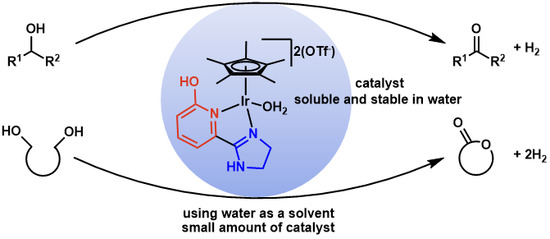
Dehydrogenative Transformation of Alcoholic Substrates in Aqueous Media Catalyzed by an Iridium Complex Having a Functional Ligand with α-Hydroxypyridine and 4,5-Dihydro-1H-imidazol-2-yl Moieties
Abstract
:1. Introduction
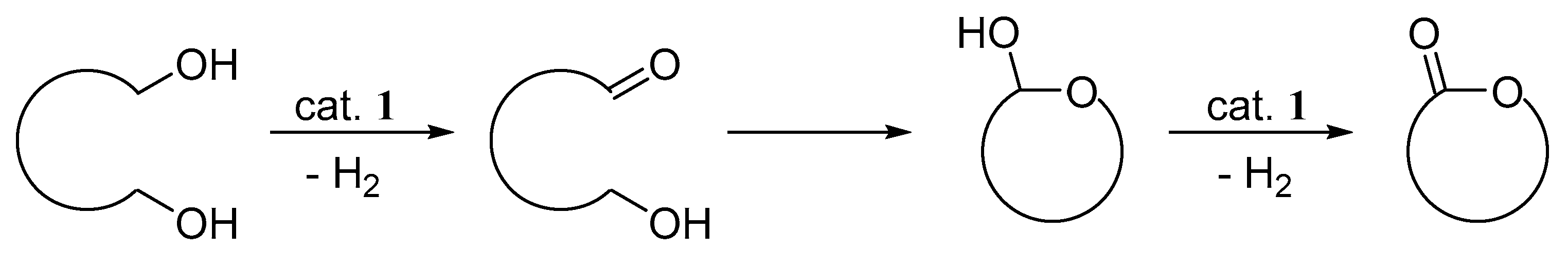
2. Results and Discussion



3. Experimental Section
3.1. General
3.2. Preparation of Dicationic Complexes 1–4
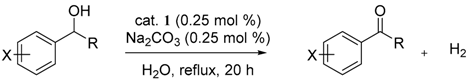
3.3. General Procedures for the Dehydrogenative Oxidation of 1-Phenylethanol (Table 1 and Table 2)
3.4. General Procedure for the Dehydrogenative Oxidation of Secondary Alcohols (Table 3)
3.5. Procedure for the Quantitative Analysis of the Evolved Hydrogen Gas in the Dehydrogenative Oxidation of 1-Indanol (Equation (1))
3.6. Preparation of Monocationic Complex 9 (Equation (2))
3.7. General Procedure for the Dehydrogenative Lactonization of Diols (Table 4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Lindström, U.M. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 102, 2751–2772. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Fokin, V.V. Organic Synthesis “On Water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, R.N.; Coyne, A.G. Water: Nature’s Reaction Enforcer—Comparative Effects for Organic Synthesis “In-Water” and “On-Water”. Chem. Rev. 2010, 110, 6302–6337. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.-O.; Li, C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Using Water-Soluble and Reusable Cp*Ir Catalysts Bearing a Functional Bipyridine Ligand. J. Am. Chem. Soc. 2012, 134, 3643–3646. [Google Scholar] [CrossRef] [PubMed]
- Toyomura, K.; Fujita, K. Synthesis of Coordinatively Unsaturated Iridium Complexes Having Functional 8-Quinolinolato Ligands: New Catalysts for Dehydrogenative Oxidation of Alcohols in Aqueous Media. Chem. Lett. 2017, 46, 808–810. [Google Scholar] [CrossRef]
- Fujita, K.; Tamura, R.; Tanaka, Y.; Yoshida, M.; Onoda, M.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Catalyzed by a Water-Soluble Dicationic Iridium Complex Bearing a Functional N-Heterocyclic Carbene Ligand without Using Base. ACS Catal. 2017, 7, 7226–7230. [Google Scholar] [CrossRef]
- Fujita, K.; Ito, W.; Yamaguchi, R. Dehydrogenative Lactonization of Diols in Aqueous Media Catalyzed by a Water—Soluble Iridium Complex Bearing a Functional Bipyridine Ligand. ChemCatChem 2013, 6, 109–112. [Google Scholar] [CrossRef]
- A number of catalytic systems for dehydrogenative transformation of alcoholic substrates have been reported by other research groups. See the references 12 to 22.
