Selective Reduction of Ketones and Aldehydes in Continuous-Flow Microreactor—Kinetic Studies
Abstract
:1. Introduction
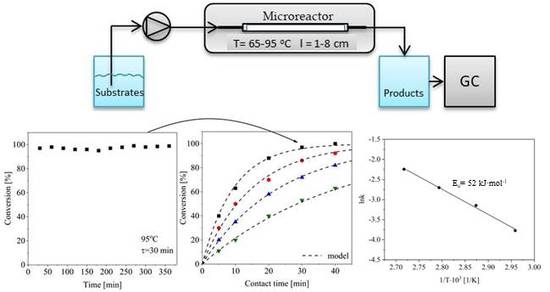
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, S.H.; Jaenicke, S.; Chuah, G.K. Hydrous zirconia as a selective catalyst for the Meerwein–Ponndorf–Verley reduction of cinnamaldehyde. J. Catal. 2002, 206, 321–330. [Google Scholar] [CrossRef]
- Plessers, E.; Fu, G.X.; Tan, C.Y.X.; De Vos, D.E.; Roeffaers, M.B.J. Zr-based MOF-808 as Meerwein–Ponndorf–Verley reduction catalyst for challenging carbonyl compounds. Catalysts 2016, 6, 104. [Google Scholar] [CrossRef]
- Axpuac, S.; Aramendía, M.A.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Montes-Jiménez, V.; Urbano, F.J.; Borau, V. Study of structure–performance relationships in Meerwein–Ponndorf–Verley reduction of crotonaldehyde on several magnesium and zirconium-based systems. Catal. Today 2012, 187, 183–190. [Google Scholar] [CrossRef]
- Zapilko, C.; Liang, Y.C.; Nerdal, W.; Anwander, R. A general strategy for the rational design of size-selective mesoporous catalysts. Chem. Eur. J. 2007, 13, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Aramendı́a, M.A.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F. Reduction of α,β-unsaturated aldehydes with basic MgO/M2O3 catalysts (M=Al, Ga, In). Appl. Catal. A Gen. 2003, 249, 1–9. [Google Scholar] [CrossRef]
- Uysal, B.; Oksal, B.S. A new method for the chemoselective reduction of aldehydes and ketones using boron tri-isopropoxide, B(OiPr)3: Comparison with boron tri-ethoxide, B(OEt)3. J. Chem. Sci. 2011, 123, 681–685. [Google Scholar] [CrossRef]
- Uysal, B.; Oksal, B.S. New heterogeneous B(OEt)3-MCM-41 catalyst for preparation of α,β-unsaturated alcohols. Res. Chem. Intermed. 2015, 41, 3893–3911. [Google Scholar] [CrossRef]
- Meerwein, H.; Schmidt, R. Ein neues verfahren zur reduktion von aldehyden und ketonen. Justus Liebigs Ann. Chem. 1925, 444, 221–238. [Google Scholar] [CrossRef]
- Ooi, T.; Miura, T.; Takaya, K.; Ichikawa, H.; Maruoka, K. Zr(OBut)4 as an effective promoter for the Meerwein–Ponndorf–Verley alkynylation and cyanation of aldehydes: Development of new asymmetric cyanohydrin synthesis. Tetrahedron 2001, 57, 867–873. [Google Scholar] [CrossRef]
- Wang, J.; Okumura, K.; Jaenicke, S.; Chuah, G.K. Post-synthesized zirconium-containing beta zeolite in Meerwein–Ponndorf–Verley reduction: Pros and cons. Appl. Catal. A Gen. 2015, 493, 112–120. [Google Scholar] [CrossRef]
- Creyghton, E.J.; Downing, R.S. Shape-selective hydrogenation and hydrogen transfer reactions over zeolite catalysts. J. Mol. Catal. A Chem. 1998, 134, 47–61. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Yuan, J.; Wang, L.; Deng, B.X. Meerwein–Ponndorf–Verley reaction of acetophenone over ZrO2-La2O3/MCM-41: Influence of loading order of ZrO2 and La2O3. Catal. Commun. 2017, 92, 46–50. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Tolborg, S.; Taarning, E. Meerwein–Ponndorf–Verley-Oppenauer reaction of crotonaldehyde with ethanol over Zr-containing catalysts. J. Catal. 2014, 316, 121–129. [Google Scholar] [CrossRef]
- Rodriguez-Castellon, E.; Jimenez-Lopez, A.; Maireles-Torres, P.; Jones, D.I.; Roziere, J.; Trombetta, M.; Busca, G.; Lenarda, M.; Storaro, L. Textural and structural properties and surface acidity characterization of mesoporous silica-zirconia molecular sieves. J. Solid State Chem. 2003, 175, 159–169. [Google Scholar] [CrossRef]
- Jimenez-Sanchidrian, C.; Ruiz, J.R. Tin-containing hydrotalcite-like compounds as catalysts for the Meerwein–Ponndorf–Verley reaction. Appl. Catal. A Gen. 2014, 469, 367–372. [Google Scholar] [CrossRef]
