The Pros and Cons of Polydopamine-Sensitized Titanium Oxide for the Photoreduction of CO2
Abstract
:1. Introduction
2. Results and Discussions
2.1. Physical and Optical Properties
2.2. Physical and Optical Properties
2.3. Photo-Stability Evaluation
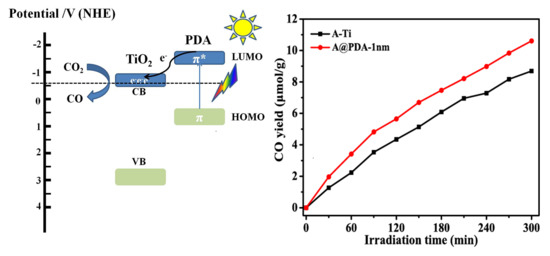
2.4. Proposed Reaction Mechanisms
3. Experimental Section
3.1. Materials and Methods
3.1.1. Preparation of TiO2@PDA Composites
3.1.2. Preparation of PDA
3.2. Characterizations
3.3. Photocatalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Yaumi, A.L.; Abu Bakar, M.Z.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Tu, W.G.; Zou, Z.G. New Materials for CO2 Photoreduction. In Photocatalysis: Fundamentals and Perspectives; Schneider, J., Bahnemann, D., Ye, J.H., Puma, G.L., Dionysiou, D.D., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 318–322. ISBN 978-1-78262-041-9. [Google Scholar]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Li, X.; Wen, J.Q.; Low, J.X.; Fang, Y.P.; Yu, J.G. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100. [Google Scholar] [CrossRef]
- Chang, X.X.; Wang, T.; Gong, J.L. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.L.; Jia, Y.S.; Chen, X.B.; Han, H.X.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Hoivik, N.; Wang, K.Y.; Jakobsen, H. Engineering TiO2nanomaterials for CO2 conversion/solar fuels. Sol. Energy Mater. Sol. Cells 2012, 105, 53–68. [Google Scholar] [CrossRef]
- Das, S.; Daud, W. Photocatalytic CO2 transformation into fuel: A review on advances in photocatalyst and photoreactor. Renew. Sustain. Energy Rev. 2014, 39, 765–805. [Google Scholar] [CrossRef]
- Zhao, H.L.; Pan, F.P.; Li, Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Materiomics 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Ozcan, O.; Yukruk, F.; Akkaya, E.U.; Uner, D. Dye sensitized artificial photosynthesis in the gas phase over thin and thick TiO2 films under UV and visible light irradiation. Appl. Catal. B Environ. 2007, 71, 291–297. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Wu, J.C.S.; Chiou, C.H. Photoreduction of CO2 over Ruthenium dye-sensitized TiO2-based catalysts under concentrated natural sunlight. Catal. Commun. 2008, 9, 2073–2076. [Google Scholar] [CrossRef]
- Qin, G.H.; Zhang, Y.; Ke, X.B.; Tong, X.L.; Sun, Z.; Liang, M.; Xue, S. Photocatalytic reduction of carbon dioxide to formic acid, formaldehyde, and methanol using dye-sensitized TiO2 film. Appl. Catal. B Environ. 2013, 129, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Won, D.I.; Lee, J.S.; Ji, J.M.; Jung, W.J.; Son, H.J.; Pac, C.; Kang, S.O. Highly Robust Hybrid Photocatalyst for Carbon Dioxide Reduction: Tuning and Optimization of Catalytic Activities of Dye/TiO2/Re(I) Organic-Inorganic Ternary Systems. J. Am. Chem. Soc. 2015, 137, 13679–13690. [Google Scholar] [CrossRef] [PubMed]
- Do, J.Y.; Tamilavan, V.; Agneeswari, R.; Hyun, M.H.; Kang, M. Synthesis and optical properties of TDQD and effective CO2 reduction using a TDQD-photosensitized TiO2 film. J. Photochem. Photobiol. A Chem. 2016, 330, 30–36. [Google Scholar] [CrossRef]
