Binary Nitrogen Precursor-Derived Porous Fe-N-S/C Catalyst for Efficient Oxygen Reduction Reaction in a Zn-Air Battery
Abstract
:1. Introduction
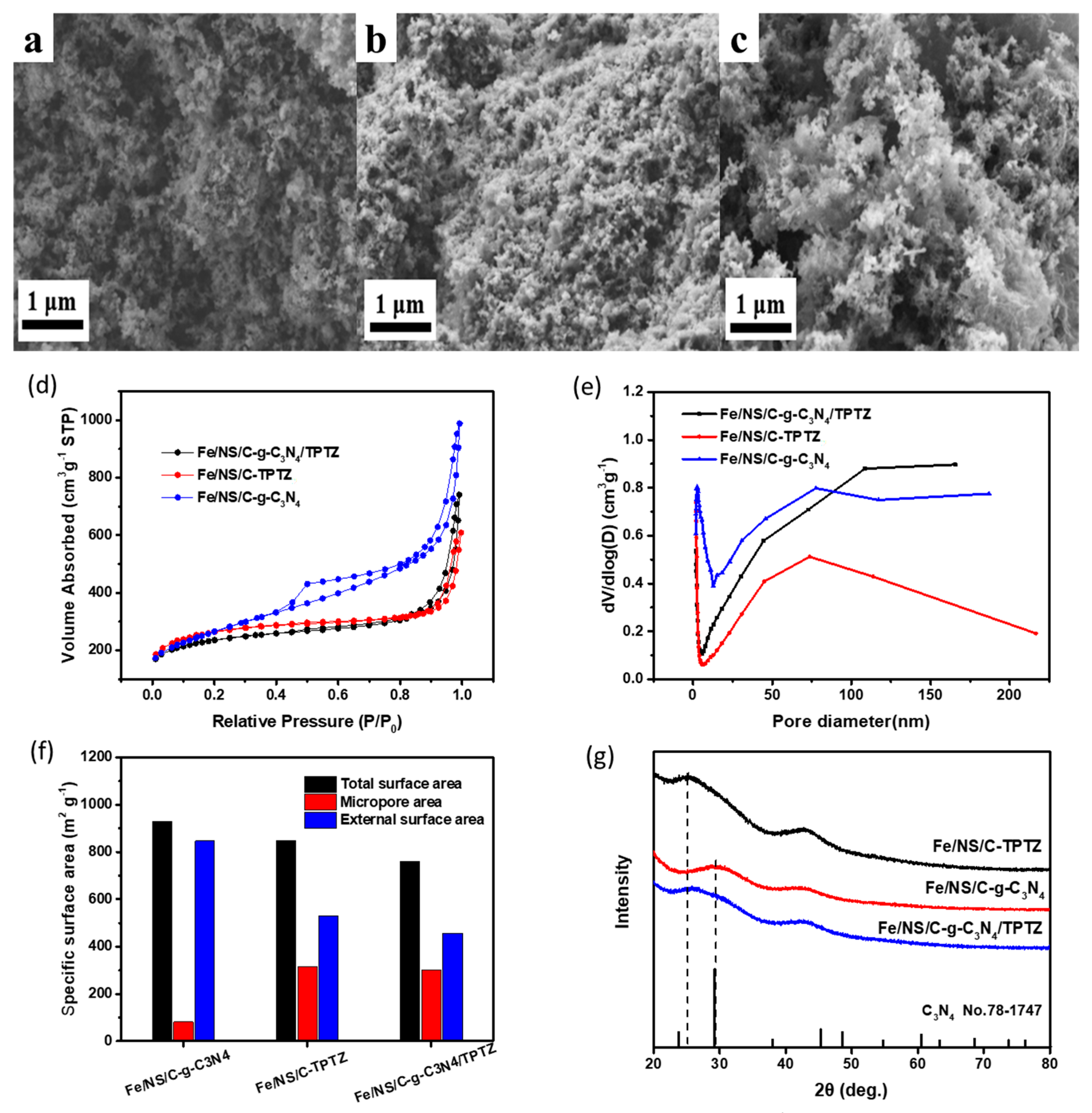
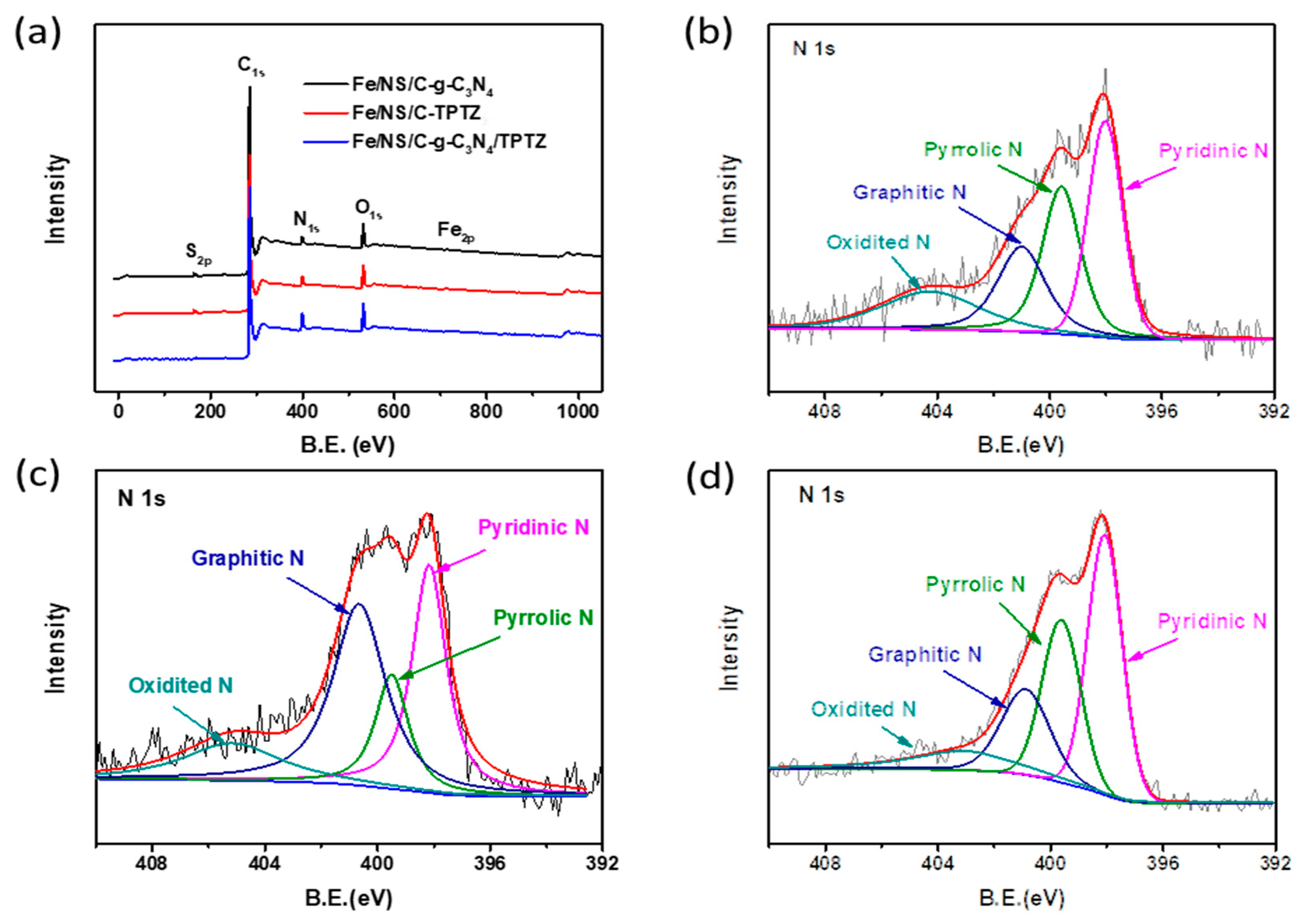
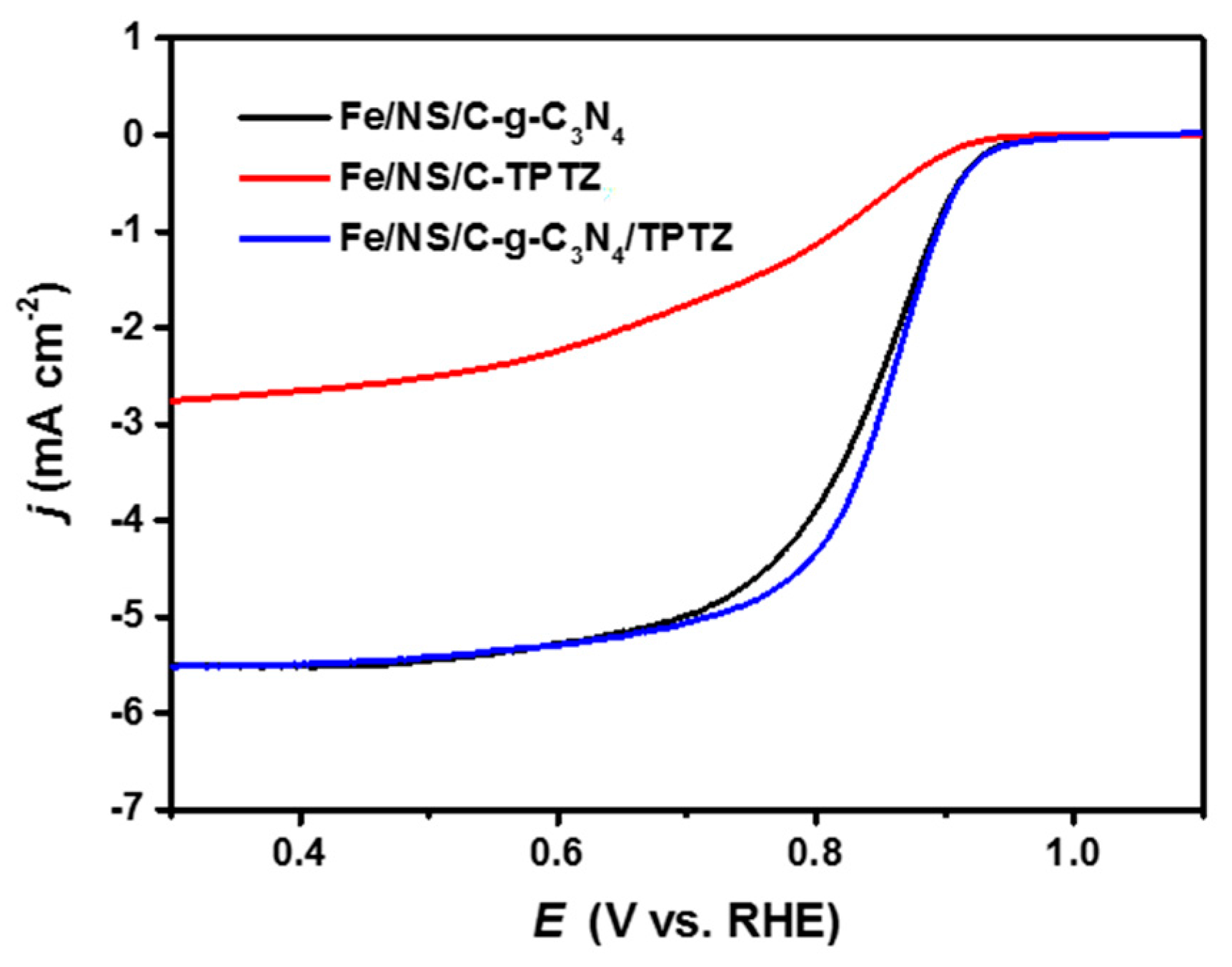
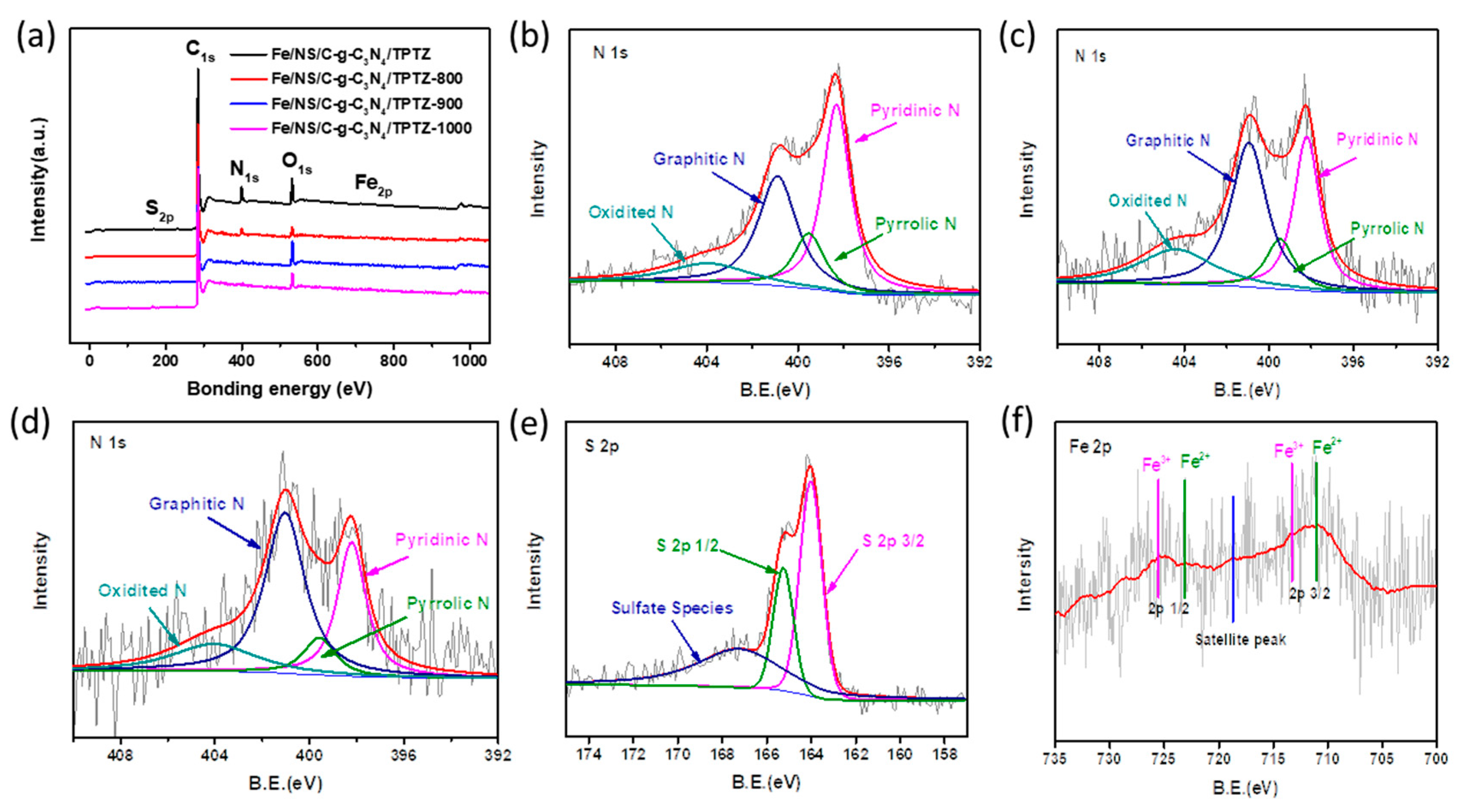
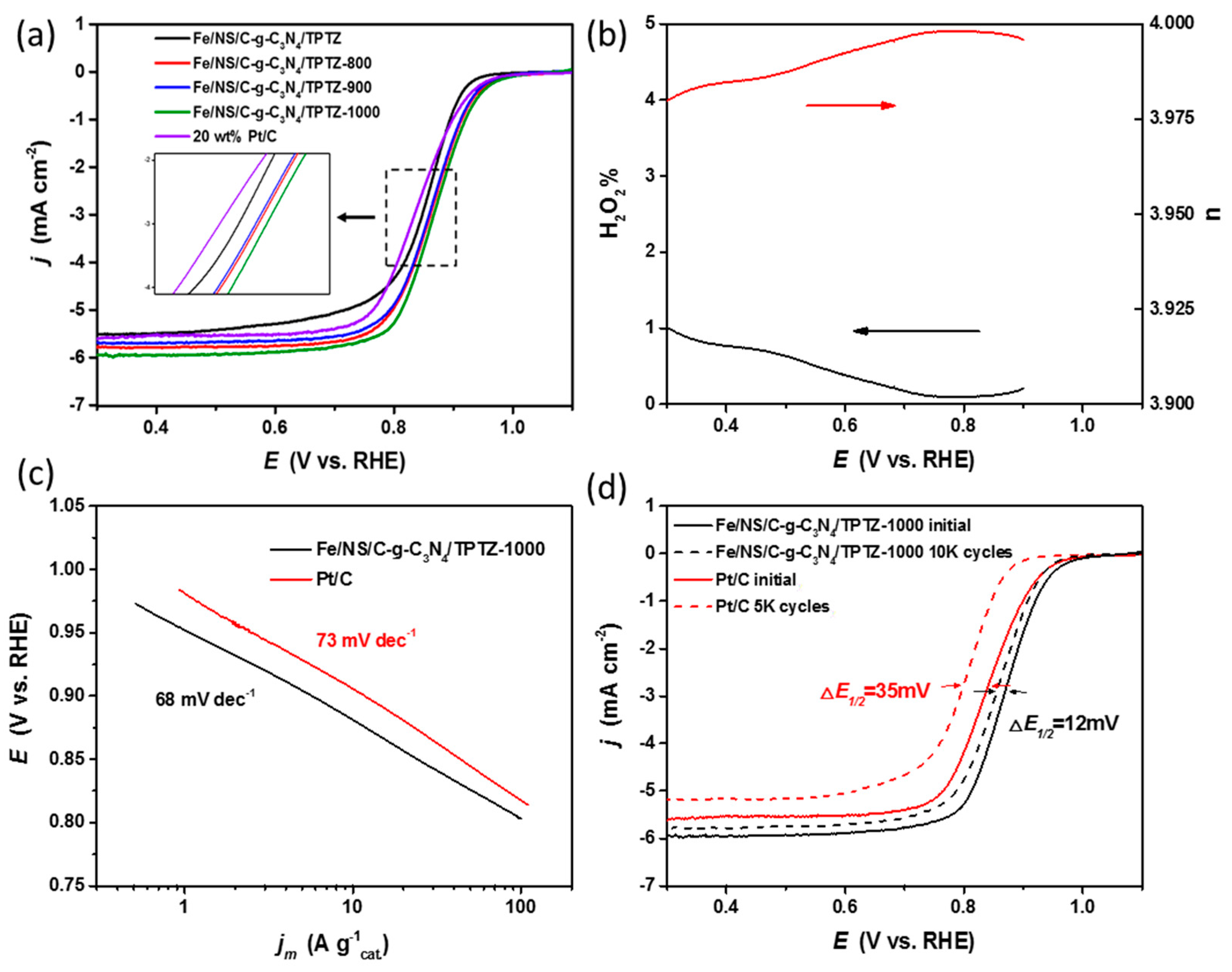
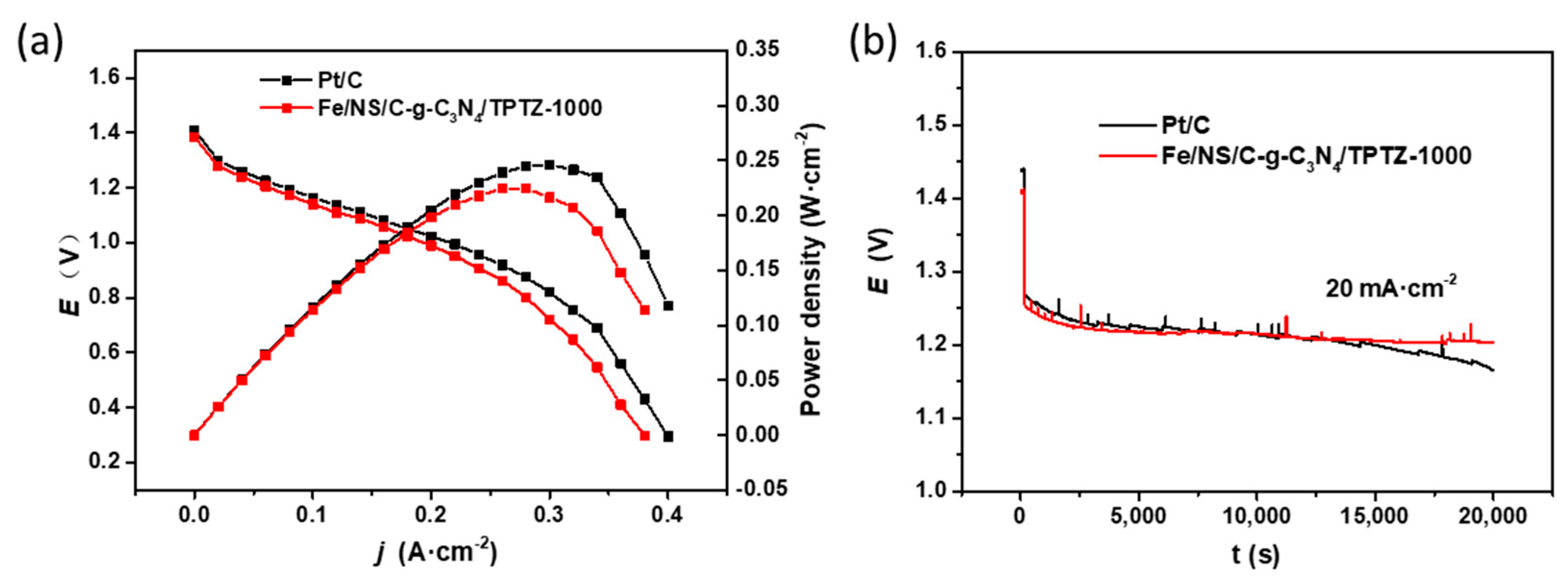
2. Results and Discussion
3. Experimental Section
3.1. Preparation of g-C3N4 Nanosheets
3.2. Catalyst Synthesis
3.3. Characterizations
3.4. Electrochemical Measurement
3.5. Primary Zn-Air Battery Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, X.; Yang, S.; Yan, X.; Leng, J.; Shuang, S.; Ajayan, P.M.; Zhang, Z. Pyridinic-Nitrogen-Dominated Graphene Aerogels with Fe-N-C Coordination for Highly Efficient Oxygen Reduction Reaction. Adv. Funct. Mater. 2016, 26, 5708–5717. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Guan, Y.; Xue, J.; Cui, L. Constructing of cobalt-embedded in nitrogen-doped carbon material with desired porosity derived from MOFs confined growth within graphene aerogel as a superior catalyst towards HER and ORR. J. Mater. Chem. A 2016, 4, 15536–15545. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.J.; Kariuki, N.N.; Chen, L.X. Aligned carbon nanotubes with built-in FeN4 active sites for electrocatalytic reduction of oxygen. Chem. Commun. 2008, 36, 329–331. [Google Scholar] [CrossRef]
