MgO-Templated Mesoporous Carbon as a Catalyst Support for Polymer Electrolyte Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carbon Characteristics
2.1.1. Heat-Treatment
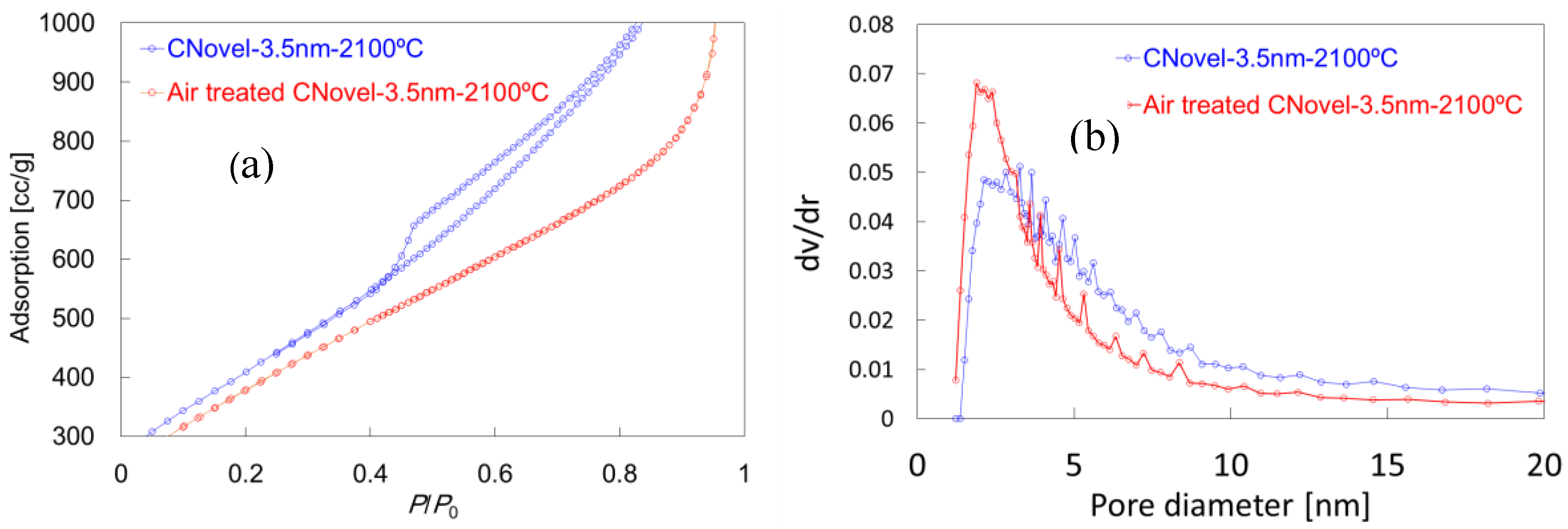
2.1.2. Air Treatment
2.1.3. Bead-Milling
2.1.4. Functionalization
2.2. Pt/C
2.3. Electrochemical Characteristics
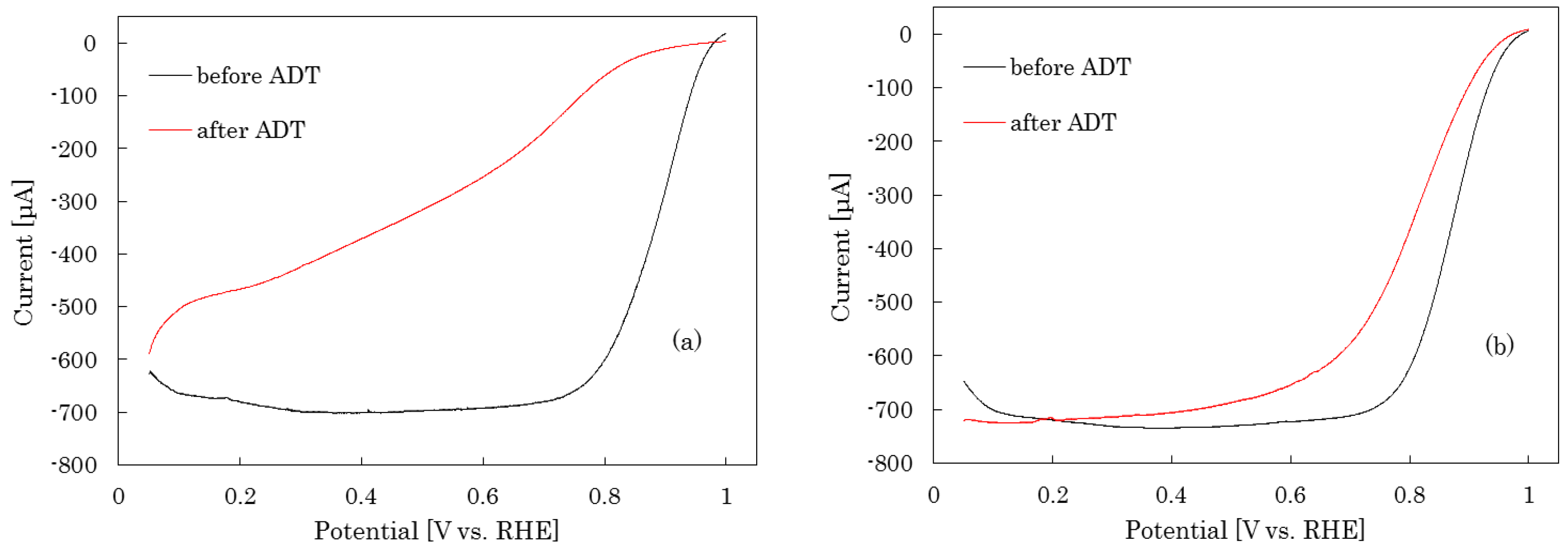
2.3.1. RDE Measurement
2.3.2. MEA Test
3. Experimental
3.1. Sample Preparation
3.1.1. Materials
3.1.2. Heat Treatment for Gratification
3.1.3. Air Treatment
3.1.4. Bead Milling
3.1.5. Functionalization
3.1.6. Platinum Deposition
3.1.7. Rotating Disk Electrode (RDE) Thin Film Preparation
3.1.8. Membrane Electrode Assembly (MEA) Preparation
3.2. Characterization
3.2.1. Carbon
3.2.2. Pt/C
3.2.3. Electrochemical Characterization by RDE
3.2.4. MEA Performance Test & Sulfonic Coverage Measurement
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. Temperature-controlled growth of single-crystal Pt nanowire arrays for high performance catalyst electrodes in polymer electrolyte fuel cells. Appl. Catal. B Environ. 2015, 164, 389–395. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. Three-dimensional catalyst electrodes based on PtPd nanodendrites for oxygen reduction reaction in PEFC applications. Appl. Catal. B Environ. 2016, 187, 108–114. [Google Scholar] [CrossRef]
- Du, S.; Millington, B.; Pollet, B.G. The effect of Nafion ionomer loading coated on gas diffusion electrodes with in-situ grown Pt nanowires and their durability in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2011, 36, 4386–4393. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Debe, M.K. Novel catalysts, catalysts support and catalysts coated membrane methods. In Handbook of Fuel Cells; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Subbaraman, R.; Strmcnik, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Oxygen Reduction Reaction at Three-Phase Interfaces. ChemPhysChem 2010, 11, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kudo, K.; Morimoto, Y. Model for investigation of oxygen transport limitation in a polymer electrolyte fuel cell. J. Power Sources 2013, 222, 379–389. [Google Scholar] [CrossRef]
