Morphology-Controlled Nitrogen-Containing Polymers as Synthetic Precursors for Electrochemical Oxygen Reduction Fe/N/C Cathode Catalysts
Abstract
:1. Introduction
2. ORR Reaction Scheme and Active Sites over NPM Catalysts
3. Pioneering Studies on Nitrogen-Containing Polymers as Precursors of NPM Catalysts
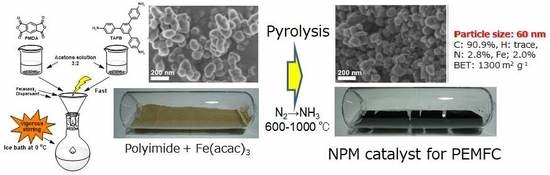
4. Fe/N/C Catalysts Prepared by Pyrolyzing Spherical Polyimides
4.1. Synthesis of Polyimide Nano-Particles
4.2. Fe/N/C Catalysts after the Carbonization of Polyimide Nano-Particles.
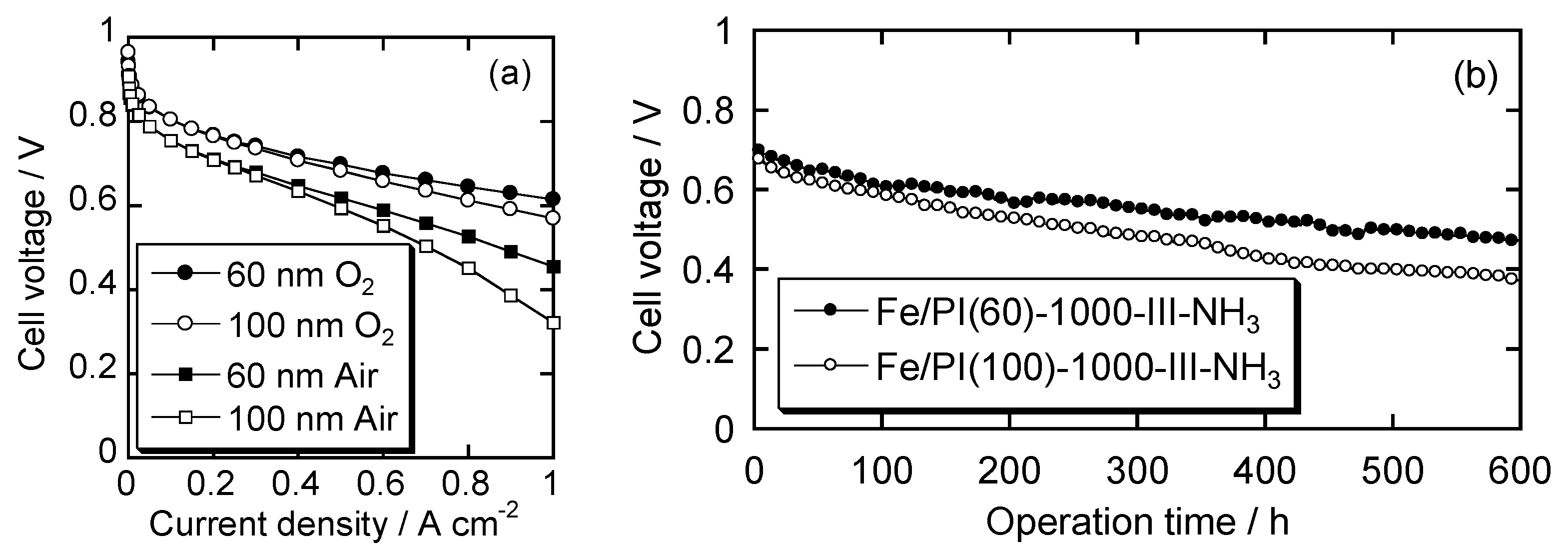
4.3. Fuel Cell Performance
5. Mesoporous Fe/N/C Catalysts for Enhanced Oxygen Diffusion
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamanaka, I.; Onizawa, T.; Takenaka, S.; Otsuka, K. Direct and continuous production of hydrogen peroxide with 93% selectivity using a fuel-cell system. Angew. Chemie Int. Ed. 2003, 42, 3653–3655. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. Highly Selective Two-Electron Oxygen Reduction Catalyzed by Mesoporous Nitrogen-Doped Carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous Nitrogen Doped Carbon for the Electrocatalytic Synthesis of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K. Zirconium-Based Compounds for Cathode of Polymer Electrolyte Fuel Cell. J. Electrochem. Soc. 2007, 154, B362. [Google Scholar] [CrossRef]
- Ishihara, A.; Tamura, M.; Ohgi, Y.; Matsumoto, M.; Matsuzawa, K.; Mitsushima, S.; Imai, H.; Ota, K. Emergence of Oxygen Reduction Activity in Partially Oxidized Tantalum Carbonitrides: Roles of Deposited Carbon for Oxygen-Reduction-Reaction-Site Creation and Surface Electron Conduction. J. Phys. Chem. C 2013, 117, 18837–18844. [Google Scholar] [CrossRef]
- Chisaka, M.; Ando, Y.; Yamamoto, Y.; Itagaki, N. A Carbon-Support-Free Titanium Oxynitride Catalyst for Proton Exchange Membrane Fuel Cell Cathodes. Electrochim. Acta 2016, 214, 165–172. [Google Scholar] [CrossRef]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Masa, J.; Ozoemena, K. I.; Schuhmann, W.; Zagal, J.H. Fundamental Studies on the Electrocatalytic Properties of Metal Macrocyclics and Other Complexes for the Electroreduction of O2. In Electrocatalysis in Fuel Cells; Springer: London, UK, 2013; pp. 157–212. [Google Scholar]
- Jahnke, H.; Schönborn, M.; Zimmermann, G. Organic dyestuffs as catalysts for fuel cells. In Physical and Chemical Applications of Dyestuffs; Springer: Berlin/Heidelberg, Germany, 1976; Volume 61, pp. 133–181. ISBN 0340-1022. [Google Scholar]
- Dodelet, J.-P. The Controversial Role of the Metal in Fe- or Co-Based Electrocatalysts for the Oxygen Reduction Reaction in Acid Medium. In Electrocatalysis in Fuel Cells; Springer: London, UK, 2013; pp. 271–338. [Google Scholar]
