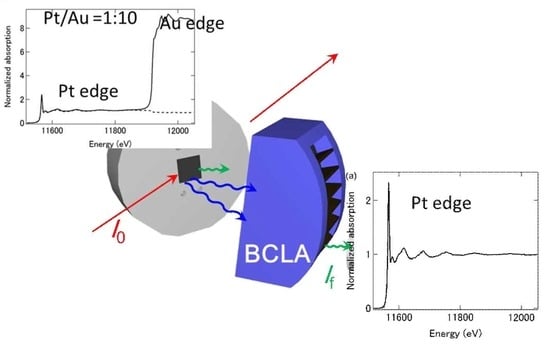
A Demonstration of Pt L3-Edge EXAFS Free from Au L3-Edge Using Log–Spiral Bent Crystal Laue Analyzers
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kristian, N.; Wang, X. Pt shell–Au core/C electrocatalyst with a controlled shell thickness and improved Pt utilization for fuel cell reactions. Electrochem. Commun. 2008, 10, 12–15. [Google Scholar] [CrossRef]
- Shuangyin, W.; Noel, K.; Sanping, J.; Xin, W. Controlled synthesis of dendritic Au@Pt core–shell nanomaterials for use as an effective fuel cell electrocatalyst. Nanotechnology 2009, 20, 025605. [Google Scholar] [CrossRef]
- Xiu, C.; Shengnan, W.; Scott, J.; Zhibing, C.; Zhenghua, W.; Lun, W.; Li, Y. The deposition of Au-Pt core-shell nanoparticles on reduced graphene oxide and their catalytic activity. Nanotechnology 2013, 24, 295402. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S. Oxygen Reduction Electrocatalyst of Pt on Au Nanoparticles through Spontaneous Deposition. ACS Appl. Mater. Interfaces 2015, 7, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Chiba, H.; Kato, T.; Endo, S.; Hayashi, T.; Todoroki, N.; Wadayama, T. Oxygen reduction reaction activity and structural stability of Pt-Au nanoparticles prepared by arc-plasma deposition. Phys. Chem. Chem. Phys. 2015, 17, 18638–18644. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, Y.; Asakura, K.; Tada, M. XAFS Techniques for Catalysts, Nanomaterials, and Surfaces; Springer: New York, NY, USA, 2016. [Google Scholar]
- Nagamatsu, S.; Arai, T.; Yamamoto, M.; Ohkura, T.; Oyanagi, H.; Ishizaka, T.; Kawanami, H.; Uruga, T.; Tada, M.; Iwasawa, Y. Potential-Dependent Restructuring and Hysteresis in the Structural and Electronic Transformations of Pt/C, Au(Core)-Pt(Shell)/C, and Pd(Core)-Pt(Shell)/C Cathode Catalysts in Polymer Electrolyte Fuel Cells Characterized by in Situ X-ray Absorption Fine Structure. J. Phys. Chem. C 2013, 117, 13094–13107. [Google Scholar]
- Yuan, Q.; Takakusagi, S.; Wakisaka, Y.; Uemura, Y.; Wada, T.; Ariga, H.; Asakura, K. Polarization-dependent Total Reflection Fluorescence X-ray Absorption Fine Structure (PTRF-XAFS) Studies on the Structure of a Pt Monolayer on Au(111) Prepared by the Surface-limited Redox Replacement Reaction. Chem. Lett. 2017, 46, 1250–1253. [Google Scholar] [CrossRef]
- Kaito, T.; Mitsumoto, H.; Sugawara, S.; Shinohara, K.; Uehara, H.; Ariga, H.; Takakusagi, S.; Hatakeyama, Y.; Nishikawa, K.; Asakura, K. K-Edge X-ray Absorption Fine Structure Analysis of Pt/Au Core–Shell Electrocatalyst: Evidence for Short Pt–Pt Distance. J. Phys. Chem. C 2014, 118, 8481–8490. [Google Scholar] [CrossRef]
- Kaito, T.; Mitsumoto, H.; Sugawara, S.; Shinohara, K.; Uehara, H.; Ariga, H.; Takakusagi, S.; Asakura, K. A new spectroelectrochemical cell for in situ measurement of Pt and Au K-edge X-ray absorption fine structure. Rev. Sci. Instrum. 2014, 85, 084104. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, P.; de Groot, F.M.F.; Manoilova, O.; Grandjean, D.; Weckhuysen, B.M.; Bergmann, U.; Barrea, R. Range-extended EXAFS at the $L$ edge of rare earths using high-energy-resolution fluorescence detection: A study of La in LaOCl. Phys. Rev. B 2005, 72, 014117. [Google Scholar] [CrossRef]
- Yano, J.; Pushkar, Y.; Glatzel, P.; Lewis, A.; Sauer, K.; Messinger, J.; Bergmann, U.; Yachandra, V. High-Resolution Mn EXAFS of the Oxygen-Evolving Complex in Photosystem II: Structural Implications for the Mn4Ca Cluster. J. Am. Chem. Soc. 2005, 127, 14974–14975. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Kawamura, N.; Mizumaki, M.; Nitta, K.; Ishii, K.; Hosokawa, S.; Teramura, K.; Tanaka, T. A feasibility study of “range-extended” EXAFS measurement at the Pt L3-edge of Pt/Al2O3 in the presence of Au2O3. J. Anal. At. Spectrom. 2018, 33, 84–89. [Google Scholar] [CrossRef]
