Synthesis of Vinyl Chloride Monomer over Carbon-Supported Tris-(Triphenylphosphine) Ruthenium Dichloride Catalysts
Abstract
:1. Introduction
2. Results and Discussion
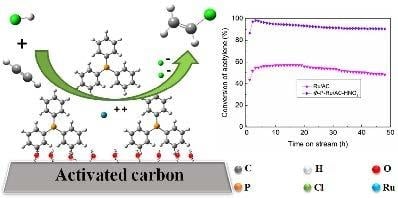
2.1. Results of the Catalytic Performance Test
2.2. The Functional Groups on the Ru-Based Catalysts
2.3. The Pore Properties of the Samples
2.4. The Coking Deposition on the Catalysts
2.5. The Dispersity of Ru Species on the Catalysts
2.6. Valence Change of Ru Species in the Synthesized Catalysts
2.7. The Adsorption Property of the Synthesized Catalysts for Reactants
2.8. The Reusability of the Catalyst with 0.1% Ru Loading
3. Experimental
3.1. Chemicals and Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Li, X.; Zhu, M.; Dai, B. AuCl3 on polypyrrole-modified carbon nanotubes as acetylene hydrochlorination catalysts. Appl. Catal. B-Environ. 2013, 142–143, 234–240. [Google Scholar] [CrossRef]
- Wang, B.; Yu, L.; Zhang, J.; Pu, Y.; Zhang, H.; Li, W. Phosphorus-doped carbon supports enhance gold-based catalysts for acetylene hydrochlorination. RSC Adv. 2014, 4, 15877–15885. [Google Scholar] [CrossRef]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsonaespriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Malta, G.; Freakley, S.J.; Kondrat, S.A.; Hutchings, G.J. Acetylene hydrochlorination using Au/carbon: A journey towards single site catalysis. Chem. Commun. 2017, 53, 11733–11746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Li, X.; Zhao, W.; Gu, J.; Qi, X.; Dong, Y.; Dai, B.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au-Ba(II)/AC catalysts. Catal. Sci. Technol. 2015, 5, 1870–1877. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Zhao, Z.; Luo, G.; Miller, J.T.; Wong, M.S.; Wei, F. Synergistic gold–bismuth catalysis for non-mercury hydrochlorination of acetylene to vinyl chloride monomer. ACS Catal. 2014, 4, 3112–3116. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Ni, J.; Zhang, T.; Xu, X.; Li, X. Activated-carbon-supported gold-cesium(I) as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride. ChemPlusChem 2014, 80, 196–201. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Zhang, T.; Di, X.; Ni, J.; Li, X. Enhancement of Au/AC acetylene hydrochlorination catalyst activity and stability via nitrogen-modified activated carbon support. Chem. Eng. J. 2015, 262, 1152–1160. [Google Scholar] [CrossRef]
- Zhou, K.; Jia, J.; Li, C.; Xu, H.; Zhao, J.; Luo, G.; Wei, F. A low content Au-based catalyst for hydrochlorination of C2H2 and its industrial scale-up for future PVC processes. Green Chem. 2014, 17, 356–364. [Google Scholar] [CrossRef]
- Wang, L.; Shen, B.; Zhao, J.; Bi, X. Trimetallic Au-Cu-K/AC for acetylene hydrochlorination. Can. J. Chem. Eng. 2017, 95, 1069–1075. [Google Scholar] [CrossRef]
- Wang, S.; Shen, B.; Song, Q. Kinetics of acetylene hydrochlorination over bimetallic Au-Cu/C catalyst. Catal. Lett. 2010, 134, 102–109. [Google Scholar] [CrossRef]
- Chao, S.; Guan, Q.; Li, W. Study of the active site for acetylene hydrochlorination in AuCl3/C catalysts. J. Catal. 2015, 330, 273–279. [Google Scholar] [CrossRef]
- Wan, F.; Chao, S.; Guan, Q.; Wang, G.; Li, W. Reaction mechanisms of acetylene hydrochlorination catalyzed by AuCl3/C catalysts: A density functional study. Catal. Commun. 2017, 101, 120–124. [Google Scholar] [CrossRef]
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination. J. Am. Chem. Soc. 2015, 137, 14548–14557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Kang, L.; Su, Y.; Zhang, S.; Dai, B. MClx (M = Hg, Au, Ru; x = 2, 3) catalyzed hydrochlorination of acetylene—A density functional theory study. Can. J. Chem. 2013, 91, 120–125. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, W.; Guo, C.; Li, W. Acetylene hydrochlorination over bimetallic Ru-based catalysts. RSC Adv. 2013, 3, 21062–21068. [Google Scholar] [CrossRef]