- Zeng, L.; Cui, X.; Chen, L.; Ye, T.; Huang, W.; Ma, R.; Zhang, X.; Shi, J. Non-noble bimetallic alloy encased in nitrogen-doped nanotubes as a highly active and durable electrocatalyst for oxygen reduction reaction. Carbon 2017, 114, 347–355. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Z.; Zhang, J.; Zhang, L.; Zhang, J. Ultrasonic spray pyrolyzed iron-polypyrrole mesoporous spheres for fuel cell oxygen reduction electrocatalysts. J. Mater. Chem. 2009, 19, 468–470. [Google Scholar] [CrossRef]
- Liang, H.W.; Wei, W.; Wu, Z.S.; Feng, X.; Müllen, K. Mesoporous Metal-Nitrogen-Doped Carbon Electrocatalysts for Highly Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, L. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysts of oxygen reduction reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Jia, Q.; Ramaswamy, N.; Hafiz, H.; Tylus, U.; Strickland, K.; Wu, G.; Barbiellini, B.; Bansil, A.; Holby, E.F.; Zelenay, P. Experimental observation of redox-induced Fe–N switching behavior as a determinant role for oxygen reduction activity. ACS Nano 2015, 9, 12496–12505. [Google Scholar] [CrossRef] [PubMed]
- Kone, I.; Xie, A.; Tang, Y.; Chen, Y.; Liu, J.; Chen, Y.; Sun, Y.; Yang, X.; Wan, P. Hierarchical Porous Carbon Doped with Iron-Nitrogen-Sulfur for Efficient Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 20963–20973. