An Efficient Photocatalyst for Fast Reduction of Cr(VI) by Ultra-Trace Silver Enhanced Titania in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
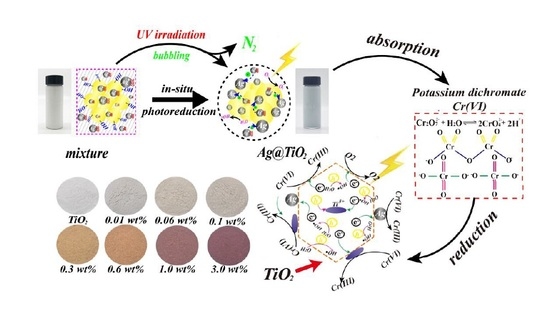
2.1. Preparation and Characterizations of Ultra-Trace Silver Enhanced Titania Photocatalyst
2.2. Catalytic Activity for Cr(VI) Photoreduction
2.3. Kinetic Modeling Study
2.4. Mechanism of Photoreduction
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ag@TiO2 Photocatalyst via Ultrasonic Impregnation Assisted Photoreduction Deposition
3.3. Batch Experiments of Photoreduction
3.4. Catalyst Characterization and Involved Analytical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Flora, S.; Bagnasco, M.; Serra, D.; Zanacchi, P. Genotoxicity of chromium compounds. A review. Mutat. Res. 1990, 238, 99–172. [Google Scholar] [CrossRef]
- Ellis, A.S.; Bullen, T.D. Chromium isotopes and the fate of hexavalent chromium in the environment. Science 2002, 295, 2060–2062. [Google Scholar] [CrossRef] [PubMed]
- Kebir, M.; Chabani, M.; Nasrallah, N.; Bensmaili, A.; Trari, M. Coupling adsorption with photocatalysis process for the Cr(VI) removal. Desalination 2011, 270, 166–173. [Google Scholar] [CrossRef]
- Cimino, G.; Passerini, A.; Toscano, G. Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res. 2000, 34, 2955–2962. [Google Scholar] [CrossRef]
- Sheng, G.; Hu, J.; Li, H.; Li, J.; Huang, Y. Enhanced sequestration of Cr(VI) by nanoscale zero-valent iron supported on layered double hydroxide by batch and XAFS study. Chemosphere 2016, 148, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, X.; Liu, M.; Luo, S.; Luo, Y.; Li, G. Fast photoelectro-reduction of Cr(VI) over MoS2@TiO2 nanotubes on Ti wire. J. Hazard. Mater. 2017, 329, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Bhowal, A.; Datta, S. Studies on transport mechanism of Cr(VI) extraction from an acidic solution using liquid surfactant membranes. J. Membr. Sci. 2001, 188, 1–8. [Google Scholar] [CrossRef]
- Chuang, S.M.; Vinh, Y.; Feng, C.L.; Lee, S.J.; Choo, K.H.; Li, C.W. Electrochemical Cr(VI) reduction using a sacrificial Fe anode: Impacts of solution chemistry and stoichiometry. Sep. Purif. Technol. 2017, 191, 167–172. [Google Scholar] [CrossRef]
- Alowitz, M.J.; Scherer, M.M. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Xiang, C.; Zhu, R.; Ma, H.; Zinchenko, A.; Chu, W. An efficient heterogeneous Fenton catalyst based on modified diatomite for degradation of cationic dye simulated wastewater. Desalin. Water Treat. 2017, 79, 378–385. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pu, S.Y.; Ma, J.; Yan, C.; Zinchenko, A.; Pei, X.J.; Chu, W. Formation of multi-layered chitosan honeycomb spheres via breath-figure-like approach in combination with co-precipitation processing. Mater. Lett. 2018, 211, 91–95. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Galán, B.; Castañeda, D.; Ortiz, I. Removal and recovery of Cr(VI) from polluted ground waters: A comparative study of ion-exchange technologies. Water Res. 2005, 39, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 1, 223–224. [Google Scholar]
- Chen, D.; Ray, A.K. Removal of toxic metal ions from wastewater by semiconductor photocatalysis. Chem. Eng. Sci. 2001, 56, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, A.D.; Pelizzetti, E. Photocatalytic Transformation of Organic Compounds in the Presence of Inorganic Ions. 2. Competitive Reactions of Phenol and Alcohols on a Titanium Dioxide-Fluoride System. Langmuir 2015, 16, 8964–8972. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Liu, S.; Xu, Y.J. Size effect induced activity enhancement and anti-photocorrosion of reduced graphene oxide/ZnO composites for degradation of organic dyes and reduction of Cr(VI) in water. Appl. Catal. B 2013, 140–141, 598–607. [Google Scholar] [CrossRef]
