Carbon Supported Multi-Branch Nitrogen-Containing Polymers as Oxygen Reduction Catalysts
Abstract
:1. Introduction
2. Result and Discussion
2.1. The Micro-Structured Surface and the Elemental Composition of the Catalysts
2.2. Electrocatalytic Characterization and Catalyst’s Study of Dynamics
3. Experimental Section
3.1. Materials
3.2. Sample Preparation
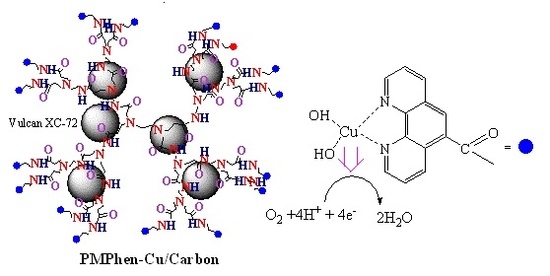
3.2.1. Synthesis of PMPhen/C
3.2.2. Preparation of the Modified Electrode with PMPhen/C, PMPhen-Cu/C and Pt/C Catalysts
3.3. Physical Characterization
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, Z.K.; Lin, L.; Liu, Y.N.; Zhou, X.; Yuan, C.Z.; Xu, A.W. Supramolecular polymers-derived nonmetal N, S-codoped carbon nanosheets for efficient oxygen reduction reaction. RSC Adv. 2016, 6, 52937–52944. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Zhang, L.Q.; Wei, W.; Li, Y.; Liu, A.R.; Zhang, Y.J.; Liu, S.Q. Effect of annealing temperature and element composition of titanium dioxide/graphene/hemin catalysts for oxygen reduction reaction. RSC Adv. 2015, 5, 82879–82886. [Google Scholar] [CrossRef]
- Tran, T.N.; Song, M.Y.; Singh, K.P.; Yang, D.S.; Yu, J.S. Iron-polypyrrole electrocatalyst with remarkable activity and stability for ORR in both alkaline and acidic conditions: A comprehensive assessment of catalyst preparation sequence. J. Mater. Chem. A 2016, 4, 8645–8657. [Google Scholar] [CrossRef]
- Nallathambi, V.; Lee, J.W.; Kumaraguru, S.P.; Wu, G.; Popov, B.N. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM proton exchange membrane fuel cells. J. Power Sources 2008, 183, 34–42. [Google Scholar] [CrossRef]
- Yadav, R.M.; Wu, J.J.; Kochandra, R.; Ma, L.L.; Tiwary, C.S.; Ge, L.H.; Ye, G.L.; Vajtai, R.; Lou, J.; Ajayan, P.M. Carbon nitrogen nanotubes as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions. ACS. Appl. Mater. Interfaces 2015, 7, 11991–12000. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.J.; Fei, H.L.; Zou, X.L.; Zhou, W.; Yang, S.B.; Ye, G.L.; Liu, Z.; Peng, Z.W.; Lou, J.; Vajtai, R.; et al. Boron and nitrogen-substituted graphene nanoribbons as efficient catalysts for oxygen reduction reaction. Chem. Mater. 2015, 27, 1181–1186. [Google Scholar] [CrossRef]
- Raj, C.R.; Samanta, A.; Noh, S.H.; Mondal, S.; Okajima, T.; Ohsaka, T. Emerging new generation electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 11156–11178. [Google Scholar] [CrossRef]
- Yan, Z.H.; Wang, M.; Liu, J.F.; Liu, R.M.; Zhao, J.S. Glycerol-stabilized NaBH4 reduction at room-temperature for the synthesis of a carbon-supported PtxFe alloy with superior oxygen reduction activity for a microbial fuel cell. Electrochim. Acta 2014, 141, 331–339. [Google Scholar] [CrossRef]
- Jung, W.S. High-performance bimetallic alloy catalyst using Ni and N co-doped composite carbon for the oxygen reduction electro-reduction. J. Colloid Interface Sci. 2018, 514, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.L.; Hu, L.Y.; Li, J.; Wang, E. Hybrid of g-C3N4 Assisted metal-organic frameworks and their derived high-efficiency oxygen reduction electrocatalyst in the whole pH range. ACS Appl. Mater. Interfaces 2016, 8, 35281–35288. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.T.; Liu, C.Y.; Wang, Z.J.; Zhang, Z.Y.; Zhang, M.N.; Chang, X.M.; Zhang, W.; Cao, R. Noncovalent immobilization of a pyrene-modified cobalt corrole on carbon supports for enhanced electrocatalytic oxygen reduction and oxygen evolution in aqueous solutions. ACS Catal. 2016, 6, 6429–6437. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Chu, Y.; Ju, X.P.; Zhao, J.S.; Kong, L.Q.; Zhang, Y. Carbon-supported copper-based nitrogen-containing supramolecule as an efficient oxygen reduction reaction catalysts in neutral medium. Catalysts 2018, 8, 53. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y.; Hayakawa, T.; Okajima, T.; Ohsaka, T. Fe-containing polyimide-based high-performance ORR catalysts in acidic medium: A kinetic approach to study the durability of catalysts. Catal. Sci. Technol. 2015, 5, 475–483. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.L.; Lu, X.P.; Li, Z.; Sun, L.L.; Song, Y.H. Dendritic copper-cobalt nanostructures/reduced graphene oxide-chitosan modified glassy carbon electrode for glucose sensing. Sens. Actuators B Chem. 2014, 195, 1–7. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Alreja, P.; Kaur, N. Recent advances in 1, 10-phenanthroline ligands for chemosensing of cations and anions. RSC Adv. 2016, 6, 23169–23217. [Google Scholar] [CrossRef]
- Lin, L.; Li, M.; Jiang, L.; Li, Y.; Liu, D.; He, X.; Cui, L. A novel iron (II) polyphthalocyanine catalyst assembled on graphene with significantly enhanced performance for oxygen reduction reaction in alkaline medium. J. Power Sources 2014, 268, 269–278. [Google Scholar] [CrossRef]
- Hyun, K.; Lee, J.H.; Yoon, C.W.; Cho, Y.H.; Kim, L.H.; Kwon, Y. Improvement in oxygen reduction activity of polypyrrole-coated PtNi alloy catalyst prepared for proton exchange membrane fuel cells. Synth. Met. 2014, 190, 48–55. [Google Scholar] [CrossRef]
- Canales, C.; Ramírez, G. Glassy carbon electrodes modified with supramolecular assemblies generated by π-stacking of Cobalt (II) octaethylporphyrins. A 4 electrons-dioxygen reduction reaction occurring at positive potentials. Electrochim. Acta 2015, 173, 636–641. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of multitudinous active sites for oxygen reduction reaction in transition metal-nitrogen-carbon electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Ding, W.; Wei, Z.D.; Chen, S.G.; Qi, X.Q.; Yang, T.; Hu, J.S.; Wang, D.; Wang, L.J.; Alvi, S.F.; Li, L. Space-Confinement-Induced Synthesis of Pyridinic- and Pyrrolic-Nitrogen-Doped Graphene for the Catalysis of Oxygen Reduction. Angew. Chem. Int. Ed. 2013, 52, 11755–11759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Niu, J.B.; Dai, L.M.; Xia, Z.H. Effect of microstructure of nitrogen-doped graphene on oxygen reduction activity in fuel cells. Langmuir 2012, 28, 7542–7550. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Wang, M.; Zhang, Y.; Zhao, J.S. Soluble conjugated polymer enriched with pyridinic nitrogen and its application as high-performance catalyst for oxygen reduction. J. Solid. State Electrochem. 2017, 21, 1639–1651. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.L.; Wang, M.; Kong, L.Q.; Zhao, J.S. 1,10-phenanthroline metal complex covalently bonding to poly-(pyrrole-3-carboxylic acid)-coated carbon: An efficient electrocatalyst for oxygen reduction. Electrochim. Acta 2015, 180, 86–95. [Google Scholar] [CrossRef]
- He, H.Y.; Wang, M.; Zhao, J.S.; Zhang, Y. Poly(10,12-bis(4-hexylthiophen-2-yl)thieno[3′,4′:5,6]pyrazino [2,3-f][1,10]-phenanthroline)-copper(II) complex(II) complex as an efficient electrocatalyst for oxygen reduction. Chem. Eng. J. 2017, 316, 680–691. [Google Scholar] [CrossRef]
- Ye, H.; Crooks, J.A.; Crooks, R.M. Effect of particle size on the kinetics of the electrocatalytic oxygen reduction reaction catalyzed by Pt dendrimer-encapsulated nanoparticles. Langmuir 2007, 23, 11901–11906. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.; Beamson, G. XPS studies of the oxygen 1s and 2s levels in a wide range of functional polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