- Huang, H.W.; Lin, J.J.; Zhu, G.B.; Weng, Y.X.; Wang, X.X.; Fu, X.Z.; Long, J.L. A Long-Lived Mononuclear Cyclopentadienyl Ruthenium Complex Grafted onto Anatase TiO2 for Efficient CO2 Photoreduction. Angew. Chem. Int. Ed. 2016, 55, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Iadonisi, A.; Valerio, S.; Panzella, L.; Napolitano, A.; Adinolfi, M.; d’Ischia, M. Disentangling Eumelanin “Black Chromophore”: Visible Absorption Changes As Signatures of Oxidation State- and Aggregation-Dependent Dynamic Interactions in a Model Water-Soluble 5,6-Dihydroxyindole Polymer. J. Am. Chem. Soc. 2009, 131, 15270–15275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and Eumelanin: From Structure-Property Relationships to a Unified Tailoring Strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Son, E.J.; Kim, J.H.; Kim, K.; Park, C.B. Quinone and its derivatives for energy harvesting and storage materials. J. Mater. Chem. A 2016, 4, 11179–11202. [Google Scholar] [CrossRef]
- Mao, W.X.; Lin, X.J.; Zhang, W.; Chi, Z.X.; Lyu, R.W.; Cao, A.M.; Wan, L.J. Core-shell structured TiO2@polydopamine for highly active visible-light photocatalysis. Chem. Commun. 2016, 52, 7122–7125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.S.; Jin, B.; Luo, J.; Xu, X.Y.; Zhang, L.L.; Li, J.J.; Guan, H.J. Dramatic visible light photocatalytic degradation due to the synergetic effects of TiO2 and PDA nanospheres. RSC Adv. 2016, 6, 64446–64449. [Google Scholar] [CrossRef]
- Kim, S.; Moon, G.H.; Kim, G.; Kang, U.; Park, H.; Choi, W. TiO2 complexed with dopamine-derived polymers and the visible light photocatalytic activities for water pollutants. J. Catal. 2017, 346, 92–100. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Yu, Y.G.; Zhou, Y.S.; Zheng, Y.; Hao, S. The synthesis of condensed C-PDA-g-C3N4 composites with superior photocatalytic performance. Chem. Commun. 2015, 51, 6824–6827. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.X.; Li, F.; Yang, Q.B.; Shi, H.; Chen, Q.; Xu, M. Nature-Mimic Method To Fabricate Polydopamine/Graphitic Carbon Nitride for Enhancing Photocatalytic Degradation Performance. ACS Sustain. Chem. Eng. 2017, 5, 7840–7850. [Google Scholar] [CrossRef]
- Zhou, X.S.; Jin, B.; Luo, J.; Gu, X.X.; Zhang, S.Q. Photoreduction preparation of Cu2O@polydopamine nanospheres with enhanced photocatalytic activity under visible light irradiation. J. Solid State Chem. 2017, 254, 55–61. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.C.; Wan, L.S.; Liang, H.Q.; Li, H.Y.; Xu, Z.K. Polydopamine-Coated Porous Substrates as a Platform for Mineralized beta-FeOOH Nanorods with Photocatalysis under Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.M.; Zhang, K.; Wu, F.; Wang, N.N.; Wang, Y.; Wang, M.Y. Polydopamine nanofilms as visible light-harvesting interfaces for palladium nanocrystal catalyzed coupling reactions. Catal. Sci. Technol. 2016, 6, 1764–1771. [Google Scholar] [CrossRef]
- Feng, J.J.; Zhang, P.P.; Wang, A.J.; Liao, Q.C.; Xi, J.L.; Chen, J.R. One-step synthesis of monodisperse polydopamine-coated silver core-shell nanostructures for enhanced photocatalysis. New J. Chem. 2012, 36, 148–154. [Google Scholar] [CrossRef]
- Ohsaka, T. Temperature dependence of the Raman spectrum in anatase TiO2. J. Phys. Soc. Jpn. 1980, 48, 1661–1668. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S.B. Size effects in the Raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Ye, W.C.; Wang, D.A.; Zhang, H.; Zhou, F.; Liu, W.M. Electrochemical growth of flowerlike gold nanoparticles on polydopamine modified ITO glass for SERS application. Electrochim. Acta 2010, 55, 2004–2009. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Magne, C.; Dufour, F.; Labat, F.; Lancel, G.; Durupthy, O.; Cassaignon, S.; Pauporte, T. Effects of TiO2 nanoparticle polymorphism on dye-sensitized solar cell photovoltaic properties. J. Photochem. Photobiol. A Chem. 2012, 232, 22–31. [Google Scholar] [CrossRef]