- Battilocchio, C.; Hawkins, J.M.; Ley, S.V. A mild and efficient flow procedure for the transfer hydrogenation of ketones and aldehydes using hydrous zirconia. Org. Lett. 2013, 15, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Koreniuk, A.; Maresz, K.; Mrowiec-Białoń, J. Supported zirconium-based continuous-flow microreactor for effective Meerwein–Ponndorf–Verley reduction of cyclohexanone. Catal. Commun. 2015, 64, 48–51. [Google Scholar] [CrossRef]
- Ciemięga, A.; Maresz, K.; Mrowiec-Białoń, J. Continuous-flow chemoselective reduction of cyclohexanone in a monolithic silica-supported Zr(OPri)4 multichannel microreactor. Microporous Mesoporous Mater. 2017, 252, 140–145. [Google Scholar] [CrossRef]
- Maresz, K.; Ciemięga, A.; Mrowiec-Białoń, J. Meervein–Ponndorf–Vereley reduction of carbonyl compounds in monolithic siliceous microreactors doped with Lewis acid centers. Appl. Catal. A Gen. 2018, 560, 111–118. [Google Scholar]
- Ciemięga, A.; Maresz, K.; Malinowski, J.J.; Mrowiec-Białoń, J. Continuous-flow monolithic silica microreactors with arenesulfonic acid groups: Structure-catalytic activity relationships. Catalysts 2017, 7, 255. [Google Scholar] [CrossRef]
- Li, G.; Fu, W.H.; Wang, Y.M. Meervvein–Ponndorf–Verley reduction of cyclohexanone catalyzed by partially crystalline zirconosilicate. Catal. Commun. 2015, 62, 10–13. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-resistant solid Lewis acid catalysts: Meerwein–Ponndorf–Verley and Oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Jiménez-Sanchidrián, C.; Hidalgo, J.M.; Marinas, J.M. Reduction of ketones and aldehydes to alcohols with magnesium–aluminium mixed oxide and 2-propanol. J. Mol. Catal. A Chem. 2006, 246, 190–194. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Activity of basic catalysts in the Meerwein–Ponndorf–Verley reaction of benzaldehyde with ethanol. J. Colloid Interface Sci. 2001, 238, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Kourti, M.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Esters of some non-steroidal anti-inflammatory drugs with cinnamyl alcohol are potent lipoxygenase inhibitors with enhanced anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2015, 25, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Schneider, Y.; Ceraline, J.; Duranton, B.; Gosse, F.; Seiler, N.; Raul, F. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J. Pharmacol. Exp. Ther. 2001, 298, 197–200. [Google Scholar] [PubMed]






| Substrate | K 1 [min−1] | Conversion 1,* [%] | Productivity 1,* [mmol·gcat−1·h−1] | Productivity 2 [mmol·gcat−1·h−1] | Productivity 3 [mmol·gcat−1·h−1] |
|---|---|---|---|---|---|
 | 0.106 | 88 | 2.22 | - | 2.28 [21] |
 | 0.021 | 35 | 0.9 | - | 0.11 [15] |
 | 0.212 | 99 | 2.64 | 1.14 [16] | 0.36 [10] |
 | 0.081 | 80 | 1.92 | 0.66 [16] | 0.48 [5] |
 | 0.031/0.047 4 | 46/61 | 0.48/0.9 | - | 0.54 [5] |
 | 0.026 | 40 | 0.96 | 0.48 [16] | 0.12 [22] |
 | 0.08 | 80 | 2.1 | - | - |
 | 0.041 | 56 | 1.32 | - | 0.72 [23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maresz, K.; Ciemięga, A.; Mrowiec-Białoń, J. Selective Reduction of Ketones and Aldehydes in Continuous-Flow Microreactor—Kinetic Studies. Catalysts 2018, 8, 221. https://doi.org/10.3390/catal8050221
Maresz K, Ciemięga A, Mrowiec-Białoń J. Selective Reduction of Ketones and Aldehydes in Continuous-Flow Microreactor—Kinetic Studies. Catalysts. 2018; 8(5):221. https://doi.org/10.3390/catal8050221
Chicago/Turabian StyleMaresz, Katarzyna, Agnieszka Ciemięga, and Julita Mrowiec-Białoń. 2018. "Selective Reduction of Ketones and Aldehydes in Continuous-Flow Microreactor—Kinetic Studies" Catalysts 8, no. 5: 221. https://doi.org/10.3390/catal8050221