- Zhong, Z.; Chapman, L.D.; Bunker, B.A.; Bunker, G.B.; Fischetti, R.; Segre, C.U. A bent Laue analyzer for fluorescence EXAFS detection. J. Synchrotron Radiat. 1999, 6, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Kujala, N.G.; Karanfil, C.; Barrea, R.A. High resolution short focal distance Bent Crystal Laue Analyzer for copper K edge X-ray absorption spectroscopy. Rev. Sci. Instrum. 2011, 82, 063106. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, M.; Glatzel, P. A tool to plan photon-in/photon-out experiments: Count rates, dips and self-absorption. J. Synchrotron Radiat. 2012, 19, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Karanfil, C.; Bunker, G.; Newville, M.; Segre, C.U.; Chapman, D. Quantitative performance measurements of bent crystal Laue analyzers for X-ray fluorescence spectroscopy. J. Synchrotron Radiat. 2012, 19, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Friebel, D.; Viswanathan, V.; Miller, D.J.; Anniyev, T.; Ogasawara, H.; Larsen, A.H.; O’Grady, C.P.; Nørskov, J.K.; Nilsson, A. Balance of Nanostructure and Bimetallic Interactions in Pt ModelFuel Cell Catalysts: In Situ XAS and DFT Study. J. Am. Chem. Soc. 2012, 134, 9664–9671. [Google Scholar] [CrossRef] [PubMed]
- Merte, L.R.; Behafarid, F.; Miller, D.J.; Friebel, D.; Cho, S.; Mbuga, F.; Sokaras, D.; Alonso-Mori, D.; Weng, T.-C.; Nordlund, D.; et al. Electrochemical Oxidation of Size-Selected Pt Nanoparticles Studied Using in Situ High-Energy-Resolution X-ray Absorption Spectroscopy. ACS Catal. 2012, 2, 2371–2376. [Google Scholar] [CrossRef]
- Cui, Y.-T.; Harada, Y.; Niwa, H.; Hatanaka, T.; Nakamura, N.; Ando, M.; Yoshida, T.; Ishii, K.; Matsumura, D.; Oji, H.; et al. Wetting Induced Oxidation of Pt-based Nano Catalysts Revealed by In Situ High Energy Resolution X-ray Absorption Spectroscopy. Sci. Rep. 2017, 7, 1482. [Google Scholar] [CrossRef] [PubMed]
- Wakisaka, Y.; Iwasaki, Y.; Uehara, H.; Mukai, S.; Kido, D.; Takakusgi, S.; Uemura, Y.; Wada, T.; Yuan, Q.; Sekizawa, O.; et al. Approach to Highly Sensitive XAFS by Means of Bent Crystal Laue Analyzers. J. Surf. Sci. Soc. Jpn. 2017, 38, 378–383. [Google Scholar] [CrossRef]
- Takahashi, S.; Takahashi, N.; Todoroki, N.; Wadayama, T. Dealloying of Nitrogen-Introduced Pt–Co Alloy Nanoparticles: Preferential Core–Shell Formation with Enhanced Activity for Oxygen Reduction Reaction. ACS Omega 2016, 1, 1247–1252. [Google Scholar] [CrossRef]
- Liu, Y.; Hangarter, C.M.; Garcia, D.; Moffat, T.P. Self-terminating electrodeposition of ultrathin Pt films on Ni: An active, low-cost electrode for H2 production. Surf. Sci. 2015, 631, 141–154. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakisaka, Y.; Kido, D.; Uehara, H.; Yuan, Q.; Takakusagi, S.; Uemura, Y.; Yokoyama, T.; Wada, T.; Uo, M.; Sakata, T.; et al. A Demonstration of Pt L3-Edge EXAFS Free from Au L3-Edge Using Log–Spiral Bent Crystal Laue Analyzers. Catalysts 2018, 8, 204. https://doi.org/10.3390/catal8050204
Wakisaka Y, Kido D, Uehara H, Yuan Q, Takakusagi S, Uemura Y, Yokoyama T, Wada T, Uo M, Sakata T, et al. A Demonstration of Pt L3-Edge EXAFS Free from Au L3-Edge Using Log–Spiral Bent Crystal Laue Analyzers. Catalysts. 2018; 8(5):204. https://doi.org/10.3390/catal8050204
Chicago/Turabian StyleWakisaka, Yuki, Daiki Kido, Hiromitsu Uehara, Qiuyi Yuan, Satoru Takakusagi, Yohei Uemura, Toshihiko Yokoyama, Takahiro Wada, Motohiro Uo, Tomohiro Sakata, and et al. 2018. "A Demonstration of Pt L3-Edge EXAFS Free from Au L3-Edge Using Log–Spiral Bent Crystal Laue Analyzers" Catalysts 8, no. 5: 204. https://doi.org/10.3390/catal8050204