- Akimov, A.V.; Neukirch, A.J.; Prezhdo, O.V. Theoretical Insights into Photoinduced Charge Transfer and Catalysis at Oxide Interfaces. Chem. Rev. 2013, 113, 4496–4565. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Fratesi, G.; Selcuk, S.; Brivio, G.P.; Selloni, A. Effects of Thermal Fluctuations on the Structure, Level Alignment, and Absorption Spectrum of Dye-Sensitized TiO2: A Comparative Study of Catechol and Isonicotinic Acid on the Anatase (101) and Rutile (110) Surfaces. J. Phys. Chem. C 2016, 120, 3899–3905. [Google Scholar] [CrossRef]
- Phua, S.L.; Yang, L.P.; Toh, C.L.; Ding, G.Q.; Lau, S.K.; Dasari, A.; Lu, X.H. Simultaneous Enhancements of UV Resistance and Mechanical Properties of Polypropylene by Incorporation of Dopamine-Modified Clay. ACS Appl. Mater. Interfaces 2013, 5, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Duan, L.; Cheng, X.J.; Ran, X.Q. Effect of polydopamine coating on improving photostability of poly(1,3,4-oxadiazole)s fiber. J. Polym. Res. 2016, 23. [Google Scholar] [CrossRef]
- Proks, V.; Brus, J.; Pop-Georgievski, O.; Vecernikova, E.; Wisniewski, W.; Kotek, J.; Urbanova, M.; Rypacek, F. Thermal-Induced Transformation of Polydopamine Structures: An Efficient Route for the Stabilization of the Polydopamine Surfaces. Macromol. Chem. Phys. 2013, 214, 499–507. [Google Scholar] [CrossRef]
- Wang, Z.H.; Tang, F.; Fan, H.L.; Wang, L.; Jin, Z.X. Polydopamine Generates Hydroxyl Free Radicals under Ultraviolet-Light Illumination. Langmuir 2017, 33, 5938–5946. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Chen, J.E.; Pang, Z.F.; Li, S.H.; Ling, D.S.; Deng, F.; Kong, X.Q. Understanding Surface and Interfacial Chemistry in Functional Nanomaterials via Solid-State NMR. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Adhyaru, B.B.; Akhmedov, N.G.; Katritzky, A.R.; Bowers, C.R. Solid-state cross-polarization magic angle spinning 13C and 15N NMR characterization of Sepia melanin, Sepia melanin free acid and Human hair melanin in comparison with several model compounds. Magn. Reson. Chem. 2003, 41, 466–474. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrowczynski, R.; Scheidt, H.A.; Filip, C.; Hadade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C: Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ai, K.L.; Liu, Y.L.; Ruan, C.P.; Lu, L.H.; Lu, G.Q. Sp2 C-Dominant N-Doped Carbon Sub-micrometer Spheres with a Tunable Size: A Versatile Platform for Highly Efficient Oxygen-Reduction Catalysts. Adv. Mater. 2013, 25, 998–1003. [Google Scholar] [CrossRef] [PubMed]





| Photocatalyst | SBET (m2/g) | Mean Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| A-Ti | 58.2 | 21.5 | 0.31 |
| A@PDA-1 nm | 60.1 | 23.7 | 0.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xia, M.; Kong, X. The Pros and Cons of Polydopamine-Sensitized Titanium Oxide for the Photoreduction of CO2. Catalysts 2018, 8, 215. https://doi.org/10.3390/catal8050215
Wang T, Xia M, Kong X. The Pros and Cons of Polydopamine-Sensitized Titanium Oxide for the Photoreduction of CO2. Catalysts. 2018; 8(5):215. https://doi.org/10.3390/catal8050215
Chicago/Turabian StyleWang, Tongyao, Ming Xia, and Xueqian Kong. 2018. "The Pros and Cons of Polydopamine-Sensitized Titanium Oxide for the Photoreduction of CO2" Catalysts 8, no. 5: 215. https://doi.org/10.3390/catal8050215