Effect of Y Modified Ceria Support in Mono and Bimetallic Pd–Au Catalysts for Complete Benzene Oxidation
Abstract
:1. Introduction
2. Results and Discussion
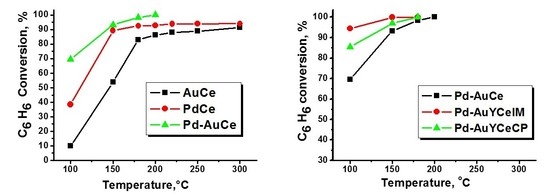
2.1. Catalytic Activity Measurements
2.2. Sample Characterization
3. Experimental
3.1. Sample Preparation
3.2. Sample Characterization
3.3. Catalytic Activity Measurements in CBO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Solsona, B.; Pérez-Cabero, M.; Vázquez, I.; Dejoz, A.; García, T.; Álvarez-Rodríguez, J.; El-Haskouri, J.; Beltrán, D.; Amorós, P. Total oxidation of VOCs on Au nanoparticles anchored on Co doped mesoporous UVM-7 silica. Chem. Eng. J. 2012, 187, 391–400. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Tidahy, H.L.; Hosseini, M.; Siffert, S.; Cousin, R.; Lamojnier, J.-F.; Aboukiais, A.; Su, B.-L.; Giraudon, J.-M.; Leclerq, G. Nanosrutuctured macro-mesoporous zirconia impregnated by noble metal for catalytic total oxidation of toluene. Catal. Today 2008, 137, 335–339. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 429–436. [Google Scholar] [CrossRef]
- Centi, G. Supported palladium catalysts in environmental catalytic technologies for gaseous emissions. J. Mol. Catal. A Chem. 2001, 173, 287–312. [Google Scholar] [CrossRef]
- Andreeva, D.; Tabakova, T.; Ilieva, L.; Naydenov, A.; Mehanjiev, D.; Abrashev, M.V. Nanosize gold catalysts promoted by vanadium oxide supported on titania and zirconia for complete benzene oxidation. Appl. Catal. A Gen. 2001, 209, 291–300. [Google Scholar] [CrossRef]
- Andreeva, D.; Nedyalkova, R.; Ilieva, L.; Abrashev, M.V. Gold-vanadia catalysts supported on ceria-alumina for complete benzene oxidation. Appl. Catal. B Environ. 2004, 52, 157–165. [Google Scholar] [CrossRef]
- Andreeva, D.; Petrova, P.; Ilieva, L.; Abrashev, M.V. Gold supported on ceria and ceria–alumina promoted by molybdena for complete benzene oxidation. Appl. Catal. B Environ. 2006, 67, 237–245. [Google Scholar] [CrossRef]
- Andreeva, D.; Petrova, P.; Ilieva, L.; Sobczak, J.W.; Abrashev, M.V. Design of new gold catalysts supported on mechanochemically activated ceria-alumina, promoted by molybdena for complete bezene oxidation. Appl. Catal. B Environ. 2008, 77, 364–372. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Tabakova, T.; Zanella, R.; Abrashev, M.V.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Andreeva, D. Relationship between structural properties and activity in complete benzene oxidation over Au/CeO2–CoOx catalysts. Catal. Today 2012, 187, 30–38. [Google Scholar] [CrossRef]
- Pretzer, L.A.; Song, H.J.; Fang, Y.L.; Zhao, Z.; Guo, N.; Wu, T.; Arslan, I.; Miller, J.T.; Wong, M.S. Hydrodechlorination catalysis of Pd-on-Au nanoparticles varies with particle size. J. Catal. 2013, 298, 206–217. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Glanville, N.; Kesavan, L.; Hammond, C.; Edwards, J.K.; Carley, A.F.; Kiely, C.J.; Hutchings, G.J. Reactivity studies of Au-Pd supported nanoparticles for catalytic applications. Appl. Catal. A Gen. 2011, 391, 400–406. [Google Scholar] [CrossRef]