- Elbaz, L.; Wu, G.; Zelenay, P. Heat-Treated Non-precious-Metal-Based Catalysts for Oxygen Reduction. In Electrocatalysis in Fuel Cells; Springer: London, UK, 2013; pp. 213–246. [Google Scholar]
- Lefvre, M.; Dodelet, J.P.; Bertrand, P.; Lefe, M. Molecular Oxygen Reduction in PEM Fuel Cells: Evidence for the Simultaneous Presence of Two Active Sites in Fe-Based Catalysts. J. Phys. Chem. B 2002, 106, 8705–8713. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Nabae, Y.; Muthukrishnan, A.; Ohsaka, T. Electrochemical deposition and dissolution of Fe species for N-doped carbon to understand the degradation mechanism of Pt-free oxygen reduction catalysts. Electrochim. Acta 2016, 214, 307–312. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-treated ordered mesoporous carbons as catalytic materials for oxygen reduction reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Dai, L. Highly Efficient Metal-Free Growth of Nitrogen-Doped Single-Walled Carbon Nanotubes on Plasma-Etched Substrates for Oxygen Reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidik, R.A.; Anderson, A.B.; Subramanian, N.P.; Kumaraguru, S.P.; Popov, B.N. O2 reduction on graphite and nitrogen-doped graphite: Experiment and theory. J. Phys. Chem. B 2006, 110, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Boero, M.; Huang, S.F.; Terakura, K.; Oshima, M.; Ozaki, J.I. Carbon alloy catalysts: Active sites for oxygen reduction reaction. J. Phys. Chem. C 2008, 112, 14706–14709. [Google Scholar] [CrossRef]
- Ikeda, T.; Hou, Z.; Chai, G.L.; Terakura, K. Possible oxygen reduction reactions for graphene edges from first principles. J. Phys. Chem. C 2014, 118, 17616–17625. [Google Scholar] [CrossRef]
- Damjanovic, A. Distinction between Intermediates Produced in Main and Side Electrodic Reactions. J. Chem. Phys. 1966, 45, 4057. [Google Scholar] [CrossRef]
- Nallathambi, V.; Lee, J.-W.; Kumaraguru, S.P.; Wu, G.; Popov, B.N. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane fuel cells. J. Power Sources 2008, 183, 34–42. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity. Adv. Energy Mater. 2014, 4, 1301735. [Google Scholar] [CrossRef]
- Iwazaki, T.; Obinata, R.; Sugimoto, W.; Takasu, Y. High oxygen-reduction activity of silk-derived activated carbon. Electrochem. Commun. 2009, 11, 376–378. [Google Scholar] [CrossRef] [Green Version]
- Olson, T.S.; Pylypenko, S.; Fulghum, J.E.; Atanassov, P. Bifunctional Oxygen Reduction Reaction Mechanism on Non-Platinum Catalysts Derived from Pyrolyzed Porphyrins. J. Electrochem. Soc. 2010, 157, B54. [Google Scholar] [CrossRef]