- Marletta, G.; Iacona, F.; Toth, A. Particle beam-induced reactions versus thermal degradation in PMDA-ODA polyimide. Macromolecules 1992, 25, 3190–3198. [Google Scholar] [CrossRef]
- Martínez, J.M.L.; Rodríguez-Castellón, E.; Sánchez, R.M.T.; Denaday, L.R.; Buldain, G.Y.; Dall’Orto, V.C. XPS studies on the Cu (I, II)–polyampholyte heterogeneous catalyst: An insight into its structure and mechanism. J. Mol. Catal. A Chem. 2011, 339, 43–51. [Google Scholar] [CrossRef]
- Yang, L.; Su, Y.; Li, W.; Kan, X. Fe/N/C Electrocatalysts for Oxygen Reduction Reaction in PEM Fuel Cells Using Nitrogen-Rich Ligand as Precursor. J. Phys. Chem. C 2015, 119, 11311–11319. [Google Scholar] [CrossRef]
- Ferrandon, M.; Kropf, A.J.; Myers, D.J.; Artyushkova, K.; Kramm, U.; Bogdanoff, P.; Wu, G.; Johnston, C.M.; Zelenay, P. Multitechnique characterization of a polyaniline–iron–carbon oxygen reduction catalyst. J. Phys. Chem. C 2012, 116, 16001–16013. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, F.B.; Xia, X.H. Bioinspired copper catalyst effective for both reduction and evolution of oxygen. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.F.; Lu, Y.Q.; Yuan, C.G.; Zhao, J.S.; Wang, M.; Liu, R.M. Carbon supported polyindole-5-carboxylic acid covalently bonded with pyridine-2, 4-diamine copper complex as a non-precious oxygen reduction catalyst. Electrochim. Acta 2014, 143, 1–9. [Google Scholar] [CrossRef]
- McCrory, C.C.; Devadoss, A.; Ottenwaelder, X.; Lowe, R.D.; Stack, T.D.P.; Chidsey, C.E. Electrocatalytic O2 reduction by covalently immobilized mononuclear copper (I) complexes: Evidence for a binuclear Cu2O2 intermediate. J. Am. Chem. Soc. 2011, 133, 3696–3699. [Google Scholar] [CrossRef] [PubMed]
- Saracini, C.; Ohkubo, K.; Suenobu, T.; Meyer, G.J.; Karlin, K.D.; Fukuzumi, S. Laser-induced dynamics of peroxodicopper (II) complexes vary with the ligand architecture. One-photon two-electron O2 ejection and formation of mixed-valent CuICuII–superoxide intermediates. J. Am. Chem. Soc. 2015, 137, 15865–15874. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhang, L.; Lui, H.S.; Hui, R.; Shi, Z.; Zhang, J.J. Oxygen reduction reaction (ORR) catalyzed by carbon-supported cobalt polypyrrole (Co-PPy/C) electrocatalysts. Electrochim. Acta 2009, 54, 4704–4711. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Ottenwaelder, X.; Stack, T.D.P.; Chidsey, C.E.D. Kinetic and mechanistic studies of the electrocatalytic reduction of O2 to H2O with mononuclear Cu complexes of substituted 1,10-phenanthrolines. J. Phys. Chem. A 2007, 111, 12641–12650. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhang, D.; Niwa, H.; Harada, Y.; Oshima, M.; Ofuchi, H.; Nabae, Y.; Okajima, T.; Ohsaka, T. Enhancement in kinetics of the oxygen reduction reaction on a nitrogen-doped carbon catalyst by introduction of iron via electrochemical methods. Langmuir 2015, 31, 5529–5536. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, A.; Nabae, Y.; Okajima, T.; Ohsaka, T. Kinetic approach to investigate the mechanistic pathways of oxygen reduction reaction on Fe-Containing N-doped carbon catalysts. ACS Catal. 2015, 5, 5194–5202. [Google Scholar] [CrossRef]
- Olson, T.S.; Pylypenko, S.; Fulghum, J.E.; Atanassov, P. Bifunctional oxygen reduction reaction mechanism on non-platinum catalysts derived from pyrolyzed porphyrins. J. Electrochem. Soc. 2010, 157, 54–63. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y. Estimation of the inherent kinetic parameters for oxygen reduction over a pt-free cathode catalyst by resolving the quasi-four-electron reduction. J. Phys. Chem. C 2016, 120, 22515–22525. [Google Scholar] [CrossRef]