- Feng, B.; Chen, C.; Yang, H.; Zhao, X.; Hua, L.; Yu, Y.; Cao, T.; Shi, Y.; Hou, Z. Ionic Liquid-Promoted Oxidant-Free Dehydrogenation of Alcohols with Water-Soluble Ruthenium Nanoparticles in Aqueous Phase. Adv. Synth. Catal. 2012, 354, 1559–1565. [Google Scholar] [CrossRef]
- Tang, L.; Sun, H.; Li, Y.; Zha, Z.; Wang, Z. Highly Active and Selective Synthesis of Imines from Alcohols and Amines or Nitroarenes Catalyzed by Pd/DNA in Water with Dehydrogenation. Green Chem. 2012, 14, 3423–3428. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Applications of Acceptorless Dehydrogenation and Related Transformations in Chemical Synthesis. Science 2013, 341, 1229712. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, E.; Khaskin, E.; Leitus, G.; Milstein, D. Catalytic Transformation of Alcohols to Carboxylic Acid Salts and H2 Using Water as the Oxygen Atom Source. Nat. Chem. 2013, 5, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Sawama, Y.; Morita, K.; Yamada, T.; Nagata, S.; Yabe, Y.; Monguchi, Y.; Sajiki, H. Rhodium-on-Carbon Catalyzed Hydrogen Scavenger-and Oxidant-Free Dehydrogenation of Alcohols in Aqueous Media. Green Chem. 2014, 16, 3439–3443. [Google Scholar] [CrossRef]
- Ngo, A.H.; Adams, M.J.; Do, L.H. Selective Acceptorless Dehydrogenation and Hydrogenation by Iridium Catalysts Enabling Facile Interconversion of Glucocorticoids. Organometallics 2014, 33, 6742–6745. [Google Scholar] [CrossRef]
- Sawama, Y.; Morita, K.; Asai, S.; Kozawa, M.; Tadokoro, S.; Nakajima, J.; Monguchi, Y.; Sajiki, H. Palladium on Carbon-Catalyzed Aqueous Transformation of Primary Alcohols to Carboxylic Acids Based on Dehydrogenation under Mildly Reduced Pressure. Adv. Synth. Catal. 2015, 357, 1205–1210. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Liu, Y.; Xiao, J. Acceptorless Dehydrogenation and Aerobic Oxidation of Alcohols with a Reusable Binuclear Rhodium(II) Catalyst in Water. Green Chem. 2016, 18, 4605–4610. [Google Scholar] [CrossRef]
- Zhang, L.; Nguyen, D.H.; Raffa, G.; Trivelli, X.; Capet, F.; Desset, S.; Paul, S.; Dumeignil, F.; Gauvin, R.M. Catalytic Conversion of Alcohols into Carboxylic Acid Salts in Water: Scope, Recycling, and Mechanistic Insights. ChemSusChem 2016, 9, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. Homogeneous Transition Metal Catalysis of Acceptorless Dehydrogenative Alcohol Oxidation: Applications in Hydrogen Storage and to Heterocycle Synthesis. Chem. Rev. 2017, 117, 9228–9246. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Muthaiah, S. Well-Defined Ruthenium Complex for Acceptorless Alcohol Dehydrogenation in Aqueous Medium. ChemistrySelect 2018, 3, 3737–3741. [Google Scholar] [CrossRef]
- Very recently, Himeda et al. Reported the synthesis of a closely related complex which has the same dicationic part of complex 1, while the anionic part of their complex is sulfate (SO42−). They applied this complex as a catalyst for hydrogenation of carbon dioxide and dehydrogenation of formic acid in water: Wang, L.; Onishi, N.; Murata, K.; Hirose, T.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Efficient Hydrogen Storage and Production Using a Catalyst with an Imidazoline-Based, Proton-Responsive Ligand. ChemSusChem 2017, 10, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- We performed the dehydrogenative oxidation of 1-phenylethanol (5a) to acetophenone (6a) by using 0.25 mol% of our previously reported water-soluble dicationic catalysts. Yield of 6a was 72% with the catalyst reported in reference 7 and 89% with the catalyst reported in reference 9, respectively.
