Synergistic Effect of Cu2O and Urea as Modifiers of TiO2 for Enhanced Visible Light Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation Conditions and Visible Light Photocatalytic Efficiency
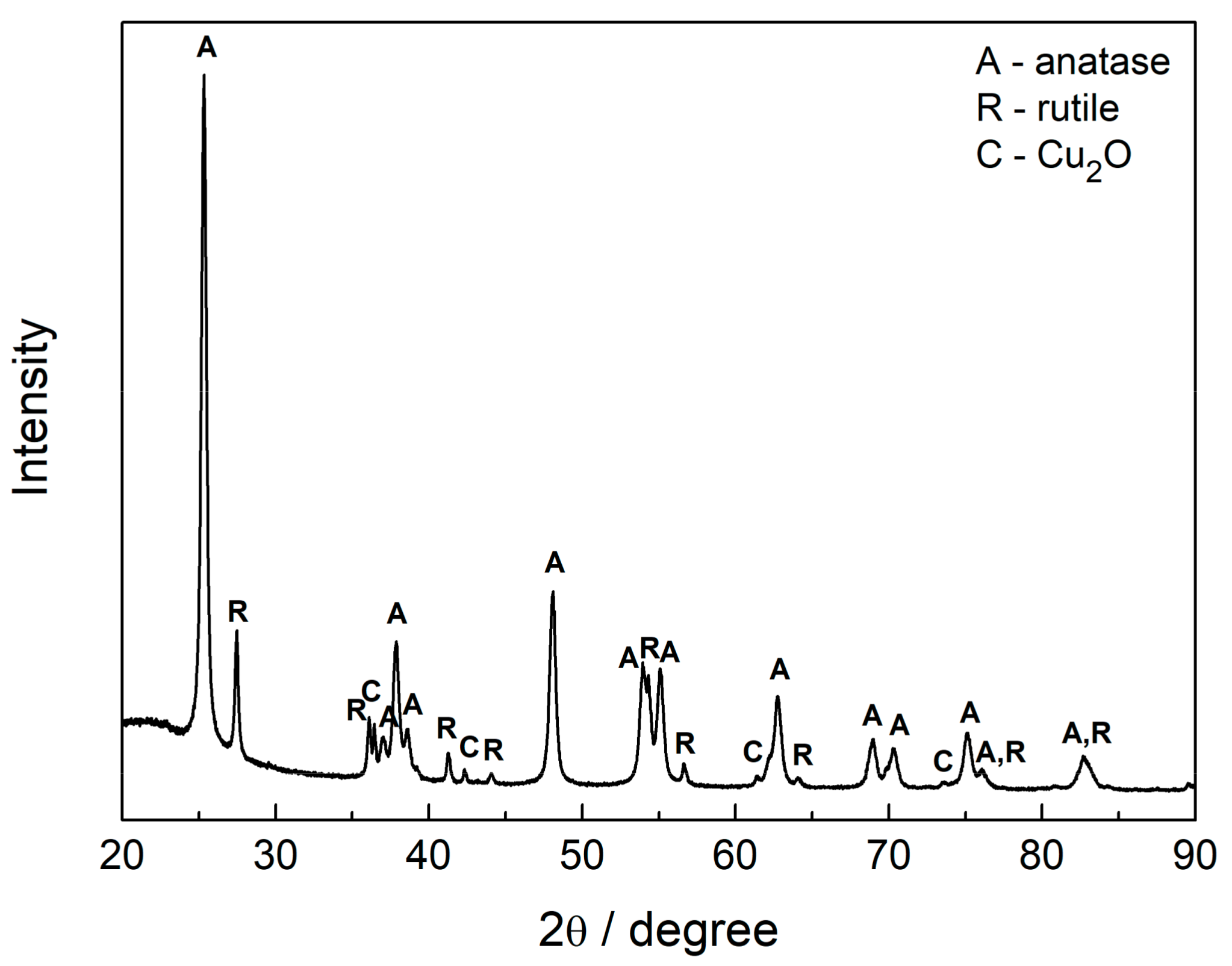
2.2. Characterization of Samples
2.3. Enhanced Visible Light Photocatalytic Activity as a Synergistic Effect of Two Modifiers
3. Materials and Methods
3.1. Preparation of Cu2O/PTr–TiO2
3.2. Sample Characterization
3.3. Photocatalytic Reaction
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bahnemann, D.W. Photocatalytic water treatment: Solar energy applications. Solar Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peng, B.S.; Peng, T.Y. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Pietron, J.J.; DeSario, P.A. Review of roles for photonic crystals in solar fuels photocatalysis. J. Photonics Energy 2016, 7, 012007. [Google Scholar] [CrossRef] [Green Version]
- Ragesh, P.; Ganesh, V.A.; Nair, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor photocatalysis—Mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, S.; Ghosh, P.; Ganesh, A.; Cheng, W.L. 1D copper nanostructures: Progress, challenges and opportunities. Small 2015, 11, 1232–1252. [Google Scholar] [CrossRef] [PubMed]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Bedford, N.M.; Ren, Y.; Zahran, E.M.; Goodin, R.C.; Chagani, F.F.; Bachas, L.G.; Knecht, M.R. Direct Synthetic Control over the Size, Composition, and Photocatalytic Activity of Octahedral Copper Oxide Materials: Correlation Between Surface Structure and Catalytic Functionality. ACS Appl. Mater. Interfaces 2015, 7, 13238–13250. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Wang, C.G.; Shao, M.H.; Xu, X.J.; Huang, J.Z. Low-temperature solution synthesis of CuO/Cu2O nanostructures for enhanced photocatalytic activity with added H2O2: Synergistic effect and mechanism insight. RSC Adv. 2017, 7, 4329–4338. [Google Scholar] [CrossRef]
- Yang, L.; Luo, S.; Li, Y.; Xiao, Y.; Kang, Q.; Cai, Q. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p-n heterojunction network catalyst. Environ. Sci. Technol. 2010, 44, 7641–7646. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Zheng, X.M.; Kong, F.; Wu, G.H.; Luo, L.L.; Guo, Y.; Liu, H.L.; Wang, Y.; Yu, H.X.; Zou, Z.G. Architecture of Cu2O@TiO2 core-shell heterojunction and photodegradation for 4-nitrophenol under simulated sunlight irradiation. Mater. Chem. Phys. 2011, 129, 1184–1188. [Google Scholar] [CrossRef]