- Mashio, T.; Ohma, A.; Yamamoto, S.; Shinohara, K. Analysis of Reactant Gas Transport in a Catalyst Layer. ECS Trans. 2007, 11, 529–540. [Google Scholar]
- Kodama, K.; Jinnouchi, R.; Suzuki, T.; Murata, H.; Hatanaka, T.; Morimoto, Y. Increase in adsorptivity of sulfonate anions on Pt (111) surface with drying of ionomer. Electrochem. Commun. 2013, 36, 26–28. [Google Scholar] [CrossRef]
- Subbaraman, R.; Strmcnik, D.; Stamenkovic, V.; Markovic, N.M. Three Phase Interfaces at Electrified Metal−Solid Electrolyte Systems 1. Study of the Pt(hkl)−Nafion Interface. J. Phys. Chem. C 2010, 114, 8414–8422. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kitano, N.; Morimoto, Y. Molecular Dynamics Simulations on O2 Permeation through Nafion Ionomer on Platinum Surface. Electrochim. Acta 2016, 188, 767–776. [Google Scholar] [CrossRef]
- Kudo, K.; Jinnouchi, R.; Morimoto, Y. Humidity and Temperature Dependences of Oxygen Transport Resistance of Nafion Thin Film on Platinum Electrode. Electrochim. Acta 2016, 209, 682–690. [Google Scholar] [CrossRef]
- Kodama, K.; Shinohara, A.; Hasegawa, N.; Shinozaki, K.; Jinnouchi, R.; Suzuki, T.; Hatanaka, T.; Morimoto, Y. Catalyst Poisoning Property of Sulfonimide Acid Ionomer on Pt (111) Surface. J. Electrochem. Soc. 2014, 161, F649–F652. [Google Scholar] [CrossRef]
- Ito, T.; Matsuwaki, U.; Otsuka, Y.; Hatta, M.; Hayakawa, K.; Matsutani, K.; Tada, T.; Jinnai, H. Three-Dimensional Spatial Distributions of Pt Catalyst Nanoparticles on Carbon Substrates in Polymer Electrolyte Fuel Cells. Electrochemistry 2011, 79, 374–376. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Yamada, H.; Morimoto, Y. Relative Humidity Dependence of Pt Utilization in Polymer Electrolyte Fuel Cell Electrodes: Effects of Electrode Thickness, Ionomer-to-Carbon Ratio, Ionomer Equivalent Weight, and Carbon Support. J. Electrochem. Soc. 2011, 158, B467–B475. [Google Scholar] [CrossRef]
