Constructing A Rational Kinetic Model of the Selective Propane Oxidation Over A Mixed Metal Oxide Catalyst
Abstract
:1. Introduction
- (a)
- Multi step process: The main steps of this process are: (i) interactions between components of the reaction mixture and the catalytic surface, i.e., the impact reaction between the molecule of gaseous propane and catalyst oxygen, adsorption of water etc; (ii) mass transfer processes, i.e., oxygen exchange between the catalyst surface and bulk; (iii) transport of charge (electrons and/or ions) within the catalytic unit.
- (b)
- Complex structure of the active site/centre: The ‘structure of the catalyst’ tackles the nature of the catalyst surface; i.e., the architecture of catalytically active site/centre, how many active centres are considered per site, and furthermore, how many sites are distinguishable etc.An empirical concept of site isolation proposed by Grasselli [19,20,21] can be used as one of basic structure pre-assumptions for the rational kinetic model. This concept is presented as follows: “The catalytic reaction sequence should occur on an ensemble of atoms (‘the active site’) that holds together with the educt molecule all other reactants and exchanges electrons between the reactants. For these operations neither the influx of active species during the unit conversion time (as long as the educt is adsorbed) nor the participation of electrons from outside of the active site are required ... The catalytic cycle is only finished when the site is regenerated into its initial active form, being here fully oxidised and holding all oxygen atoms belonging to its initial structure” [22].In this concept, many additional processes are not considered, such as processes of the formation of active sites, processes of spill-over, rather to say, all processes external regarding the isolated sites. From the kinetic point of view, the process over the isolated site will be characterised by the specific relationship between the rate of reactants and products which participate in this process. However, these relationships are still not recognised.In the literature, there are many considerations on the complex functional structure of the active sites which is responsible for generation of different products. It makes sense to distinguish: a ‘whole active site’ and ‘active centre’ (or ‘centres’) within this site, which generates (or generate) the specific product(s). One ‘active site’ consists of at least one ‘active centre’. There are speculations about dimer- and trimer structures within the whole active site. These different centres can be considered as ingredients over which different catalytic routes are performed and different products are generated. In particular, it was assumed that an active site for oxidation of propane may consist of dimer- and trimer-ingredients. Dimers and more complex structures (trimers and even ‘dimers of dimers’) can store redox-equivalents as a part of the process of oxygen activation.It is clear that structures of active sites and/or active centres mean surface structures. The surface of a catalyst material (e.g., a metal or metal oxide) may significantly differ from its bulk structure and composition, and may additionally be affected strongly by the gas phase surrounding it [12,23,24,25,26,27].
- (c)
- Multi-scale temporal behaviour: As for multi-scale temporal behaviour of the catalytic process, at least three characteristic times have to be distinguished; ‘fast’ and ‘moderate’ intrinsic times of the catalytic cycle, and the ‘slow’ time, which is external. The latter is caused by the modification and evolution of the catalytic centre/site under the influence of reactive medium. In the catalytic literature, this phenomenon was described and conceptualised by Boreskov [28], see also Yablonskii et al. [29]. A variety of physical methods for the investigation of the surface and bulk of catalysts and different methods of kinetic analysis, mostly steady state methods, have been used to investigate this process.
1.1. Investigations of the Selective Oxidation of Propane. Methods and Results
1.1.1. Physical Methods
1.1.2. Kinetic Methods. Model-Free Kinetic Data
1.1.3. Mechanisms
- Is the mechanism parallel or consecutive, or parallel-consecutive, regarding the different gas products, in particular propene?
- Does this complex reaction occur on a single site or multiple sites?
- What is the nature of an active site?