- Hsueh, K.L.; Chin, D.T.; Srinivasan, S. Electrode kinetics of oxygen reduction. A theoretical and experimental analysis of the rotating ring-disc electrode method. J. Electroanal. Chem. 1983, 153, 79–95. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y.; Hayakawa, T.; Okajima, T.; Ohsaka, T. Fe-containing polyimide-based high-performance ORR catalysts in acidic medium: A kinetic approach to study the durability of catalysts. Catal. Sci. Technol. 2015, 5, 475–483. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y.; Okajima, T.; Ohsaka, T. Kinetic Approach to Investigate the Mechanistic Pathways of Oxygen Reduction Reaction on Fe-Containing N-Doped Carbon Catalysts. ACS Catal. 2015, 5. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y. Estimation of the inherent kinetic parameters for oxygen reduction over a Pt-free cathode catalyst by resolving the quasi-four-electron reduction. J. Phys. Chem. C 2016, 120, 22515–22525. [Google Scholar] [CrossRef]
- van Veen, J.A.R.; Colijn, H.A.; van Baar, J.F. On the effect of a heat treatment on the structure of carbon-supported metalloporphyrins and phthalocyanines. Electrochim. Acta 1988, 33, 801–804. [Google Scholar] [CrossRef]
- Gojkovic, S.L.; Gupta, S.; Savinell, R.F. Heat-Treated Iron(III) Tetramethoxyphenyl Porphyrin Supported on High-Area Carbon as an Electrocatalyst for Oxygen Reduction. J. Electrochem. Soc. 1998, 145, 3493. [Google Scholar] [CrossRef]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Bron, M.; Radnik, J.; Fieber-Erdmann, M.; Bogdanoff, P.; Fiechter, S. EXAFS, XPS and electrochemical studies on oxygen reduction catalysts obtained by heat treatment of iron phenanthroline complexes supported on high surface area carbon black. J. Electroanal. Chem. 2002, 535, 113–119. [Google Scholar] [CrossRef]
- Lalande, G.; Coté, R.; Guay, D.; Dodelet, J.P.; Weng, L.T.; Bertrand, P. Is nitrogen important in the formulation of Fe-based catalysts for oxygen reduction in solid. Electrochim. Acta 1997, 42, 1379–1388. [Google Scholar] [CrossRef]
- Herranz, J.; Lefèvre, M.; Larouche, N.; Stansfield, B.; Dodelet, J.P. Step-by-step synthesis of non-noble metal electrocatalysts for O2 reduction under proton exchange membrane fuel cell conditions. J. Phys. Chem. C 2007, 111, 19033–19042. [Google Scholar] [CrossRef]
- Alves, M.C.M.; Dodelet, J.P.; Guay, D.; Ladouceur, M.; Tourillon, G. Origin of the electrocatalytic properties for oxygen reduction of some heat-treated polyacrylonitrile and phthalocyanine cobalt compounds adsorbed on carbon black as probed by electrochemistry and x-ray absorption spectroscopy. J. Phys. Chem. 1992, 96, 10898–10905. [Google Scholar] [CrossRef]
- Chokai, M.; Taniguchi, M.; Moriya, S.; Matsubayashi, K.; Shinoda, T.; Nabae, Y.; Kuroki, S.; Hayakawa, T.; Kakimoto, M.; Ozaki, J.-I.; et al. Preparation of carbon alloy catalysts for polymer electrolyte fuel cells from nitrogen-containing rigid-rod polymers. J. Power Sources 2010, 195, 5947–5951. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.E.; Tan, Z.; Schmoeckel, A.K.; O’Neill, D.; Atanasoski, R. Non-precious metal oxygen reduction catalyst for PEM fuel cells based on nitroaniline precursor. J. Power Sources 2008, 178, 510–516. [Google Scholar] [CrossRef]