- Venezia, A.M.; La Parola, V.; Pawelec, B.; Fierro, J. Hydrogenation of aromatics over Au-Pd/SiO2-Al2O3 catalysts; support acidity effect. Appl. Catal. A Gen. 2004, 264, 43–51. [Google Scholar] [CrossRef]
- Venezia, A.M.; Murania, R.; Pantaleo, G.; Deganello, G. Pd and PdAu on mesoporous silica for methane oxidation: Effect of SO2. J. Catal. 2007, 251, 94–102. [Google Scholar] [CrossRef]
- Bonarowska, M.; Malinowski, A.; Juszczyk, W.; Karpinski, Z. Hydrodechlorination of CCl2F2 (CFC-12) over silica-supported palladium-gold catalysts. Appl. Catal. B Environ. 2001, 30, 187–193. [Google Scholar] [CrossRef]
- Alshammari, A.; Kalevaru, V.N.; Martin, A. Bimetallic catalysts containing gold and palladium for environmentally important reactions. Catalysts 2016, 6, 97. [Google Scholar] [CrossRef]
- Shao, S.S.; Wang, H.; Zhang, M.; Ma, D.D.; Lee, S.T. The mutual promotional effect of Au–Pd bimetallic nanoparticles on silicon nanowires: A study of preparation and catalytic activity. Appl. Phys. Lett. 2008, 93, 243110. [Google Scholar] [CrossRef]
- Lee, D.S.; Che, Y.W. The mutual promotional effect of Au–Pd/CeO2 bimetallic catalysts on destruction of toluene. J. Taiwan Inst. Chem. Eng. 2013, 44, 40–44. [Google Scholar] [CrossRef]
- Hosseini, M.; Siffert, S.; Cousin, R.; Aboukaïs, A.; Hadj-Sadok, Z.; Su, B.-L. Total oxidation of VOCs on Pd and/or Au supported on TiO2/ZrO2 followed by “operando” DRIFT. C. R. Chim. 2009, 12, 654–659. [Google Scholar] [CrossRef]
- Takatani, H.; Kago, H.; Kobayashi, Y.; Hori, F.; Oshima, R. Properties of Au-Pd nanoparticles prepared by sono-chemical technique. Trans. Mater. Res. Soc. Jpn. 2003, 28, 871–874. [Google Scholar]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-Free oxidation of primary alcohols aldhehides using au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Barakat, T.; Cousin, R.; Aboukaïs, A.; Su, B.-L.; De Weireld, G.; Siffert, S. Catalytic performance of core-shell and alloy Pd-Au nanoparticles for total oxidation of VOC: The effect of metal deposition. Appl. Catal. B Environ. 2012, 111–112, 218–224. [Google Scholar] [CrossRef]
- Tabakova, T.; Ilieva, L.; Petrova, P.; Venezia, A.M.; Avdeev, G.; Zanella, R.; Karakirova, Y. Complete benzene oxidation over mono and bimetallic Au-Pd catalysts supported on Fe-modified ceria. Chem. Eng. J. 2015, 260, 133–141. [Google Scholar] [CrossRef]
- Centeno, M.A.; Paulis, M.; Montes, M.; Odriozola, J.A. Catalytic combustion of volatile organic compounds on Au/CeO2/Al2O3 and Au/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 234, 65–78. [Google Scholar] [CrossRef]