- Shinozaki, K.; Morimoto, Y.; Pivovar, B.S.; Kocha, S.S. Suppression of oxygen reduction reaction activity on Pt-based electrocatalysts from ionomer incorporation. J. Power Sources 2016, 325, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Iden, H.; Mashio, T.; Ohma, A. Gas transport inside and outside carbon supports of catalyst layers for PEM fuel cells. J. Electroanal. Chem. 2013, 708, 87–94. [Google Scholar] [CrossRef]
- Iden, H.; Ohma, A. An in situ technique for analyzing ionomer coverage in catalyst layers. J. Electroanal. Chem. 2013, 693, 34–41. [Google Scholar] [CrossRef]
- Chan, K.; Eikerling, M. A Pore-Scale Model of Oxygen Reduction in Ionomer-Free Catalyst Layers of PEFCs. J. Electrochem. Soc. 2011, 158, B18–B28. [Google Scholar] [CrossRef]
- Ding, J.; Chan, K.-Y.; Ren, J.; Xiao, F.-S. Platinum and platinum–ruthenium nanoparticles supported on ordered mesoporous carbon and their electrocatalytic performance for fuel cell reactions. Electrochim. Acta 2005, 50, 3131–3141. [Google Scholar] [CrossRef]
- Joo, S.H.; Pak, C.; You, D.J.; Lee, S.-A.; Lee, H.I.; Kim, J.M.; Chang, H.; Seung, D. Ordered mesoporous carbons (OMC) as supports of electrocatalysts for direct methanol fuel cells (DMFC): Effect of carbon precursors of OMC on DMFC performances. Electrochim. Acta 2006, 52, 1618–1626. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Quintana, J.J.; Calvillo, L.; Lazaro, M.J.; Cabot, P.L.; Esparbe, I.; Pastor, E. Carbon monoxide and methanol oxidation at platinum catalysts supported on ordered mesoporous carbon: The influence of functionalization of the support. Phys. Chem. Chem. Phys. 2008, 10, 6796–6806. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liang, Y.; Li, Z.; Wang, Y.; Fu, R.; Wu, D.; Tsiakaras, P. Effect of pore morphology of mesoporous carbons on the electrocatalytic activity of Pt nanoparticles for fuel cell reactions. Appl. Catal. B Environ. 2010, 98, 132–137. [Google Scholar] [CrossRef]
- Hori, M.; Kato, H.; Matsumoto, S.; Nishi, N. FC Catalyst with Mesoporous Carbon. Meet. Abstr. 2013, MA2013-02, 1500. Available online: https://ecs.confex.com/ecs/224/webprogram/Paper22769.html (accessed on 31 May 2018).
