Nitrogen-Doped Porous Carbon Derived from Bamboo Shoot as Solid Base Catalyst for Knoevenagel Condensation and Transesterification Reactions
Abstract
:1. Introduction
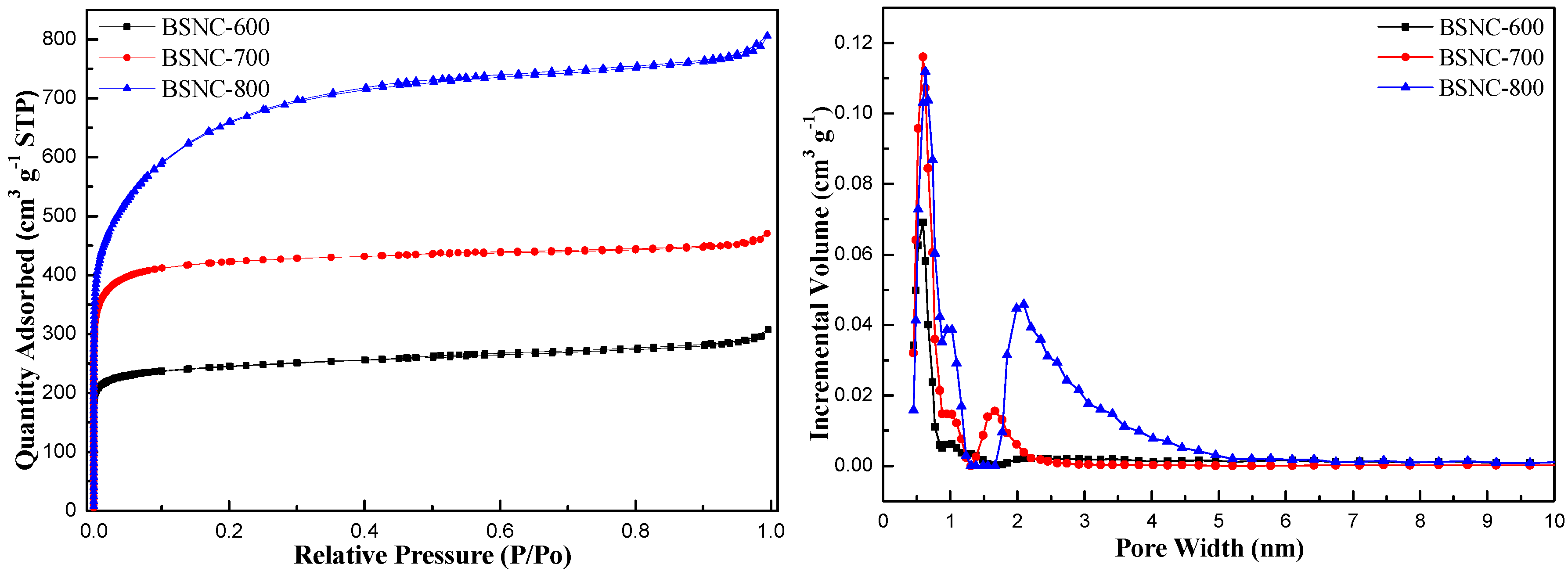
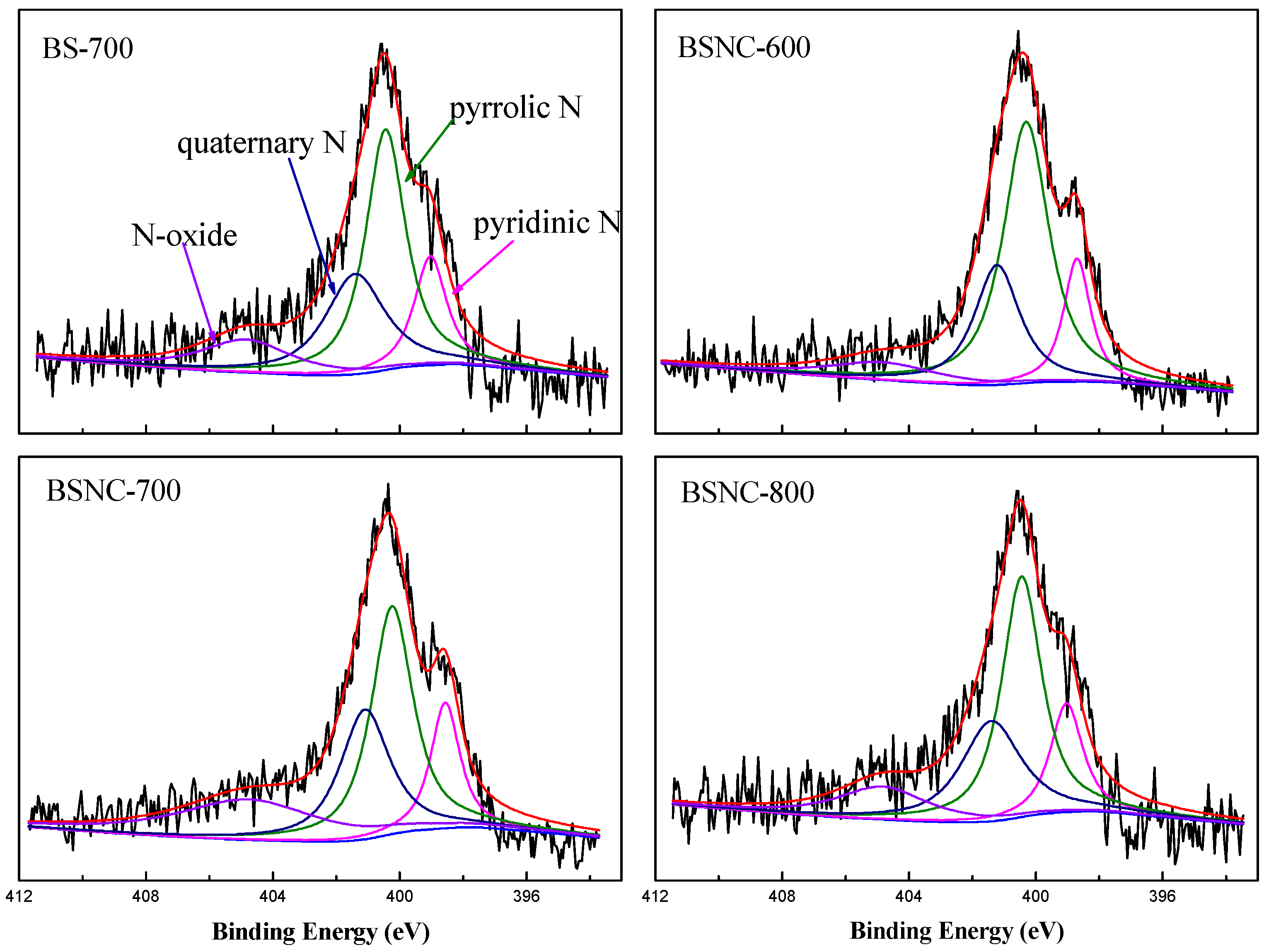
2. Results and Discussion
2.1. Catalyst Activity of the Carbon Materials for Knoevenagel Condensation
2.2. Catalyst Activity of the Deprotonation of BSNC-700
2.3. Recyclability of BSNC-700-tBu (0.1 M)
2.4. The Catalyst Activities of BSNC-700-tBu (0.1 M) for Transesterification Reactions
3. Materials and Methods
3.1. Preparation of BSNCs
3.2. Catalytic Test
3.3. Characterization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ying, A.; Liu, L.; Wu, G.; Chen, X.; Ye, W. Knoevenagel condensation catalyzed by DBU Br(ö)nsted ionic liquid without solvent. Chem. Res. Chin. Univ. 2009, 25, 876–881. [Google Scholar]
- Siddiqui, Z.N.; Tarannum, S. TsOH-SiO2, as an efficient and eco-friendly catalyst for Knoevenagel condensation. Tetrahedron Lett. 2014, 55, 2612–2617. [Google Scholar] [CrossRef]
- Singh, M.S.; Raghuvanshi, K. Recent advances in InCl3-catalyzed one-pot organic synthesis. Tetrahedron 2012, 68, 8683–8697. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Khan, K. [Et3NH][HSO4]-catalyzed efficient, eco-friendly, and sustainable synthesis of quinoline derivatives via Knoevenagel condensation. ACS Sustain. Chem. Eng. 2014, 2, 1187–1194. [Google Scholar] [CrossRef]
- Shu, K.; Masaharu, S.; Hidetoshi, K.; William, L. ChemInform Abstract: Rare-earth metal triflates in organic synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar]
- Mohite, A.R.; Bhat, R.G. A practical and convenient protocol for the synthesis of (E)-α, β-unsaturated acids. Org. Lett. 2013, 15, 4564–4567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cortés-Concepción, J.A.; Mustian, M.; Amiridis, M.D. Effect of basic properties of MgO on the heterogeneous synthesis of flavanone. Appl. Catal. 2006, 302, 232–236. [Google Scholar] [CrossRef]