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fisher, A.; Cheng, D.; Cao, D. Cu, N-codoped Hierarchical Porous Carbons as Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 21431–21439. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Tang, C.; He, G.; Qiu, K.; Binions, R.; Parkin, I.; Zhang, Q.; Guo, Z.; Titirici, M. Graphene/nitrogen-doped porous carbon sandwiches for the metal-free oxygen reduction reaction: Conductivity versus active sites. J. Mater. Chem. A 2016, 4, 12658–12666. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, H.; Wei, L.; Jiang, W.J.; Wu, M.; Hu, J.S. Lamellar Metal Organic Framework-Derived Fe–N–C Non-Noble Electrocatalysts with Bimodal Porosity for Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2017, 9, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Zhao, S.; Ogoke, O.; Lin, Y.; Xu, H.; Wu, G. Engineering Favorable Morphology and Structure of Fe-N-C Oxygen-Reduction Catalysts through Tuning of Nitrogen/Carbon Precursors. ChemSusChem 2017, 10, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zamani, P.; Choi, J.Y.; Hassan, F.M.; Jiang, G.; Higgins, D.C.; Zhang, Y.; Hoque, M.A.; Chen, Z. In Situ Polymer Graphenization Ingrained with Nanoporosity in a Nitrogenous Electrocatalyst Boosting the Performance of Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2016, 29, 1604456. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lai, Y.J.; Song, L.; Zhou, Z.Y.; Liu, J.G.; Wang, Q.; Yang, X.D.; Chen, C.; Shi, W.; Zheng, Y.P.; et al. S-Doping of an Fe/N/C ORR Catalyst for Polymer Electrolyte Membrane Fuel Cells with High Power Density. Angew. Chem. Int. Ed. 2015, 127, 10045–10048. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, T.; Xing, L.; Xu, K.; Tong, Y.; Xie, H.; Zhang, L.; Yan, W.; Chu, W.; Wu, C. Atomically Dispersed Iron-Nitrogen Species as Electrocatalysts for Bifunctional Oxygen Evolution and Reduction Reactions. Angew. Chem. Int. Ed. 2017, 56, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Morozan, A.; Sougrati, M.T.; Chenitz, R.; Dodelet, J.P.; Jones, D.; Jaouen, F. Optimized synthesis of Fe/N/C cathode catalysts for PEM fuel cells: A matter of iron-ligand coordination strength. Angew. Chem. Int. Ed. 2013, 52, 6867–6870. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kong, J.; Zhou, D.; Ang, J.M.; Phua, S.L.; Yee, W.A.; Liu, H.; Huang, Y.; Lu, X. Transition-Metal-Ion-Mediated Polymerization of Dopamine: Mussel-Inspired Approach for the Facile Synthesis of Robust Transition-Metal Nanoparticle–Graphene Hybrids. Chem. Eur. J. 2014, 20, 7776–7783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, L.; Yu, L.; Kong, J.; Yao, X.; Liu, W.; Xu, Z.; Lu, X. Fe/N/C hollow nanospheres by Fe(III)-dopamine complexation-assisted