- Toyo Tanso New Developed Product Porous Carbon (CNovel®). Available online: http://www.toyotanso.com/Products/new_developed_products/cnovel.html (accessed on 28 January 2018).
- Toyo Tanso Carbon Products. Available online: http://www.toyotanso.co.jp/Products/faq/cnovel/1701.cnv1.html (accessed on 20 May 2018). (In Japanese).
- Dollimore, D.; Heal, G.R. Pore-size distribution in typical adsorbent systems. J. Colloid Interface Sci. 1970, 33, 508–519. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W.K. Adsorption, Surface Area, and Porosity; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Sugita, Y.; Ito, K. Platinum Black Powder, Platinum Black Colloid, Method for Producing Platinum Black Powder, and Method for Producing Platinum Black Colloid. WO2010082443A1, 22 July 2010. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Furuya, Y.; Mashio, T.; Ohama, A.; Shinohara, K. Evaluation of Anion Adsorption on Pt Surface in MEA. Meet. Abstr. 2012, MA2012-02, 1272. Available online: http://ma.ecsdl.org/content/MA2012-02/13/1272.full.pdf (accessed on 31 May 2018).










| Sample | Specific Surface Area [m2/g] |
|---|---|
| Ketjen black | 800 |
| Vulcan XC | 250 |
| CNovel 3.5 nm as-is | 1270 |
| CNovel 5 nm as-is | 1550 |
| CNovel 10 nm as-is | 1630 |
| CNovel 5 nm @1700 °C | 1550 |
| CNovel 5 nm @1900 °C | 1490 |
| CNovel 5 nm @2100 °C | 1270 |
| CNovel 5 nm @2100 °C Air treated@500 °C | 1380 |
| CNovel 5 nm @2100 °C Air treated@530 °C | 1280 |
| CNovel 5 nm @2100 °C Air treated@540 °C | 890 |
| CNovel 5 nm @2100 °C Air treated@550 °C | 540 |
| Sample | Acidic Entity Density [mmol/g] |
|---|---|
| CNovel 5 nm as-is | 0.34 |
| 2100 °C treated | Not detected |
| KMnO4 treated | 2.0 |
| HNO3 treated | 0.073 |
| Catalyst | ECSA | Particle Size [m] | Mass Activity | Specific Activity | |
|---|---|---|---|---|---|
| [m2/gPt] | TEM | ECSA * | [A/gPt] | [μA/cmPt2] | |
| Pt/Vulcan | 80 ± 1 | 2.6 | 3.5 ± 0.1 | 550 ± 20 | 690 ± 30 |
| Pt/CNovel-3.5 nmHT | 121 ± 3 | 2.6 | 2.3 ± 0.1 | 680 ± 70 | 560 ± 60 |
| Pt/CNovel-5 nmHT | 93 ± 9 | 2.7 | 3.0 ± 0.3 | 410 ± 40 | 440 ± 60 |
| Pt/CNovel-5 nmAsIs | 74 ± 2 | 2.4 | 3.8 ± 0.1 | 250 ± 40 | 340 ± 60 |
| Pt/CNovel-10 nmHT | 110 ± 15 | 2.8 | 2.5 ± 0.3 | 620 ± 80 | 600 ± 100 |
| Condition | Detail |
|---|---|
| Apparatus | Micro media MMPC-X1, Bühler |
| Dispersion medium | 50 wt. % EtOH in water |
| Concentration | 12 g/L |
| Dispersion Volume | 1 L |
| Bead | YSZ (0.3 mm in diameter) |