- Jiang, X.; Hua, J.; Deng, H.; Wu, Z. Influence of pre-added NaOH on the microstructure of Au–CeO2 catalyst and its activity for benzene oxidation. J. Mol. Catal. A Chem. 2014, 383–384, 188–193. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Cristiani, C.; Lietti, L.; Groppi, G. The influence of ceria and other rare earth promoters on palladium-based methane combustion catalysts. Catal. Today 2012, 180, 124–130. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Leetmaa, M.; Vekilova, O.Y.; Simak, S.I.; Skorodumova, N.V. Oxygen diffusion in ceria doped with rare-earth elements. Phys. Chem. Phys. 2017, 19, 13723–13730. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, L.; Petrova, P.; Pantaleo, G.; Zanella, R.; Liotta, L.F.; Georgiev, V.; Boghosian, S.; Kaszkur, Z.; Sobczak, J.W.; Lisowski, W.; et al. Gold catalysts supported on Y-modified ceria for CO free hydrogen production via PROX. Appl. Catal. B Environ. 2016, 188, 154–168. [Google Scholar] [CrossRef]
- Ou, D.R.; Mori, T.; Ye, F.; Takahashi, M.; Zou, J.; Drennan, J. Microstructures and electrolytic properties of yttrium-doped ceria electrolytes: Dopant concentration and grain size dependences. Acta Mater. 2006, 54, 3737–3746. [Google Scholar] [CrossRef]
- Burbano, M.; Norberg, S.T.; Hull, S.; Eriksson, S.G.; Marrocchelli, D.; Madden, P.A.; Watson, G.W. Oxygen vacancy ordering and the conductivity maximum in Y2O3-doped CeO2. Chem. Mater. 2012, 24, 222–229. [Google Scholar] [CrossRef]
- Yan, P.F.; Mori, T.; Suzuki, A.; Wu, Y.Y.; Auchterlonie, G.J.; Zou, J.; Drennan, J. Grain boundary’s conductivity in heavily yttrium doped ceria. Solid State Ion. 2012, 222, 31–37. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Liotta, L.F.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Munteanu, G.; Tabakova, T. Gold catalysts on Y-doped ceria supports for complete benzene oxidation. Catalysts 2016, 6, 99. [Google Scholar] [CrossRef]
- Ousmane, M.; Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G.; Retailleau, L.; Boreave, A.; Giroir-Fendler, A. Supported Au catalysts for low-temperature abatment of propene and toluene, as model VOC: Support effect. Appl. Catal. B Environ. 2011, 101, 629–637. [Google Scholar] [CrossRef]
- Barakat, T.; Idakiev, V.; Cousin, R.; Shao, G.-S.; Yuan, Z.-Y.; Tabakova, T.; Siffert, S. Total oxidation of toluene over noble metal based Ce, Fe, and Ni doped Titanium oxide. Appl. Catal. B Environ. 2014, 146, 138–146. [Google Scholar] [CrossRef]
- Luo, M.F.; He, M.; Xie, Y.L.; Fang, P.; Jin, L.Y. Toluene oxidation on Pd catalysts supported by CeO2-Y2O3 washcoat cordierite honeycomb. Appl. Catal. B Environ. 2007, 69, 213–218. [Google Scholar] [CrossRef]
- Guzman, J.; Corma, A. Nanocrystalline and mesostructured Y2O3 as supports for gold catalysts. Chem. Commun. 2005, 6, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Kaszkur, Z.; Juszczyk, W.; Łomot, D. Self diffusion in nanocrystalline alloys. Phys. Chem. Chem. Phys. 2015, 17, 28250–28255. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M.; Carabineiro, S.A.; Tavares, P.B.; Figueiredo, J.L. Redox properties and VOC oxidation activity of Cu catalysts supported on Ce1−xSmxOδ mixed oxides. J. Hazard. Mater. 2013, 261, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Fu, Q.; Weber, A.; Flytzani-Stephanopoulos, M. Nanostructured Au-CeO2 catalysts for low-temperature water-gas shift. Catal. Lett. 2001, 77, 87–95. [Google Scholar] [CrossRef]