- Li, M.; Tang, M.; Deng, J.; Wang, Y. Nitrogen-doped flower-like porous carbon materials directed by in situ hydrolysed MgO: Promising support for Ru nanoparticles in catalytic hydrogenations. Nano Res. 2016, 9, 3129–3140. [Google Scholar] [CrossRef]
- Sakthivel, B.; Dhakshinamoorthy, A. Chitosan as a reusable solid base catalyst for Knoevenagel condensation reaction. J. Colloid Interface Sci. 2017, 485, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Thitsartarn, W.; Kawi, S. An active and stable CaO-CeO2 catalyst for transesterification of oil to biodiesel. Green Chem. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Sebti, S.; Solhy, A.; Tahir, R.; Smahi, A. Modified hydroxyapatite with sodium nitrate: An efficient new solid catalyst for the claisen-schmidt condensation. Appl. Catal. A 2002, 235, 273–281. [Google Scholar] [CrossRef]
- Wang, X.; Tseng, Y.H.; Chan, J.C.C.; Cheng, S. Catalytic applications of aminopropylated mesoporous silica prepared by a template-free route in flavanones synthesis. J. Catal. 2005, 233, 266–275. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Biodiesel production from acid oils and ethanol using a solid basic resin as catalyst. Biomass Bioenergy 2010, 34, 272–277. [Google Scholar] [CrossRef]
- Liu, F.; Li, W.; Sun, Q.; Zhu, L.; Meng, X.; Guo, Y.H.; Xiao, F.S. Transesterification to biodiesel with superhydrophobic porous solid base catalysts. ChemSusChem 2011, 4, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Calvino-Casilda, V.; Lopez-Peinado, A.J.; Duran-Valle, C.J.; Martin-Aranda, R.M. Cheminform abstract: Last decade of research on activated carbons as catalytic support in chemical processes. Catal. Rev. 2010, 41, 325–380. [Google Scholar] [CrossRef]
- Jin, X.; Balasubramanian, V.; Selvan, S.; Sawant, D.; Chari, M.; Lu, G.; Vinu, A. Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content: A metal-free basic catalyst. Angew. Chem. 2009, 48, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Sarkar, B.; Sameer, S.; Singhal, N.; Bordoloi, A. Role of pyridinic nitrogen on base catalyzed knoevenagel condensation over pristine CNx. ChemistrySelect 2017, 2, 8086–8090. [Google Scholar] [CrossRef]