one-pot doping as nonprecious-metal electrocatalysts for oxygen reduction. Nanoscale 2015, 7, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, F.; Herranz, J.; Lefèvre, M.; Dodelet, J.P.; Kramm, U.I.; Herrmann, I.; Bogdanoff, P.; Maruyama, J.; Nagaoka, T.; Garsuch, A. Cross-laboratory experimental study of non-noble-metal electrocatalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2009, 1, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, F.; Lefèvre, M.; Dodelet, J.P.; Cai, M. Heat-Treated Fe/N/C Catalysts for O2 Electroreduction: Are Active Sites Hosted in Micropores? J. Phys. Chem. B 2006, 110, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Furuya, A.; Uchibori, Y.; Kim, J.; Murakoshi, K. Iron–Nitrogen-Doped Vertically Aligned Carbon Nanotube Electrocatalyst for the Oxygen Reduction Reaction. Adv. Funct. Mater. 2016, 26, 738–744. [Google Scholar] [CrossRef]
- Choi, I.A.; Kwak, D.H.; Han, S.B.; Park, J.Y.; Park, H.S.; Ma, K.B.; Kim, D.H.; Won, J.E.; Park, K.W. Doped porous carbon nanostructures as non-precious metal catalysts prepared by amino acid glycine for oxygen reduction reaction. Appl. Catal. B Environ. 2017, 211, 235–244. [Google Scholar] [CrossRef]
- Liang, W.; Chen, J.; Liu, Y.; Chen, S. Density-functional-theory calculation analysis of active sites for four-electron reduction of O2 on Fe/N-doped graphene. ACS Catal. 2014, 4, 4170–4177. [Google Scholar] [CrossRef]