- Andreeva, D.; Idakiev, V.; Tabakova, T.; Ilieva, L.; Falaras, P.; Bourlinos, A.; Travlos, A. Low-temperature water-gas shift reaction over Au/CeO2 catalysts. Catal. Today 2002, 72, 51–57. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Gazquez, J.L. Oxygen vacancy model in strong metal-support interaction. J. Catal. 1987, 104, 120–135. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Bardi, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; El Faallah, J.; Hilaire, L.; Le Normand, F.; Quemere, E.; et al. Reduction of CeO2 by hydrogen. Magnetic susceptibility and Fourier-transform infrared, ultraviolet and X-ray photoelectron spectroscopy measurements. J. Chem. Soc. Faraday Trans. 1991, 87, 1601–1609. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS stydy of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Monte, M.; Munuera, G.; Costa, D.; Conesa, J.C.; Martínez-Arias, A. Near-ambient XPS characterization of interfacial copper species in ceria-supported copper catalysts. Phys. Chem. Chem. Phys. 2015, 17, 29995–30004. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, H. A novel strategy to the synthesis of Na3YSi2O7 from natural palygorskite. Appl. Clay Sci. 2014, 101, 339–344. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Roudesly, F.; Oble, J.; Poli, G. Metal-catalyzed CH activation/functionalization: The fundamentals. J. Mol. Catal. A Chem. 2017, 426, 275–296. [Google Scholar] [CrossRef]
- Moritani, I.; Fujiwara, Y. Aromatic substitution of styrene-palladium chloride complex. Tetrahedron Lett. 1967, 8, 1119–1122. [Google Scholar] [CrossRef]
- Cimino, S.; Casaletto, M.P.; Lisi, L.; Russo, G. Pd–LaMnO3 as dual site catalysts for methane combustion. Appl. Catal. A Gen. 2007, 327, 238–246. [Google Scholar] [CrossRef]
- Hea, C.; Li, J.; Li, P.; Cheng, J.; Hao, Z.; Xu, Z.-P. Comprehensive investigation of Pd/ZSM-5/MCM-48 composite catalysts with enhanced activity and stability for benzene oxidation. Appl. Catal. B Environ. 2010, 96, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Mote, V.D.; Purushotham, Y.; Dole, B.N. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Sherwood, P.M.A. Practical Surface Analysis; Briggs, D., Seah, M.P., Eds.; Wiley: New York, NY, USA, 1990; pp. 555–585. [Google Scholar]
- Monti, D.A.M.; Baiker, A. Temperature-programmed reduction. Parametric sensitivity and estimation of kinetic parameters. J. Catal. 1983, 83, 323–335. [Google Scholar] [CrossRef]







| Catalyst | SBET (m2/g) | d (nm) | v (cm3/g) | aXRD (CeO2) (nm) | DXRD (CeO2) (nm) | DHRTEM (Au) (nm) |
|---|---|---|---|---|---|---|
| Desorption Branch | ||||||