- Fujita, S.I.; Katagiri, A.; Watanabe, H.; Asano, S.; Yoshida, H.; Arai, M. Preparation of nitrogen-doped carbon from polyacrylonitrile and its application as a solid-base catalyst. ChemCatChem 2015, 7, 2965–2970. [Google Scholar] [CrossRef]
- Makowski, P.; Weber, J.; Thomas, A.; Goettmann, F. A mesoporous poly(benzimidazole) network as a purely organic heterogeneous catalyst for the Knoevenagel condensation. Catal. Commun. 2009, 10, 243–247. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, Y.; Wang, J.; Su, D.; Tang, M.; Mao, S.; Wang, Y. Cobalt encapsulated in N-doped graphene layers: An efficient and stable catalyst for hydrogenation of quinoline compounds. ACS Catal. 2016, 6, 5816–5822. [Google Scholar] [CrossRef]
- Ansari, M.B.; Jin, H.; Parvin, M.N.; Park, S.E. Mesoporous carbon nitride as a metal-free base catalyst in the microwave assisted Knoevenagel condensation of ethylcyanoacetate with aromatic aldehydes. Catal. Today 2012, 185, 211–216. [Google Scholar] [CrossRef]
- Kan-Nari, N.; Okamura, S.; Fujita, S.I.; Ozaki, J.I.; Arai, M. Nitrogen-doped carbon materials prepared by ammoxidation as solid base catalysts for Knoevenagel condensation and transesterification reactions. Adv. Synth. Catal. 2010, 352, 1476–1484. [Google Scholar] [CrossRef]
- Van, D.S.; Jong, K.P.D.; Bitter, J.H. Nitrogen-containing carbon nanotubes as solid base catalysts. Chem. Commun. 2006, 46, 4859–4861. [Google Scholar]
- Sun, Y.B.; Cao, C.; Huang, P.; Yang, S.; Song, W. Amines functionalized c60 as solid base catalysts for knoevenagel condensation with high activity and stability. RSC Adv. 2015, 5, 86082–86087. [Google Scholar] [CrossRef]
- Karagoz, S.; Tay, T.; Ucar, S.M. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 2008, 99, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Ning, Z.; Lei, S.; Rui, Y.; Tian, X.; Wang, J.; Song, Y.; Xu, D.; Guo, Q.; Liu, L. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar]