- Asao, K. Preparation and Application of Polyimide Particles. J. Photopolym. Sci. Technol. 2014, 27, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Sawai, T.; Uchida, T.; Yamazaki, S.; Kimura, K. Preparation of Novel Naphthalene Polyimide and Its Morphology. J. Photopolym. Sci. Technol. 2013, 26, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Sawai, T.; Wakabayashi, K.; Yamazaki, S.; Uchida, T.; Sakaguchi, Y.; Yamane, R.; Kimura, K. Synthesis and morphology control of self-condensable naphthalene-containing polyimide by using reaction-induced crystallization. Eur. Polym. J. 2013, 49, 2334–2343. [Google Scholar] [CrossRef]
- Chokai, M.; Nabae, Y.; Kuroki, S.; Hayakawa, T.; Kakimoto, M.; Miyata, S. Preparation of Carbon-Based Catalysts for PEFC from Nitrogen-Containing Aromatic Polymers. J. Photopolym. Sci. Technol. 2011, 24, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Nabae, Y.; Kuang, Y.; Chokai, M.; Ichihara, T.; Isoda, A.; Hayakawa, T.; Aoki, T. High performance Pt-free cathode catalysts for polymer electrolyte membrane fuel cells prepared from widely available chemicals. J. Mater. Chem. A 2014, 2, 11561–11564. [Google Scholar] [CrossRef] [Green Version]
- Nabae, Y.; Nagata, S.; Hayakawa, T.; Niwa, H.; Harada, Y.; Oshima, M.; Isoda, A.; Matsunaga, A.; Tanaka, K.; Aoki, T. Pt-free carbon-based fuel cell catalyst prepared from spherical polyimide for enhanced oxygen diffusion. Sci. Rep. 2016, 6, 23276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabae, Y.; Nagata, S. Oxygen Reduction Catalytic Activity of Carbon-based Cathode Catalyst Prepared from Polyimide Nano-Particles Containing Fe-Phenanthroline Complex. J. Photopolym. Sci. Technol. 2016, 29, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Nabae, Y.; Sonoda, M.; Yamauchi, C.; Hosaka, Y.; Isoda, A.; Aoki, T. Highly durable Pt-free fuel cell catalysts prepared by multi-step pyrolysis of Fe phthalocyanine and phenolic resin. Catal. Sci. Technol. 2014, 4, 1400. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal–nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banham, D.; Kishimoto, T.; Zhou, Y.; Sato, T.; Bai, K.; Ozaki, J.; Imashiro, Y.; Ye, S. Critical advancements in achieving high power and stable nonprecious metal catalyst-based MEAs for real-world proton exchange membrane fuel cell applications. Sci. Adv. 2018, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Ariga, K.; Mori, T.; Nakanishi, T.; Hishita, S.; Golberg, D.; Bando, Y. Preparation and characterization of well-ordered hexagonal mesoporous carbon nitride. Adv. Mater. 2005, 17, 1648–1652. [Google Scholar] [CrossRef]