- Xiao, H.; Shao, Z.G.; Zhang, G.; Gao, Y.; Lu, W.; Yi, B. Fe–N–carbon black for the oxygen reduction reaction in sulfuric acid. Carbon 2013, 57, 443–451. [Google Scholar] [CrossRef]
- Saidi, W.A. Oxygen reduction electrocatalysis using N-doped graphene quantum-dots. J. Phys. Chem. Lett. 2013, 4, 4160–4165. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Y.; Wang, K.; Liang, Y.; Wu, D.; Tsiakaras, P.; Song, S. A novel sulfur-nitrogen dual doped ordered mesoporous carbon electrocatalyst for efficient oxygen reduction reaction. Appl. Catal. B Environ. 2016, 189, 1–11. [Google Scholar] [CrossRef]
- Ai, W.; Luo, Z.; Jiang, J.; Zhu, J.; Du, Z.; Fan, Z.; Xie, L.; Zhang, H.; Huang, W.; Yu, T. Nitrogen and sulfur codoped graphene: Multifunctional electrode materials for high-performance Li-ion batteries and oxygen reduction reaction. Adv. Mater. 2014, 26, 6186–6192. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, X.D.; Zhou, Z.Y.; Lai, Y.J.; Rauf, M.; Wang, Y.; Pan, J.; Zhuang, L.; Wang, Q.; Wang, Y.C. Aminothiazole-derived N, S, Fe-doped graphene nanosheets as high performance electrocatalysts for oxygen reduction. Chem. Commun. 2015, 51, 17092–17095. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gracia-Espino, E.; Ma, J.; Zang, K.; Luo, J.; Wang, L.; Gao, S.; Mamat, X.; Hu, G.; Wagberg, T. Synergistic Effects between Atomically Dispersed Fe−N–C and C–S–C for the Oxygen Reduction Reaction in Acidic Media. Angew. Chem. Int. Ed. 2017, 56, 13800–13804. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Lyu, F.; Nie, H.; Zhan, Y.; Bian, H.; Tian, Y.; Li, Z.; Wang, A.; Lu, J.; Li, Y.Y. Facile fabrication of N/S-doped carbon nanotubes with Fe3O4 nanocrystals enchased for lasting synergy as efficient oxygen reduction catalysts. J. Mater. Chem. A 2017, 5, 13189–13195. [Google Scholar] [CrossRef]
- Ren, G.; Lu, X.; Li, Y.; Zhu, Y.; Dai, L.; Jiang, L. Porous Core–Shell Fe3C Embedded N-doped Carbon Nanofibers as an Effective Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Z.; Gu, Y.; Li, D.; Su, K.; Yang, D.; Cheng, B. Rational design of N-doped carbon nanobox-supported Fe/Fe2N/Fe3C nanoparticles as efficient oxygen reduction catalysts for Zn–air batteries. J. Mater. Chem. A 2017, 5, 11340–11347. [Google Scholar] [CrossRef]
- Zhao, Y.; Lai, Q.; Wang, Y.; Zhu, J.; Liang, Y.Y. Interconnected Hierarchically Porous Fe, N-Codoped Carbon Nanofibers as Efficient Oxygen Reduction Catalysts for Zn-Air Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16178–16186. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Toshimitsu, F.; Yang, Z.; Fujigaya, T.; Nakashima, N. Pristine carbon nanotube/iron phthalocyanine hybrids with a well-defined nanostructure show excellent efficiency and durability for oxygen reduction reaction. J. Mater. Chem. A 2016, 5, 1184–1191. [Google Scholar] [CrossRef]