| Bead volume (Volume%) | 90vol% |
| Rotor and Stator material | SiC |
| Rotor speed | 10 m/s * |
| Temperature | 18–27 °C |
| Duration | 60 min |
| Step | Condition |
|---|---|
| 1 Electrolyte deaeration | Ar bubbling for 30 min at 30 °C |
| 2 Electrode cleaning | Potential cycles: 0.05–1.2 V, 500 mV/s, 50 cycles |
| 3 Electrochemical surface area (ECSA) evaluation | Cyclic voltammetry: 0.05–1.0 V, 100 mV/s, 5 cycles |
| 4 Gas exchange | O2 bubbling for 30 min. |
| 5 ORR current measurement | Cyclic voltammetry: 0.05–1.0 V, 10 mV/s, 400 rpm * |
| 6 Reevaluation of ECSA | Repeat Step 1–3 |
| 7 Background evaluation | Linear sweep voltammetry: 0.05–1.0 V, 10 mV/s, 400 rpm |
| Step | Condition |
|---|---|
| 1 ORR activity measurement | procedures shown Table 5 |
| 2 Set the water bath temperature | 60 °C |
| 3 Potential cycles | between: 1.0–1.5 V, 100 mV/s, 10,000 cycles |
| 4 ORR activity measurement | procedures shown Table 5 at 30 °C |
| Step | Condition |
|---|---|
| 1. Voltage cycles | 0.115–1.2 V, 50 mV/s, 25 cycles, |
| Cell Temperature: 55 °C | |
| Cathode gas: N2 1 L/min, RH 100% | |
| Anode gas: (H2:N2 1:9 v/v) 1 L/min, RH 100% | |
| 2. Current sweeps | 0 A (Open circuit) to 5A or to 0.1 V, 10 mA/s, step back to open circuit, 5 sweeps |
| Cell Temperature: 55 °C | |
| Cathode gas: Air 2 L/min at RH 100%, 40 kPag | |
| Anode gas: H2 0.5 L/min RH 100%, 40 kPag |
| Step | Condition |
|---|---|
| 1. Presetting | Cell Temperature: 55, 60, 82 °C (=RH100, 80, 30%) Cathode gas: N2 1 L/min Anode gas: (H2:N2 1:9 v/v) 1 L/min |
| 2. Cleaning: Potential cycle | 0.115–1.0 V at 20 mV/s, 3 cycles |
| 3. Potential Holding | 0.015 V (CO adsorption) for 20 min 0.4 V (for sulfonate coverage) for 20 min |
| 4. Cathode gas switch | 5% CO in N2 0.4 L/min |
| 5. CO stripping potential sweep | 0.4–1.0 V 20 mV/s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamitaka, Y.; Takeshita, T.; Morimoto, Y. MgO-Templated Mesoporous Carbon as a Catalyst Support for Polymer Electrolyte Fuel Cells. Catalysts 2018, 8, 230. https://doi.org/10.3390/catal8060230
Kamitaka Y, Takeshita T, Morimoto Y. MgO-Templated Mesoporous Carbon as a Catalyst Support for Polymer Electrolyte Fuel Cells. Catalysts. 2018; 8(6):230. https://doi.org/10.3390/catal8060230
Chicago/Turabian StyleKamitaka, Yuji, Tomohiro Takeshita, and Yu Morimoto. 2018. "MgO-Templated Mesoporous Carbon as a Catalyst Support for Polymer Electrolyte Fuel Cells" Catalysts 8, no. 6: 230. https://doi.org/10.3390/catal8060230