| AuCe | 102.4 | 1.7 | 0.15 | 0.5412(7) | 6.1 | 2.1 |
| PdCe | 101.9 | 1.7 | 0.15 | 0.5419(4) | 8.4 | |
| Pd–AuCe | 101.0 | 1.7 | 0.15 | 0.5419(4) | 7.6 | 2.2/2.6 * |
| AuYCeIM | 103.0 | 1.6 | 0.16 | 0.5410(5) | 6.9 | 2.6 |
| PdYCeIM | 105.0 | 1.6 | 0.15 | 0.5417(6) | 9.0 | |
| Pd–AuYCeIM | 105.5 | 1.6 | 0.16 | 0.5412(4) | 8.4 | 2.4/4.9 ** |
| AuYCeCP | 90.0 | 3.8 | 0.12 | 0.5411(4) | 7.5 | 3.2 |
| PdYCeCP | 89.6 | 3.7 | 0.11 | 0.5416(5) | 7.9 | |
| Pd–AuYCeCP | 90.0 | 3.8 | 0.12 | 0.5414(11) | 8.8 | 2.6/2.6 * |
| Catalyst | HC (mmol g−1) | |
|---|---|---|
| CP | IM | |
| AuCe | 0.5 a,b | 0.5 a,b |
| AuYCe | 0.6 a,b | 0.6 a,b |
| PdCe | 0.6 a/1.0 b | 0.6 a/1.0 b |
| PdYCe | 0.7 a/1.4 b | 0.7 a/1.2 b |
| Pd–AuCe | 0.6 a/1.2 b | 0.6 a/1.2 b |
| Pd–AuYCe | 0.7 a/1.1 b | 0.5 a/1.1 b |
| Sample | Au 4f7/2 eV | Pd 3d5/2 eV | Y 3d5/2 eV | O 1s eV | Pd/Ce (0.016) * | Au/Ce (0.03) * | Y/Ce (0.016) * |
|---|---|---|---|---|---|---|---|
| AuCe | 84.3 | 529.3 (57%) 531.6 (43%) | 0.06 | ||||
| AuYCeCP | 85.1 | 157.7 (66%) 153.8 (34%) | 529.5 (79%) 532.0 (21%) | 0.06 | 0.09 | ||
| AuYCeIM | 84.5 | 157.4 | 529.4 (77%) 532.0 (23%) | 0.06 | 0.15 | ||
| PdCe | 337.1 | 529.6 (80%) 531.4 (20%) | 0.14 | ||||
| PdYCeCP | 337.2 | 157.5 (57%) 153.4 (43%) | 529.6 (73%) 532.2 (27%) | 0.49 | 0.25 | ||
| PdYCeIM | 337.1 | 157.7 (81%) 153.2 (19%) | 529.4 (78%) 531.7 (22%) | 0.36 | 0.21 | ||
| Pd–AuCe | 84.5 | 337.6 | 529.5 (76%) 531.8 (24%) | 0.25 | 0.03 | ||
| Pd–AuCe spent in CBO | 84.2 | 337.7 (88%) 335.3 (12%) | 529.5 (76%) 531.6 (24%) | 0.15 | 0.02 | ||
| Pd–AuYCeCP | 84.8 | 337.5 | 157.4 (70%) 153.2 (30%) | 529.4 (82%) 531.5 (18%) | 0.32 | 0.04 | 0.04 |
| Pd–AuYCeCP spent in CBO | 84.6 | 337.8 (66%) 336.3 (34%) | 157.6 | 529.7 (74%) 531.9 (26%) | 0.19 | 0.02 | 0.09 |
| Pd–AuYCeIM | 84.8 | 337.4 | 157.6 (63%) 153.7 (37%) | 529.3 (64%) 531.4 (36%) | 0.33 | 0.04 | 0.07 |
| Pd–AuYCeIM spent in CBO | 84.5 | 337.7 (52%) 336.4 (48%) | 158.0 | 529.6 (74%) 531.8 (26%) | 0.16 | 0.02 | 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, L.; Venezia, A.M.; Petrova, P.; Pantaleo, G.; Liotta, L.F.; Zanella, R.; Kaszkur, Z.; Tabakova, T. Effect of Y Modified Ceria Support in Mono and Bimetallic Pd–Au Catalysts for Complete Benzene Oxidation. Catalysts 2018, 8, 283. https://doi.org/10.3390/catal8070283
Ilieva L, Venezia AM, Petrova P, Pantaleo G, Liotta LF, Zanella R, Kaszkur Z, Tabakova T. Effect of Y Modified Ceria Support in Mono and Bimetallic Pd–Au Catalysts for Complete Benzene Oxidation. Catalysts. 2018; 8(7):283. https://doi.org/10.3390/catal8070283
Chicago/Turabian StyleIlieva, Lyuba, Anna Maria Venezia, Petya Petrova, Giuseppe Pantaleo, Leonarda Francesca Liotta, Rodolfo Zanella, Zbigniew Kaszkur, and Tatyana Tabakova. 2018. "Effect of Y Modified Ceria Support in Mono and Bimetallic Pd–Au Catalysts for Complete Benzene Oxidation" Catalysts 8, no. 7: 283. https://doi.org/10.3390/catal8070283