- Jin, Y.; Li, G.; Zhang, J.; Pu, Y.; Li, W. Effects of potassium additive on the activity of Ru catalyst for acetylene hydrochlorination. RSC Adv. 2015, 5, 37774–37779. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Jin, Y.; Sheng, W.; Hu, M.; Wang, X.; Zhang, J. Ru-Co(III)-Cu(II)/SAC catalyst for acetylene hydrochlorination. Appl. Catal. B-Environ. 2016, 189, 56–64. [Google Scholar] [CrossRef]
- Gu, J.; Gao, Y.; Zhang, J.; Li, W.; Dong, Y.; Han, Y. Hydrochlorination of acetylene catalyzed by an activated carbon-supported ammonium hexachlororuthenate complex. Catalysts 2017, 7, 17. [Google Scholar] [CrossRef]
- Shang, S.; Zhao, W.; Wang, Y.; Li, X.; Zhang, J.; Han, Y.; Li, W. Highly efficient Ru@IL/AC to substitute mercuric catalyst for acetylene hydrochlorination. ACS Catal. 2017, 7, 3510–3520. [Google Scholar] [CrossRef]
- Man, B.; Zhang, H.; Zhang, J.; Li, X.; Xu, N.; Dai, H.; Zhu, M.; Dai, B. Oxidation modification of Ru-based catalyst for acetylene hydrochlorination. RSC Adv. 2017, 7, 23742–23750. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Dai, B.; Wang, Y.; Yu, F. Nitrogen-doped pitch-based spherical active carbon as a nonmetal catalyst for acetylene hydrochlorination. ChemCatChem 2014, 6, 2339–2344. [Google Scholar] [CrossRef]
- Zhong, J.; Xu, Y.; Liu, Z. Heterogeneous non-mercury catalysts for acetylene hydrochlorination: Progress, Challenges, and Opportunities. Green Chem. 2018, 20, 2412–2427. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Man, B.; Hou, L.; Zhang, C.; Dai, H.; Zhu, M.; Dai, B.; Dong, Y.; Zhang, J. Activated carbon-supported tetrapropylammonium perruthenate catalysts for acetylene hydrochlorination. Catalysts 2017, 7, 311. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Tkowski, A.Ś.J. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Potts, W.J. Infrared absorptions characteristic of the terminal acetylenic group (–C C–H). Spectrochim. Acta 1960, 16, 419–427. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Yu, L.; Jin, Y.; Li, W. Active ruthenium species in acetylene hydrochlorination. Appl. Catal. A-Gen. 2014, 488, 28–36. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.J.; Hutchings, G.J.; Adams, M.D.; Friedl, J.; Wagner, F.E. Hydrochlorination of acetylene using gold catalysts: A study of catalyst deactivation. J. Catal. 1991, 128, 366–377. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Li, W.; Han, Y.; Gu, J.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au–Co(III)/SAC catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–109. [Google Scholar] [CrossRef]
- Gao, P.; Wang, A.; Wang, X.; Zhang, T. Synthesis and catalytic performance of highly ordered Ru-containing mesoporous carbons for hydrogenation of cinnamaldehyde. Catal. Lett. 2008, 125, 289–295. [Google Scholar] [CrossRef]
- Zgolicz, P.D.; Stassi, J.P.; Yañez, M.J.; Scelza, O.A.; Miguel, S.R.D. Influence of the support and the preparation methods on the performance in citral hydrogenation of Pt-based catalysts supported on carbon nanotubes. J. Catal. 2012, 290, 37–54. [Google Scholar] [CrossRef]
- Man, B.; Zhang, H.; Zhang, C.; Li, X.; Dai, H.; Zhu, M.; Dai, B.; Zhang, J. Effect of Ru/Cl ratio on the reaction of acetylene hydrochlorination. New J. Chem. 2017, 41, 23–49. [Google Scholar] [CrossRef]
- Fuente, J.L.; Martínez-Huerta, M.V.; Rojas, S.; Hernández-Fernández, P.; Terreros, P.; Fierro, J.L.; Peña, M.A. Tailoring and structure of PtRu nanoparticles supported on functionalized carbon for DMFC applications: New evidence of the hydrous ruthenium oxide phase. Appl. Catal. B-Environ. 2009, 88, 505–514. [Google Scholar] [CrossRef]
- Suñol, J.J.; Bonneau, M.E.; Roué, L.; Guay, D.; Schulz, R. XPS surface study of nanocrystalline Ti-Ru-Fe materials. Appl. Surf. Sci. 2000, 158, 252–262. [Google Scholar] [CrossRef]
