.3 | 13.8 | 8.1 | 4.4 |
| Al2O3-800 °C | 20.2 | 13.0 | 5.1 | 2.1 |
| Commercial alumina | 20.1 | 16.8 | 0.8 | 2.5 |
| ZnCl2/Al2O3-E | 137.8 | 93.7 | 28.8 | 15.3 |
| FeCl3/Al2O3-E | 85.8 | 40.8 | 26.9 | 18.1 |
| ZnCl2/Al2O3-W | 97.9 | 60.1 | 24.3 | 13.5 |
| FeCl3/Al2O3-W | 60.1 | 29.7 | 20.2 | 10.2 |
| Sample | Cl/M (UV/ICP) | Cl/M (XPS) |
|---|---|---|
| ZnCl2/Al2O3-E | 1.60 | 1.35 |
| FeCl3/Al2O3-E | 1.95 | 1.73 |
| ZnCl2/Al2O3-W | 0.88 | 0.90 |
| FeCl3/Al2O3-W | 0.85 | 0.92 |
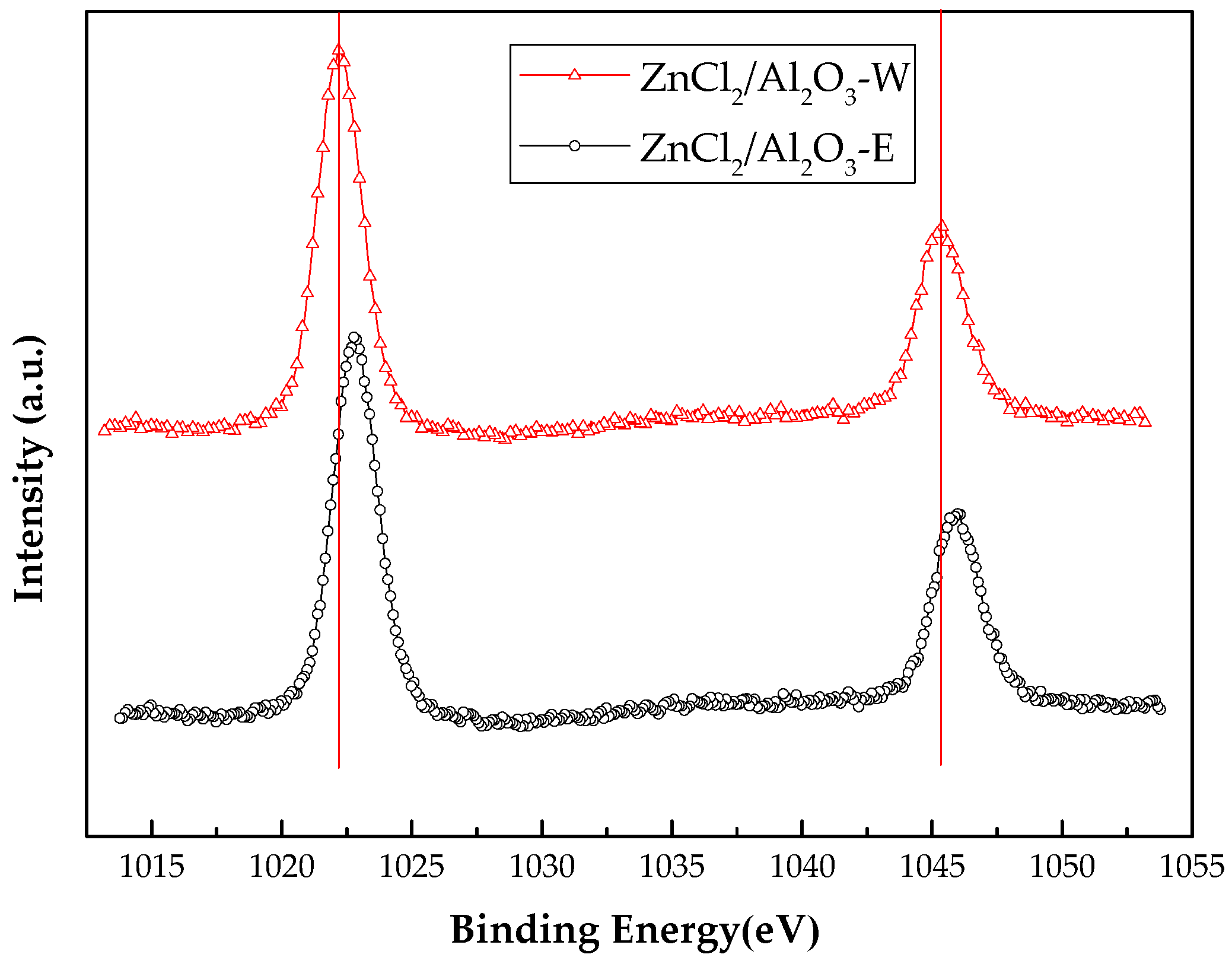
| XPS Peaks | Binding Energy Eb (eV) in ZnCl2/Al2O3-E | Binding Energy Eb (eV) in ZnCl2/Al2O3-W | Binding Energy Eb (eV) in Pure ZnCl2 | Binding Energy Eb (eV) in Pure ZnO | ∆Eb (eV) between ZnCl2/Al2O3-E and Pure ZnCl2 | ∆Eb (eV) between ZnCl2/Al2O3-W and Pure ZnCl2 |
|---|---|---|---|---|---|---|
| Zn 2p1/2 | 1022.88 | 1022.29 | 1023.0 | 1022.0 | −0.12 | −0.71 |
| Zn 2p3/2 | 1046.08 | 1045.39 | 1046.2 | 1045.1 | −0.12 | −0.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhang, F.; Yu, F.; Zhang, J.; Zhang, J. Methyl Chloride Synthesis over Metal Chlorides-Modified Mesoporous Alumina Catalyst. Catalysts 2018, 8, 99. https://doi.org/10.3390/catal8030099
Ji Y, Zhang F, Yu F, Zhang J, Zhang J. Methyl Chloride Synthesis over Metal Chlorides-Modified Mesoporous Alumina Catalyst. Catalysts. 2018; 8(3):99. https://doi.org/10.3390/catal8030099
Chicago/Turabian StyleJi, Yuwen, Feilong Zhang, Feng Yu, Jianshu Zhang, and Jinli Zhang. 2018. "Methyl Chloride Synthesis over Metal Chlorides-Modified Mesoporous Alumina Catalyst" Catalysts 8, no. 3: 99. https://doi.org/10.3390/catal8030099