- Ball, R.G.; Graham, W.A.G.; Heinekey, D.M.; Hoyano, J.K.; McMaster, A.D.; Mattson, B.M.; Michel, S.T. Synthesis and Structure of Dicarbonylbis(η-pentamethylcyclopentadienyl)diiridium. Inorg. Chem. 1990, 29, 2023–2025. [Google Scholar] [CrossRef]
- Ogo, S.; Makihara, N.; Watanabe, Y. pH-Dependent Transfer Hydrogenation of Water-Soluble Carbonyl Compounds with [Cp*IrIII(H2O)3]2+ (Cp* = η5-C5Me5) as a Catalyst Precursor and HCOONa as a Hydrogen Donor in Water. Organometallics 1999, 18, 5470–5474. [Google Scholar] [CrossRef]
- Uyanik, M.; Fukatsu, R.; Ishihara, K. Bromine-Catalyzed Aerobic Oxidation of Alcohols. Chem. Asian J. 2010, 5, 456–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchwald, S.L.; Watson, B.T.; Lum, R.T.; Nugent, W.A. A General Method for the Preparation of Zirconocene Complexes of Substituted Benzynes: In Situ Generation, Coupling Reactions, and Use in the Synthesis of Polyfunctionalized Aromatic Compounds. J. Am. Chem. Soc. 1987, 109, 7137–7141. [Google Scholar] [CrossRef]
- Rios, M.Y.; Salazar, E.; Olivo, H.F. Chemo-enzymatic Baeyer–Villiger Oxidation of Cyclopentanone and Substituted Cyclopentanones. J. Mol. Catal. B Enzym. 2008, 54, 61–66. [Google Scholar] [CrossRef]
- Herbivo, C.; Comel, A.; Kirsch, G.; Raposo, M.M.M. Synthesis of 5-Aryl-5′-formyl-2,2′-bithiophenes as New Precursors for Nonlinear Optical (NLO) Materials. Tetrahedron 2009, 65, 2079–2086. [Google Scholar] [CrossRef]
- Ruan, J.; Li, X.; Saidi, O.; Xiao, J. Oxygen and Base-Free Oxidative Heck Reactions of Arylboronic Acids with Olefins. J. Am. Chem. Soc. 2008, 130, 2424–2425. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Takenaga, N.; Goto, A.; Fujioka, H.; Kita, Y. Clean and Efficient Benzylic C−H Oxidation in Water Using a Hypervalent Iodine Reagent: Activation of Polymeric Iodosobenzene with KBr in the Presence of Montmorillonite-K10. J. Org. Chem. 2008, 73, 7365–7368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Shi, B.-F.; Yu, J.-Q. Palladium(II)-Catalyzed ortho Alkylation of Benzoic Acids with Alkyl Halides. Angew. Chem. Int. Ed. 2009, 48, 6097–6100. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, J.; Parthasarathy, K.; Cheng, C.-H. Synthesis of Biarylketones and Phthalides from Organoboronic Acids and Aldehydes Catalyzed by Cobalt Complexes. Chem. Commun. 2011, 47, 10461–10463. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.M.; Stahl, S.S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omura, S.; Fukuyama, T.; Murakami, Y.; Okamoto, H.; Ryu, I. Hydroruthenation Triggered Catalytic Conversion of Dialdehydes and Keto Aldehydes to Lactones. Chem. Commun. 2009, 45, 6741–6743. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.W.; Kinloch, S.A.; McCormick, A.S.; Russell, R.A.; Warrener, R.N. Anthracyclines XVII: The Synthesis of 2-Fluoro and 3-Fluoro-4-demethoxydaunomycin. Tetrahedron 1988, 44, 4591–4604. [Google Scholar] [CrossRef]