- Mirghani, M.; Al-Mubaiyedh, U.A.; Nasser, M.S.; Shawabkeh, R. Experimental study and modeling of photocatalytic reduction of Pb2+ by WO3/TiO2 nanoparticles. Sep. Purif. Technol. 2015, 14, 285–293. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lai, C.W.; Zaki, S.M. The Influence of Lead Concentration on Photocatalytic Reduction of Pb(II) Ions Assisted by Cu-TiO2 Nanotubes. Int. J. Photoenergy 2014, 2014, 839106. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Marzorati, S.; Longhi, M.; Coduri, M.; Artiglia, L.; Selli, E. Photocatalytic activity of TiO2–WO3 mixed oxides in relation to electron transfer efficiency. Appl. Catal. B 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Saccomanni, A.; Selli, E. Cr(VI) photocatalytic reduction: Effects of simultaneous organics oxidation and of gold nanoparticles photodeposition on TiO2. J. Hazard. Mater. 2012, 99, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Matamoros-Ambrocio, M.; Ruiz-Peralta, M.; Chigo-Anota, E.; García-Serrano, J.; Pérez-Centeno, A.; Sánchez-Cantú, M.; Rubio-Rosas, E.; Escobedo-Morales, A. A Comparative Study of Gold Impregnation Methods for Obtaining Metal/Semiconductor Nanophotocatalysts: Direct Turkevich, Inverse Turkevich, and Progressive Heating Methods. Catalysts 2018, 8, 161. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.L. Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; NavıO, J.A. Photocatalytic deactivation of commercial TiO2 samples during simultaneous photoreduction of Cr(VI) and photooxidation of salicylic acid. J. Photochem. Photobiol. 2001, 138, 79–85. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.H. Facile in situ synthesis of visible-light plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag) and evaluation of their photocatalytic oxidation of benzene to phenol. J. Mater. Chem. 2011, 21, 9079–9087. [Google Scholar] [CrossRef]
- Cheng, B.; Le, Y.; Yu, J. Preparation and enhanced photocatalytic activity of Ag@TiO2 core-shell nanocomposite nanowires. J. Hazard. Mater. 2010, 177, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Kamat, P.V. Photoinduced electron storage and surface plasmon modulation in Ag@TiO2 clusters. Langmuir 2004, 20, 5645–5647. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Cheng, P.; Ong, H.C. Microwave-Hydrothermal Preparation and Visible-Light Photoactivity of Plasmonic Photocatalyst Ag-TiO2 Nanocomposite Hollow Spheres. Chem. Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol-gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chao, Y.; Lei, C.; Zhang, Y.; Yang, J. Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application. Nanomaterials 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Guin, D.; Manorama, S.V.; Latha, J.N.L.; Singh, S. Photoreduction of Silver on Bare and Colloidal TiO2 Nanoparticles/Nanotubes: Synthesis, Characterization, and Tested for Antibacterial Outcome. J. Phys. Chem. C 2007, 111, 13393–13397. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Shokri, M.; Rad, B. Enhancement of photocatalytic activity of TiO2 nanoparticles by Silver doping: Photodeposition versus liquid impregnation methods. Glob. NEST J. 2007, 10, 1–7. [Google Scholar]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.Y.; Hui, M.; Zinchenko, A.; Wei, C. Novel highly porous magnetic hydrogel beads composed of chitosan and sodium citrate: An effective adsorbent for the removal of heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. 2017, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Ning, J.; Su, Z.; Xu, S.; Xing, Z.; Wang, R.; Lu, S.; Dong, J.; Zhang, B.; Yang, H. Structural Dependences of Localization and Recombination of Photogenerated Carriers in the top GaInP Subcells of GaInP/GaAs Double-Junction Tandem Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Wang, S.F.; Tsai, H.Y.; Lin, S.Y. Intrinsic photocatalytic oxidation of the dye adsorbed on TiO2 photocatalysts by diffuse reflectance infrared Fourier transform spectroscopy. Appl. Catal. B 2001, 30, 293–301. [Google Scholar] [CrossRef]