- Guo, S.; Dong, X.; Zhu, C.; Han, Y.; Ma, F.; Wu, T. Pyrolysis behaviors and thermodynamics properties of hydrochar from bamboo (phyllostachys heterocycla cv. pubescens) shoot shell. Bioresour. Technol. 2017, 233, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ding, Z.Q.; Gao, Q.; Xun, H.; Tang, F.; Xia, E.D. Major chemical constituents of bamboo shoots (Phyllostachys pubescens): Qualitative and quantitative research. J. Agric. Food Chem. 2016, 64, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Jimura, K.; Hayashi, S.; Kikuchi, R.; Takagaki, A. Utilization of hexagonal boron nitride as a solid acid-b bifunctional catalyst. J. Catal. 2017, 355, 176–184. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, J.Y.; Zhang, B.; Dong, S.M.; Guo, X.C.; Mu, X.D.; Fei, B.H. A novel hierarchical porous nitrogen-doped carbon derived from bamboo shoot for high performance supercapacitor. Sci. Rep. 2017, 7, 7362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Boero, M.; Huang, S.F.; Terakura, K.; Oshima, M.; Ozaki, J. Carbon alloy catalysts: Active sites for oxygen reduction reaction. J. Phys. Chem. C 2008, 112, 14706–14709. [Google Scholar] [CrossRef]



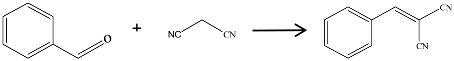
| Entry | Catalyst | Benzaldehyde Conversion (%) | Target Product Selectivity (%) |
|---|---|---|---|
| 1 | / | 54.5 | 13.3 |
| 2 | BS-700 | 2.2 | 100 |
| 3 | Commercial activated carbon | 7.5 | 0 |
| 4 | BSNC-600 | 8.3 | 100 |
| 5 | BSNC-700 | 16.1 | 100 |
| 6 | BSNC-800 | 8.2 | 100 |
| Samples | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | C a (wt.%) | N a (wt.%) | Surface O b (at.%) | Surface N b (at.%) | N b/O b |
|---|---|---|---|---|---|---|---|---|
| Bamboo shoot | 0.4 [32] | 0.002 [32] | / | 41.67 | 4.27 | / | / | / |
| BS-700 | 4.6 | 0.01 | 7.13 | 63.36 | 4.40 | 18.3 | 6.10 | 0.333 |
| BSNC-600 | 962 | 0.48 | 1.97 | 69.18 | 4.65 | 13.31 | 4.56 | 0.343 |
| BSNC-700 | 1475 | 0.73 | 1.97 | 80.37 | 2.79 | 7.41 | 4.92 | 0.664 |
| BSNC-800 | 2271 | 1.25 | 2.19 | 43.43 | 1.06 | 10.53 | 3.73 | 0.354 |
| Samples | Pyridinic N (%) 398.7 eV | Pyrrolic N (%) 400.3 eV | Quaternary N (%) 401.2 eV | N-Oxide (%) 404.7 eV |
|---|---|---|---|---|
| BS-700 | 18.1 | 43.6 | 19.9 | 18.4 |
| BSNC-600 | 14.0 | 58.8 | 19.1 | 8.1 |