- Chan, H.Y.; Takoudis, C.G.; Weaver, M.J. High-pressure oxidation of ruthenium as probed by surface-enhanced Raman and X-ray photoelectron spectroscopies. J. Catal. 1997, 172, 336–345. [Google Scholar] [CrossRef]
- Ke, J.; Zhao, Y.; Yin, Y.; Chen, K.; Duan, X.; Ye, L.; Yuan, Y. Yttrium chloride-modified Au/AC catalysts for acetylene hydrochlorination with improved activity and stability. J. Rare Earth 2017, 35, 1083–1091. [Google Scholar] [CrossRef]
- Cao, H.; Xing, L.; Wu, G.; Xie, Y.; Shi, S.; Zhang, Y.; Minakata, D.; Crittenden, J.C. Promoting effect of nitration modification on activated carbon in the catalytic ozonation of oxalic acid. Appl. Catal. B-Environ. 2014, 146, 169–176. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Davies, T.E.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Modifications of the metal and support during the deactivation and regeneration of Au/C catalysts for the hydrochlorination of acetylene. Catal. Sci. Technol. 2013, 3, 128–134. [Google Scholar] [CrossRef] [Green Version]








| Samples | SBET a (m2 g−1) | ΔSBET (%) | Smicro. b (m2 g−1) | Sext. c (m2 g−1) | VP d (cm3 g−1) | ΔVP (%) | Vmicro. e (cm3 g‒1) | Vext. (cm3 g‒1) | Dpore f (nm) |
|---|---|---|---|---|---|---|---|---|---|
| Fresh AC | 1238 | 25.1 | 704 | 534 | 0.69 | 27.5 | 0.39 | 0.30 | 2.22 |
| Used AC | 927 | 514 | 413 | 0.50 | 0.29 | 0.21 | 2.17 | ||
| Fresh Ru/AC | 1160 | 31.7 | 698 | 462 | 0.64 | 31.3 | 0.39 | 0.25 | 2.22 |
| Spent Ru/AC | 792 | 401 | 391 | 0.44 | 0.23 | 0.21 | 2.22 | ||
| Fresh Ф-P-Ru/AC | 957 | 34.2 | 496 | 461 | 0.53 | 32.1 | 0.28 | 0.25 | 2.23 |
| Spent Ф-P-Ru/AC | 630 | 255 | 375 | 0.36 | 0.15 | 0.21 | 2.26 | ||
| Fresh Ф-P-Ru/AC-HCl | 845 | 51.9 | 462 | 382 | 0.47 | 51.1 | 0.26 | 0.21 | 2.20 |
| Spent Ф-P-Ru/AC-HCl | 406 | 143 | 263 | 0.23 | 0.09 | 0.14 | 2.23 | ||
| Fresh Ф-P-Ru/AC-HNO3 | 927 | 56.6 | 478 | 449 | 0.52 | 55.8 | 0.27 | 0.25 | 2.24 |
| Spent Ф-P-Ru/AC-HNO3 | 402 | 144 | 258 | 0.23 | 0.09 | 0.14 | 2.27 |
| Catalysts | Amount of Carbon Deposition (%) |
|---|---|
| Ru/AC | 2.8 [25] |
| Ф-P-Ru/AC | 3.9 |
| Ф-P-Ru/AC-HCl | 4.1 |
| Ф-P-Ru/AC-HNO3 | 7.2 |
| Samples | Binding Energy (eV), (Area%) | (Area%) | |||
|---|---|---|---|---|---|
| Ru | RuCl3 | RuO2 | RuOx | RuOx + RuO2 | |
| Fresh Ru/AC | 461.7 (14.0) | 463.4 (49.6) | 464.6 (19.0) | 466.1 (17.4) | (36.4) |
| Fresh Ф-P-Ru/AC | 461.2 (14.9) | 462.8 (37.9) | 464.2 (24.5) | 465.1 (22.7) | (45.2) |
| Fresh Ф-P-Ru/AC-HNO3 | 461.3 (9.9) | 463.0 (38.5) | 464.4 (34.9) | 465.7 (16.7) | (53.6) |
| Fresh Ф-P-Ru/AC-HCl | 461.9 (12.5) | 463.5 (39.1) | 464.6 (28.2) | 465.7 (20.2) | (48.4) |
| Spent Ru/AC | 461.0 (26.2) | 463.0 (44.5) | 465.1 (14.6) | 466.5 (14.7) | (29.3) |
| Spent Ф-P-Ru/AC | 461.7 (18.6) | 463.1 (44.5) | 464.4 (19.8) | 465.1 (17.1) | (36.9) |
| Spent Ф-P-Ru/AC-HNO3 | 461.3 (19.4) | 463.1 (35.3) | 464.8 (31.1) | 466.3 (14.2) | (45.3) |
| Spent Ф-P-Ru/AC-HCl | 461.5 (16.2) | 463.2 (39.5) | 464.2 (27.8) | 465.8 (16.5) | (44.3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, H.; Man, B.; Zhang, C.; Dai, H.; Dai, B.; Zhang, J. Synthesis of Vinyl Chloride Monomer over Carbon-Supported Tris-(Triphenylphosphine) Ruthenium Dichloride Catalysts. Catalysts 2018, 8, 276. https://doi.org/10.3390/catal8070276
Li X, Zhang H, Man B, Zhang C, Dai H, Dai B, Zhang J. Synthesis of Vinyl Chloride Monomer over Carbon-Supported Tris-(Triphenylphosphine) Ruthenium Dichloride Catalysts. Catalysts. 2018; 8(7):276. https://doi.org/10.3390/catal8070276
Chicago/Turabian StyleLi, Xing, Haiyang Zhang, Baochang Man, Chuanming Zhang, Hui Dai, Bin Dai, and Jinli Zhang. 2018. "Synthesis of Vinyl Chloride Monomer over Carbon-Supported Tris-(Triphenylphosphine) Ruthenium Dichloride Catalysts" Catalysts 8, no. 7: 276. https://doi.org/10.3390/catal8070276