- Liu, L.; Gu, X.; Sun, C.; Li, H.; Deng, Y.; Gao, F.; Dong, L. In situ loading of ultra-small Cu2O particles on TiO2 nanosheets to enhance the visible-light photoactivity. Nanoscale 2012, 4, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Yang, W.Y.; Li, Q.; Gao, S.A.; Shang, J.K. Synthesis of Cu2O nanospheres decorated with TiO2 nanoislands, their enhanced photoactivity and stability under visible light illumination, and their post-illumination catalytic memory. ACS Appl. Mater. Interfaces 2014, 6, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.B.; Yang, F.; Yan, L.L.; Yan, N.N.; Yang, X.; Qiu, M.Q.; Yu, Y. Bifunctional photocatalysis of TiO2/Cu2O composite under visible light: Ti3+ in organic pollutant degradation and water splitting. J. Phys. Chem. Solids. 2011, 72, 1104–1109. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mucklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zheng, M.; Li, R.; Wang, Y. Morphology transformation of Cu2O by adding TEOA and their antibacterial activity. J. Nanopart. Res. 2016, 18, 342. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.; Park, S.H.; Park, H.; Huh, Y.D. Morphology-dependent antibacterial activities of Cu2O. Mater. Lett. 2011, 65, 818–820. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A.Y. Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Bacsa, R.; Kiwi, J.; Ohno, T.; Albers, P.; Nadtochenko, V. Preparation, testing and characterization of doped TiO2 active in the peroxidation of biomolecules under visible light. J. Phys. Chem. B 2005, 109, 5994–6003. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H.; Sakthivel, S.; Janczarek, M.; Mitoraj, D. A low-band gap, nitrogen-modified titania visible-light photocatalyst. J. Phys. Chem. C 2007, 111, 11445–11449. [Google Scholar] [CrossRef]
- Beranek, R.; Neumann, B.; Sakthivel, S.; Janczarek, M.; Dittrich, T.; Tributsch, H.; Kisch, H. Exploring the electronic structure of nitrogen-modified TiO2 photocatalysts through photocurrent and surface photovoltage studies. Chem. Phys. 2007, 339, 11–19. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. The nature of nitrogen-modified titanium dioxide photocatalysts active in visible light. Angew. Chem. Int. Ed. 2008, 47, 9975–9978. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj, D.; Kisch, H. On the mechanism of urea-Induced titania modification. Chem. Eur. J. 2010, 16, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj, D.; Kisch, H. Surface modified titania visible light photocatalyst powders. Solid State Phenom. 2010, 162, 49–75. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Lee, S.C.; Lintang, H.O.; Yuliati, L. A urea precursor to synthesize carbon nitride with mesoporosity for enhanced activity in the photocatalytic removal of phenol. Chem. Asian J. 2012, 7, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Senthil, R.A.; Theerthagiri, J.; Selvi, A.; Madhavan, J. Synthesis and characterization of low-cost g-C3N4/TiO2 composite with enhanced photocatalytic performance under visible-light rradiation. Opt. Mater. 2017, 64, 533–539. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, S.; Yang, S.; Wang, H.; Yu, H.; Zhang, S.; Peng, F. Synthesis and characterization of g-C3N4/Cu2O composite catalyst with enhanced photocatalytic activity under visible light irradiation. Mater. Res. Bull. 2014, 56, 19–24. [Google Scholar] [CrossRef]