- Park, S.S.; Chu, S.-W.; Xue, C.; Zhao, D.; Ha, C.-S. Facile synthesis of mesoporous carbon nitrides using the incipient wetness method and the application as hydrogen adsorbent. J. Mater. Chem. 2011, 21, 10801. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Jaroniec, M.; Liang, C.; Dai, S. Polypyrrole-based nitrogen-doped carbon replicas of SBA-15 and SBA-16 containing magnetic nanoparticles. J. Phys. Chem. C 2008, 112, 13126–13133. [Google Scholar] [CrossRef]
- Carroll, N.J.; Pylypenko, S.; Atanassov, P.B.; Petsev, D.N. Microparticles with bimodal nanoporosity derived by microemulsion templating. Langmuir 2009, 25, 13540–13544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ohnishi, K.; Sugimoto, S.; Okuhara, K.; Maeda, R.; Nabae, Y.; Kakimoto, M.; Wang, X.; Hayakawa, T. Well-ordered mesoporous polymers and carbons based on imide-incorporated soft materials. Polym. Chem. 2014, 5, 6452–6460. [Google Scholar] [CrossRef]
- Nabae, Y.; Nagata, S.; Ohnishi, K.; Liu, Y.; Sheng, L.; Wang, X.; Hayakawa, T. Block copolymer templated carbonization of nitrogen containing polymer to create fine and mesoporous carbon for oxygen reduction catalyst. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 464–470. [Google Scholar] [CrossRef]
- Gao, L.; Chandra, A.; Nabae, Y.; Hayakawa, T. Inducing defects in ordered mesoporous carbons via the block copolymer-templated high-temperature carbonization of nitrogen-containing polymeric precursors. Polym. J. 2018. [Google Scholar] [CrossRef]













| Catalyst [Ref.] | PI Source | Fe Source | Protocol | Composition (wt %) | BET 3 Surface Area (m2/g) | |||
|---|---|---|---|---|---|---|---|---|
| C 1 | H 1 | N 1 | Fe 2 | |||||
| Fe(2.0)/PI(100) [44] | PMDA 4 ODA 5 | Fe(acac)3 2 wt % | 1. 600-N2-aw 2. 800-NH3-aw 3. 1000-NH3 | 90.6 | trace | 3.1 | 1.1 | 1050 |
| Fe(2.0)/PI(60) [45] | PMDA TAPB 6 | Fe(acac)3 2 wt % | 1. 600-N2-aw 2. 800-NH3-aw 3. 1000-NH3 | 90.9 | trace | 2.8 | 2.0 | 1300 |
| Fe(0.3)/PI(60) [46] | PMDA TAPB | Fe(acac)3 0.3 wt % | 1. 900-N2 2. 800-NH3 3. 1000-NH3 | 85.5 | 0.2 | 2.2 | 1.0 | 1380 |
| Fe(0.3)(amph)/PI(60) [46] | PMDA TAPB | Fe(amph)32+ 0.3 wt % | 1. 900-N2 2. 800-NH3 3. 1000-NH3 | 91.0 | trace | 3.3 | 0.8 | 1390 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabae, Y. Morphology-Controlled Nitrogen-Containing Polymers as Synthetic Precursors for Electrochemical Oxygen Reduction Fe/N/C Cathode Catalysts. Catalysts 2018, 8, 324. https://doi.org/10.3390/catal8080324
Nabae Y. Morphology-Controlled Nitrogen-Containing Polymers as Synthetic Precursors for Electrochemical Oxygen Reduction Fe/N/C Cathode Catalysts. Catalysts. 2018; 8(8):324. https://doi.org/10.3390/catal8080324
Chicago/Turabian StyleNabae, Yuta. 2018. "Morphology-Controlled Nitrogen-Containing Polymers as Synthetic Precursors for Electrochemical Oxygen Reduction Fe/N/C Cathode Catalysts" Catalysts 8, no. 8: 324. https://doi.org/10.3390/catal8080324