- Sheelam, A.; Ramanujam, K. Iron (III) chloride-benzotriazole adduct for oxygen reduction reaction in alkaline medium. Int. J. Hydrogen Energy 2018, 43, 4754–4762. [Google Scholar] [CrossRef]
- Ou, Z.P.; Lü, A.X.; Meng, D.Y.; Huang, S.; Fang, Y.Y.; Lu, G.F.; Kadish, K.M. Molecular oxygen reduction electrocatalyzed by meso-substituted cobalt corroles coated on edge-plane pyrolytic graphite electrodes in acidic media. Inorg. Chem. 2012, 51, 8890–8896. [Google Scholar] [CrossRef] [PubMed]
- Thorseth, M.A.; Letko, C.S.; Tse, E.C.M.; Rauchfuss, T.B.; Gewirth, A.A. Ligand effects on the overpotential for dioxygen reduction by tris(2-pyridylmethyl) amine derivatives. Inorg. Chem. 2013, 52, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Iwase, K.; Yoshioka, T.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Copper-modified covalent trizazine frameworks as non-noble-metal electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 11068–11072. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Oyaizu, N.; Shimazu, K.; Yagi, I. Oxygen reduction reaction catalyzed by self-assembled monolayers of copper-based electrocatalysts on a polycrystalline gold surface. J. Phys. Chem. C 2016, 120, 15814–15822. [Google Scholar] [CrossRef]
- Thippani, T.; Mandal, S.; Wang, G.; Ramani, V.K.; Kothandaraman, R. Probing oxygen reduction and oxygen evolution reactions on bifunctional non-precious metal catalysts for metal-air batteries. RSC Adv. 2016, 6, 71122–71133. [Google Scholar] [CrossRef]
- Malko, D.; Lopes, T.; Symianakis, E.; Kucernak, A.R. The intriguing poison tolerance of non-precious metal oxygen reduction reaction (ORR) catalysts. J. Mater. Chem. A 2016, 4, 142–152. [Google Scholar] [CrossRef] [Green Version]
















| Samples and Parameters | Vulcan XC-72 Carbon | PMPhen/C | PMPhen-Cu/C |
|---|---|---|---|
| BET Surface Area (m2/g) | 166.67 | 42.96 | 56.58 |
| Pore Volume (cm3/g) | 0.3 | 0.26 | 0.27 |
| Catalyst | Eonset (V) | E1/2 (V) | n | Average H2O2 (%) | Electrolyte Solution | Reference Electrode | Ref. |
|---|---|---|---|---|---|---|---|
| PMPhen-Cu/C | 0.61 | 0.35 | 3.95 | 3.0% | PBS (pH = 7) | RHE | this study |
| PMPhen/C | 0.45 | 0.33 | 2.35 | 71.5% | PBS (pH = 7) | RHE | this study |
| Pt/C (20% Pt content) | 0.90 | 0.63 | 4.0 | 1.6% | PBS (pH = 7) | RHE | this study |
| Cu-SOCBP/C | 0.62 | 0.44 | 3.8 | 9% | PBS (pH = 7) | RHE | [12] |
| FeCl3(btaH)2 | 0.89 | 0.78 | 3.8 | 10% | 0.1 M KOH | RHE | [43] |
| CuPPyPhen/C | 0.62 | - | 4.0 | 7.8% | PBS (pH = 7) | RHE | [25] |
| FePPyPhen/C | 0.56 | - | 3.83 | 19.5% | PBS (pH = 7) | RHE | [25] |
| Corrole-Co/MWCNT | 0.75 | 0.40 | 4.0 | - | 0.5 M H2SO4 | NHE | [44] |
| [Cu(TPA)(L)]2+/C | 0.69 | 0.23 | 3.8 | - | Britton–Robinson buffer (pH = 7) | NHE | [45] |
| Cu–CTF/CPS | 0.81 | - | 3.85 | - | PBS (pH = 7) | NHE | [46] |
| Cu–HT/Au | 0.74 | - | 3.7 | - | Britton–Robinson buffer (pH = 7) | NHE | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Gu, L.; Ju, X.; Du, H.; Zhao, J.; Qu, K. Carbon Supported Multi-Branch Nitrogen-Containing Polymers as Oxygen Reduction Catalysts. Catalysts 2018, 8, 245. https://doi.org/10.3390/catal8060245
Chu Y, Gu L, Ju X, Du H, Zhao J, Qu K. Carbon Supported Multi-Branch Nitrogen-Containing Polymers as Oxygen Reduction Catalysts. Catalysts. 2018; 8(6):245. https://doi.org/10.3390/catal8060245
Chicago/Turabian StyleChu, Ya, Lin Gu, Xiuping Ju, Hongmei Du, Jinsheng Zhao, and Konggang Qu. 2018. "Carbon Supported Multi-Branch Nitrogen-Containing Polymers as Oxygen Reduction Catalysts" Catalysts 8, no. 6: 245. https://doi.org/10.3390/catal8060245