- Cai, P.; Hong, Y.; Ci, S.; Wen, Z. In situ integration of CoFe alloy nanoparticles with nitrogen-doped carbon nanotubes as advanced bifunctional cathode catalysts for Zn-air batteries. Nanoscale 2016, 8, 20048–20055. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Choi, J.Y.; Hsu, R.S.; Chen, Z. Highly active porous carbon-supported nonprecious metal-N electrocatalyst for oxygen reduction reaction in PEM fuel cells. J. Phys. Chem. C 2010, 114, 8048–8053. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, C.; Cheng, Q.; Zou, L.; Zou, Z.; Yang, H. Binary Nitrogen Precursor-Derived Porous Fe-N-S/C Catalyst for Efficient Oxygen Reduction Reaction in a Zn-Air Battery. Catalysts 2018, 8, 158. https://doi.org/10.3390/catal8040158
Liu X, Chen C, Cheng Q, Zou L, Zou Z, Yang H. Binary Nitrogen Precursor-Derived Porous Fe-N-S/C Catalyst for Efficient Oxygen Reduction Reaction in a Zn-Air Battery. Catalysts. 2018; 8(4):158. https://doi.org/10.3390/catal8040158
Chicago/Turabian StyleLiu, Xiao, Chi Chen, Qingqing Cheng, Liangliang Zou, Zhiqing Zou, and Hui Yang. 2018. "Binary Nitrogen Precursor-Derived Porous Fe-N-S/C Catalyst for Efficient Oxygen Reduction Reaction in a Zn-Air Battery" Catalysts 8, no. 4: 158. https://doi.org/10.3390/catal8040158