| BSNC-700 | 14.4 | 37.6 | 25.4 | 22.6 |
| BSNC-800 | 15.9 | 43.5 | 27.0 | 13.6 |
| Entry | Catalysts | Benzaldehyde Conversion (%) | Target Product Selectivity (%) |
|---|---|---|---|
| 1 | BSNC-700 | 16.1 | 100 |
| 2 | BSNC-700-H (1.0 M) | 1.0 | 100 |
| 3 | BSNC-700-OH (1.0 M) | 34.4 | 100 |
| 4 | BSNC-700-OH (0.1 M) | 32.2 | 100 |
| 5 | BSNC-700-tBu (1.0 M) | 79.9 | 100 |
| 6 | BSNC-700-tBu (0.1 M) | 76.0 | 100 |
| Entry | Alcohols | β-Ketoester | Time (h) | Alcohol Conversion (%) | Target Product’s Selectivity (%) |
|---|---|---|---|---|---|
| 1 | 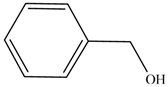 | 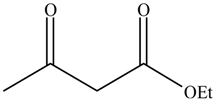 | 16 | 70.6 | 100 |
| 2 | 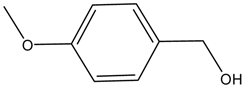 | 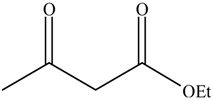 | 16 | 63.7 | 100 |
| 3 | 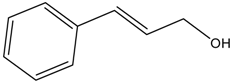 | 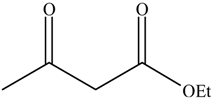 | 16 | 75.1 | 100 |
| 4 | 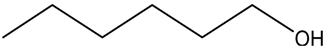 | 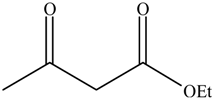 | 16 | 74.4 | 100 |
| 5 | 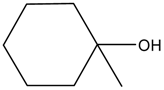 | 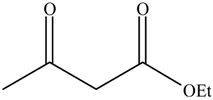 | 16 | / | / |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, B.; Chen, X.; Jiang, C.; Wang, J.; Chen, X.; Zhang, B.; Liu, X.; Liu, Z.; Fei, B. Nitrogen-Doped Porous Carbon Derived from Bamboo Shoot as Solid Base Catalyst for Knoevenagel Condensation and Transesterification Reactions. Catalysts 2018, 8, 232. https://doi.org/10.3390/catal8060232
Mi B, Chen X, Jiang C, Wang J, Chen X, Zhang B, Liu X, Liu Z, Fei B. Nitrogen-Doped Porous Carbon Derived from Bamboo Shoot as Solid Base Catalyst for Knoevenagel Condensation and Transesterification Reactions. Catalysts. 2018; 8(6):232. https://doi.org/10.3390/catal8060232
Chicago/Turabian StyleMi, Bingbing, Xiufang Chen, Changle Jiang, Jingxin Wang, Xiujuan Chen, Bo Zhang, Xianmiao Liu, Zhijia Liu, and Benhua Fei. 2018. "Nitrogen-Doped Porous Carbon Derived from Bamboo Shoot as Solid Base Catalyst for Knoevenagel Condensation and Transesterification Reactions" Catalysts 8, no. 6: 232. https://doi.org/10.3390/catal8060232