- Min, Z.; Wang, X.; Li, Y.; Jiang, J.; Li, J.; Qian, D.; Li, J. A highly efficient visible-light-responding Cu2O-TiO2/g-C3N4 photocatalyst for instantaneous discolorations of organic dyes. Mater. Lett. 2017, 193, 18–21. [Google Scholar] [CrossRef]
- Kisch, H.; Macyk, W. Visible-light photocatalysis by modified titania. Chem. Phys. Chem. 2002, 3, 399–400. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Dementjev, A.P.; de Graaf, A.; van den Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Guo, X.; Xie, Y.; Wang, X.; Zhang, S.; Hou, T.; Lv, S. Synthesis of carbon nitride nanotubes with the C3N4 stoichiometry via a benzene-thermal process at low temperatures. Chem. Commun. 2004, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J. Photonics Energy 2017, 7, 012008. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.-V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Yu, H.; Wang, H. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion. Solid State Sci. 2009, 11, 129–138. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vazquez, P.; Rodriguez, J.; Avendano, J.R.; Alfaro, S.; Tirado, S.; Garduno, A.; De la Rosa, J.M. Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–148. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-scheme photocatalytic systems for promoting photocatalytic performance: Recent progress and future challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A review of direct Z-scheme photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]








| Samples | Ti 2p3/2 (%) | O 1s (%) | Ratio | Valent State (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti4+ | Ti3+ | TiO2 a | Ti-OH b | Ti-OH c | O/Ti | C/Ti | Cu2+ | Cu+ | Cu(0) | |
| TiO2 | 98.5 | 1.5 | 57.9 | 26.7 | 15.4 | 2.6 | 3.5 | – | – | – |
| PTr-TiO2 | 97.2 | 2.8 | 62.3 | 23.7 | 14.0 | 2.5 | 4.1 | – | – | – |
| Cu2O/PTr-TiO2 | 97.0 | 3.0 | 61.8 | 24.1 | 14.1 | 2.5 | 4.3 | 0.9 | 99.1 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, M.; Wang, K.; Kowalska, E. Synergistic Effect of Cu2O and Urea as Modifiers of TiO2 for Enhanced Visible Light Activity. Catalysts 2018, 8, 240. https://doi.org/10.3390/catal8060240
Janczarek M, Wang K, Kowalska E. Synergistic Effect of Cu2O and Urea as Modifiers of TiO2 for Enhanced Visible Light Activity. Catalysts. 2018; 8(6):240. https://doi.org/10.3390/catal8060240
Chicago/Turabian StyleJanczarek, Marcin, Kunlei Wang, and Ewa Kowalska. 2018. "Synergistic Effect of Cu2O and Urea as Modifiers of TiO2 for Enhanced Visible Light Activity" Catalysts 8, no. 6: 240. https://doi.org/10.3390/catal8060240