- Davis, R.J.; Gainer, J.L.; O’Neal, G.; Wu, I.W. Photocatalytic Decolorization of Wastewater Dyes. Water Environ. Res. 1994, 66, 50–53. [Google Scholar] [CrossRef]
- Pu, S.; Wang, M.; Wang, K.; Hou, Y.; Yu, J.; Shi, Q.; Pei, X.; Chu, W. Preparation of CS-Fe@Fe3O4 nanocomposite as an efficient and recyclable adsorbent for azo dyes removal. Desalin. Water Treat. 2018, 95, 319–332. [Google Scholar] [CrossRef]
- Li, Y.; Bian, Y.; Qin, H.; Zhang, Y.; Bian, Z. Photocatalytic reduction behavior of hexavalent chromium on hydroxyl modified titanium dioxide. Appl. Catal. B 2017, 206, 293–299. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr(VI) under visible light. Appl. Surf. Sci. 2014, 321, 396–403. [Google Scholar] [CrossRef]
- Aguado, M.A.; GimãNez, J.; Cervera-March, S. Continuous photocatalytic treatment of Cr(VI) effluents with semiconductor powders. Chem. Eng. Commun. 1991, 104, 71–85. [Google Scholar] [CrossRef]










| Mass/g | Volume/mL | Dilution Factor | Element | Readings mg/L | Conversion mg/kg | Ag/TiO2 wt % |
|---|---|---|---|---|---|---|
| 0.0164 | 50 | 1 | Ag | 0.1778 | 542.03 | 0.0648 |
| 50 | Ti | 3.2883 | 501260.67 |
| Kinetics Equation | kap (min−1) | r0 (mg/L·min) | R2 | |
|---|---|---|---|---|
| 0.01 wt % | ln (Ct/C0) = 0.0377t − 0.0549 | 0.0377 | 0.3770 | 0.9947 |
| 0.03 wt % | ln(Ct/C0) = 0.0470t − 0.1046 | 0.0470 | 0.4700 | 0.9954 |
| 0.06 wt % | ln(Ct/C0) = 0.0534t − 0.1572 | 0.0534 | 0.5340 | 0.9966 |
| 0.1 wt % | ln(Ct/C0) = 0.0497t − 0.1149 | 0.0497 | 0.4970 | 0.9895 |
| 0.3 wt % | ln(Ct/C0) = 0.0525t − 0.1183 | 0.0525 | 0.5250 | 0.9963 |
| 0.6 wt % | ln(Ct/C0) = 0.0304t − 0.2233 | 0.0304 | 0.3040 | 0.9731 |
| 1 wt % | ln(Ct/C0) = 0.0281t − 0.2356 | 0.0281 | 0.2810 | 0.9858 |
| 3 wt % | ln(Ct/C0) = 0.0211t − 0.1137 | 0.0211 | 0.2110 | 0.9909 |
| Bare | ln(Ct/C0) = 0.0209t − 0.0562 | 0.0209 | 0.2090 | 0.9937 |
| pH = 3 | ln(Ct/C0) = 0.0786t − 0.1070 | 0.0786 | 0.7860 | 0.9954 |
| pH = 4 | ln(Ct/C0) = 0.615t − 0.1347 | 0.0615 | 0.6150 | 0.9989 |
| pH = 5 | ln(Ct/C0) = 0.0552t − 0.0652 | 0.0552 | 0.5520 | 0.9920 |
| pH = 7 | ln(Ct/C0) = 0.0131t − 0.1523 | 0.0131 | 0.1310 | 0.9213 |
| pH = 9 | ln(Ct/C0) = 0.0086t − 0.1151 | 0.0086 | 0.0860 | 0.9039 |
| pH = 11 | ln(Ct/C0) = − 0.0222 | 0.0001 | 0.0010 | 0.5126 |
| Na+ | ln(Ct/C0) = 0.0189t − 0.0560 | 0.0189 | 0.1890 | 0.9948 |
| K+ | ln(Ct/C0) = 0.0175t − 0.0836 | 0.0175 | 0.1750 | 0.9881 |
| Ca2+ | ln(Ct/C0) = 0.0202t − 0.0607 | 0.0202 | 0.2020 | 0.9915 |
| Mg2+ | ln(Ct/C0) = 0.0209t − 0.0890 | 0.0209 | 0.2090 | 0.9895 |
| SO42− | ln(Ct/C0) = 0.0234t − 0.1560 | 0.0234 | 0.2340 | 0.9808 |
| NO3− | ln(Ct/C0) = 0.0320t − 0.1758 | 0.0320 | 0.3202 | 0.9843 |
| Cl− | ln(Ct/C0) = 0.0190t − 0.0600 | 0.0190 | 0.1900 | 0.9948 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, S.; Hou, Y.; Chen, H.; Deng, D.; Yang, Z.; Xue, S.; Zhu, R.; Diao, Z.; Chu, W. An Efficient Photocatalyst for Fast Reduction of Cr(VI) by Ultra-Trace Silver Enhanced Titania in Aqueous Solution. Catalysts 2018, 8, 251. https://doi.org/10.3390/catal8060251
Pu S, Hou Y, Chen H, Deng D, Yang Z, Xue S, Zhu R, Diao Z, Chu W. An Efficient Photocatalyst for Fast Reduction of Cr(VI) by Ultra-Trace Silver Enhanced Titania in Aqueous Solution. Catalysts. 2018; 8(6):251. https://doi.org/10.3390/catal8060251
Chicago/Turabian StylePu, Shengyan, Yaqi Hou, Hongyu Chen, Daili Deng, Zeng Yang, Shengyang Xue, Rongxin Zhu, Zenghui Diao, and Wei Chu. 2018. "An Efficient Photocatalyst for Fast Reduction of Cr(VI) by Ultra-Trace Silver Enhanced Titania in Aqueous Solution" Catalysts 8, no. 6: 251. https://doi.org/10.3390/catal8060251