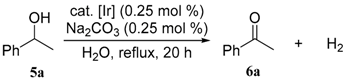
| entry | cat. | conv. of 5a (%) a | yield of 6a (%) a |
| 1 | 1 | 92 | 92 |
| 2 | 2 | 89 | 89 |
| 3 | 3 | 14 | 14 |
| 4 | 4 | 25 | 25 |
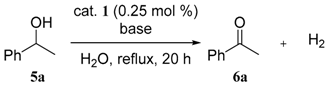
| entry | base (mol %) | conv. of 5a (%) a | yield of 6a (%) a |
| 1 | none | 16 | 16 |
| 2 | Na2CO3 (0.25) | 92 | 92 |
| 3 | Na2CO3 (0.50) | 81 | 81 |
| 4 | NaOH (0.50) | 83 | 83 |
| 5 | NaHCO3 (0.50) | 82 | 82 |
| 6 | Li2CO3 (0.25) | 83 | 82 |
| 7 | K2CO3 (0.25) | 83 | 83 |
| 8 | Cs2CO3 (0.25) | 86 | 86 |
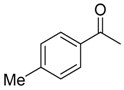 | 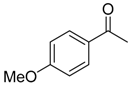 | 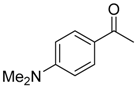 |  |
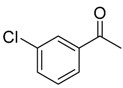
| 6b 87% (84%) | 6c 95% (92%) | 6d 62% (59%) | 6e 63% (57%) |
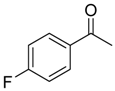 | 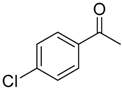 |  | 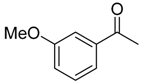 |
| 6f 83% (74%) | 6g 80% (75%) | 6h 83% (81%) | 6i 80% (78%) |
 | 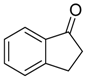 | 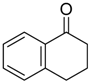 |  |
| 6j 80% (75%) | 6k 98% (98%) | 6l 98% (98%) | 6m 73% (71%) a,b |
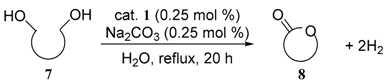
| entry | diol | product | yield (%) a | |
| 1 | 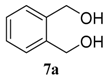 | 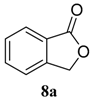 | 98 (81) | |
| 2 b |  | 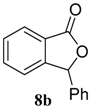 | 98 (91) | |
| 3 | 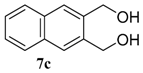 |  | 78 (73) | |
| 4 | 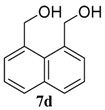 |  | 99 (71) | |
| 5 b,c | 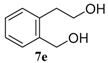 | 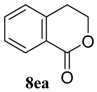 |  | 91, 82 : 18 d (91, 82 : 18 d) |
| 6 | 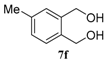 | 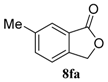 |  | 99, 48 : 52 d (86, 47 : 53 d) |
| 7 |  | 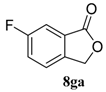 |  | 98, 45 : 55 d (88, 48 : 52 d) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, M.; Wang, H.; Shimbayashi, T.; Fujita, K.-i. Dehydrogenative Transformation of Alcoholic Substrates in Aqueous Media Catalyzed by an Iridium Complex Having a Functional Ligand with α-Hydroxypyridine and 4,5-Dihydro-1H-imidazol-2-yl Moieties. Catalysts 2018, 8, 312. https://doi.org/10.3390/catal8080312
Yoshida M, Wang H, Shimbayashi T, Fujita K-i. Dehydrogenative Transformation of Alcoholic Substrates in Aqueous Media Catalyzed by an Iridium Complex Having a Functional Ligand with α-Hydroxypyridine and 4,5-Dihydro-1H-imidazol-2-yl Moieties. Catalysts. 2018; 8(8):312. https://doi.org/10.3390/catal8080312
Chicago/Turabian StyleYoshida, Masato, Han Wang, Takuya Shimbayashi, and Ken-ichi Fujita. 2018. "Dehydrogenative Transformation of Alcoholic Substrates in Aqueous Media Catalyzed by an Iridium Complex Having a Functional Ligand with α-Hydroxypyridine and 4,5-Dihydro-1H-imidazol-2-yl Moieties" Catalysts 8, no. 8: 312. https://doi.org/10.3390/catal8080312