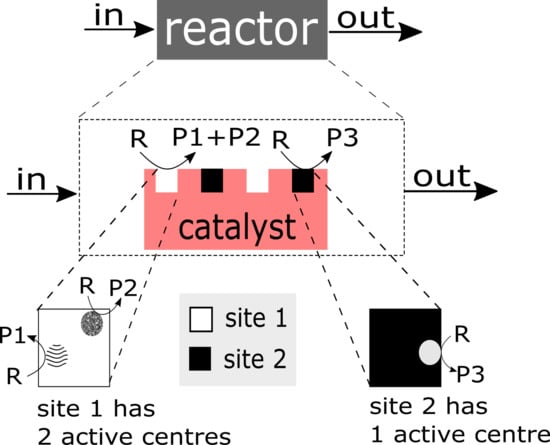
1.1.4. Catalyst Structure and Nature of the Active Site
1.2. Aim
2. Results
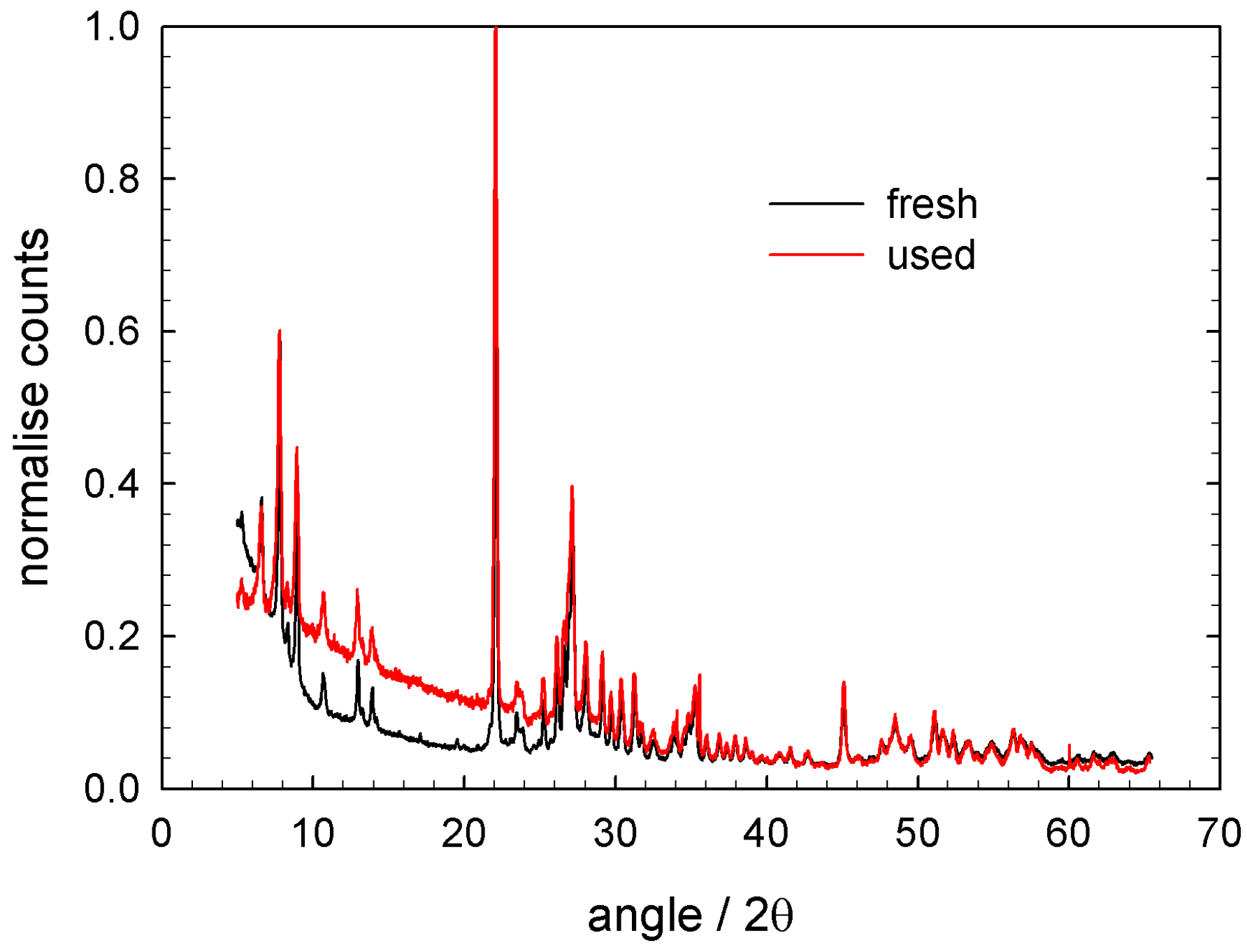
2.1. Characterisation
2.2. Reaction Equations
2.3. Justification of Kinetic Data
2.4. Kinetic Experiments under Catalyst Steady-State Conditions
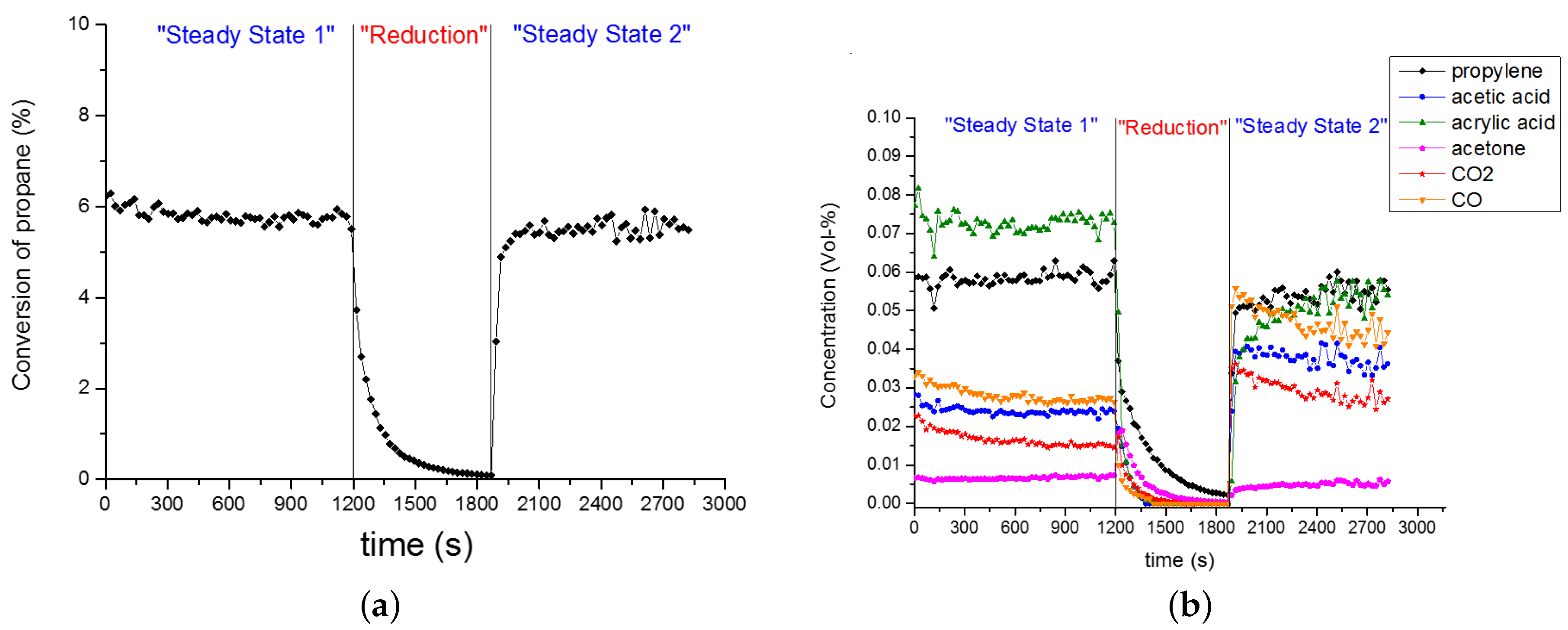
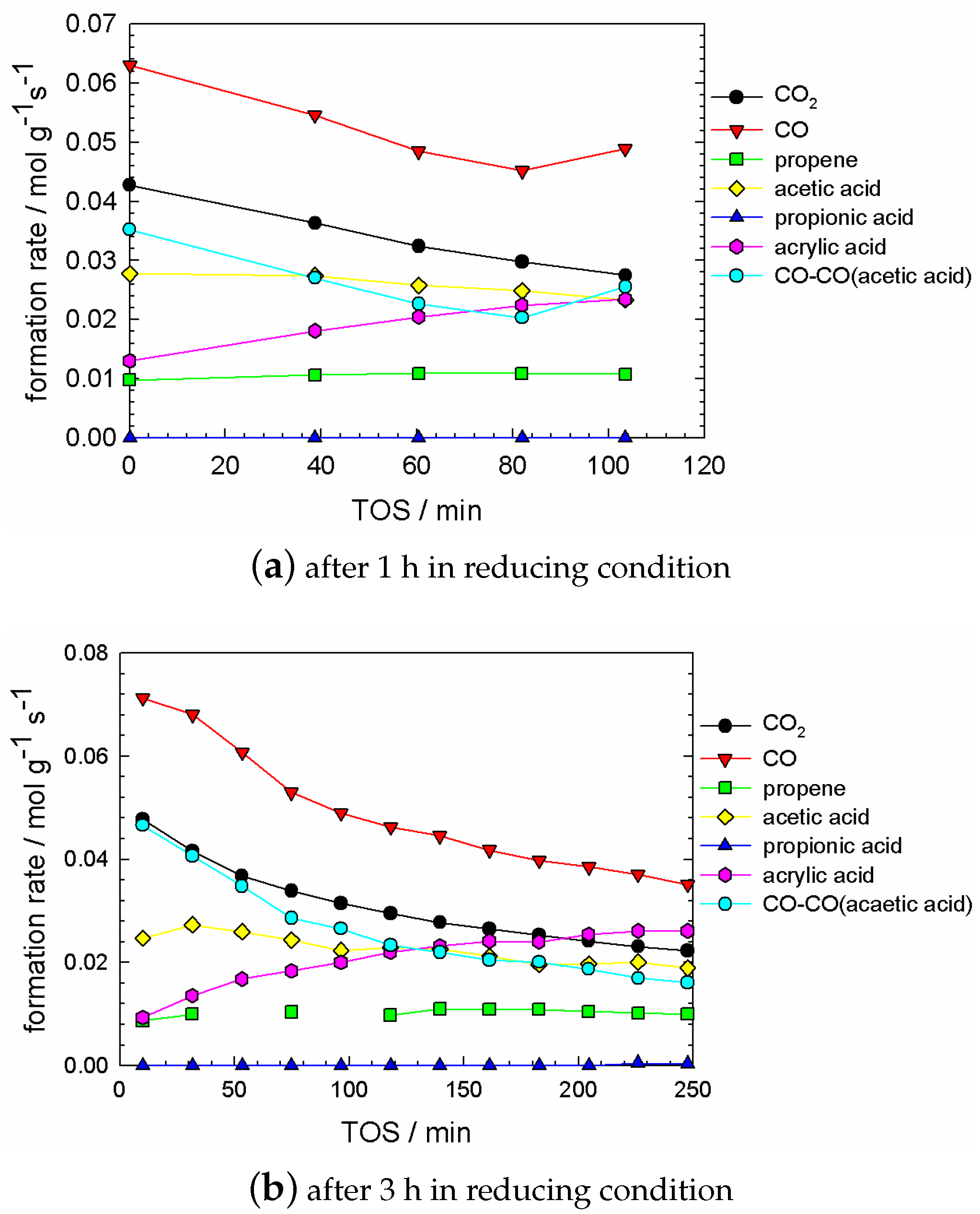
2.5. Kinetic Experiments during Catalyst Evolution (‘Slow Dynamics’ Scale of Min and Hours)
2.6. Data Interpretation and Rational Mechanism
2.6.1. Data Interpretation
- no temporal trend for propene concentration;
- a strongly decreasing trend in CO and CO concentration; and a strongly increasing trend in acrylic acid concentration;
- some trend in acetic acid concentration, which can be considered between ‘small’ and ‘significant’, depending on the degree of reduction, which is governed by the pretreatment time. This trend was small if the pretreatment time was up to 2–3 h (cf. Figure A2).
2.6.2. Rational Mechanism
2.6.3. Kinetic Equations
3. Discussion
3.1. Kinetics and Mechanism
3.2. Structure-Kinetic Interrelationship
3.3. Presenting Logics in Developing a Rational Mechanism
- 1.
- Do we have an isolated site or not?
- 2.
- Does this site consist of one centre or more?
- 3.
- Do we have a single- or a multi-route process for a centre?
- 4.
- Is there hierarchy in times of reaction or not?
- 5.
- What kind of limiting steps are there for single- or multi-route reaction?
- 6.
- What are the kinetic equations and kinetic orders?
4. Materials and Methods
4.1. Synthesis and Characterisation
4.2. Reactor Set-Up and Reaction Conditions
4.3. Types of Kinetic Experiments
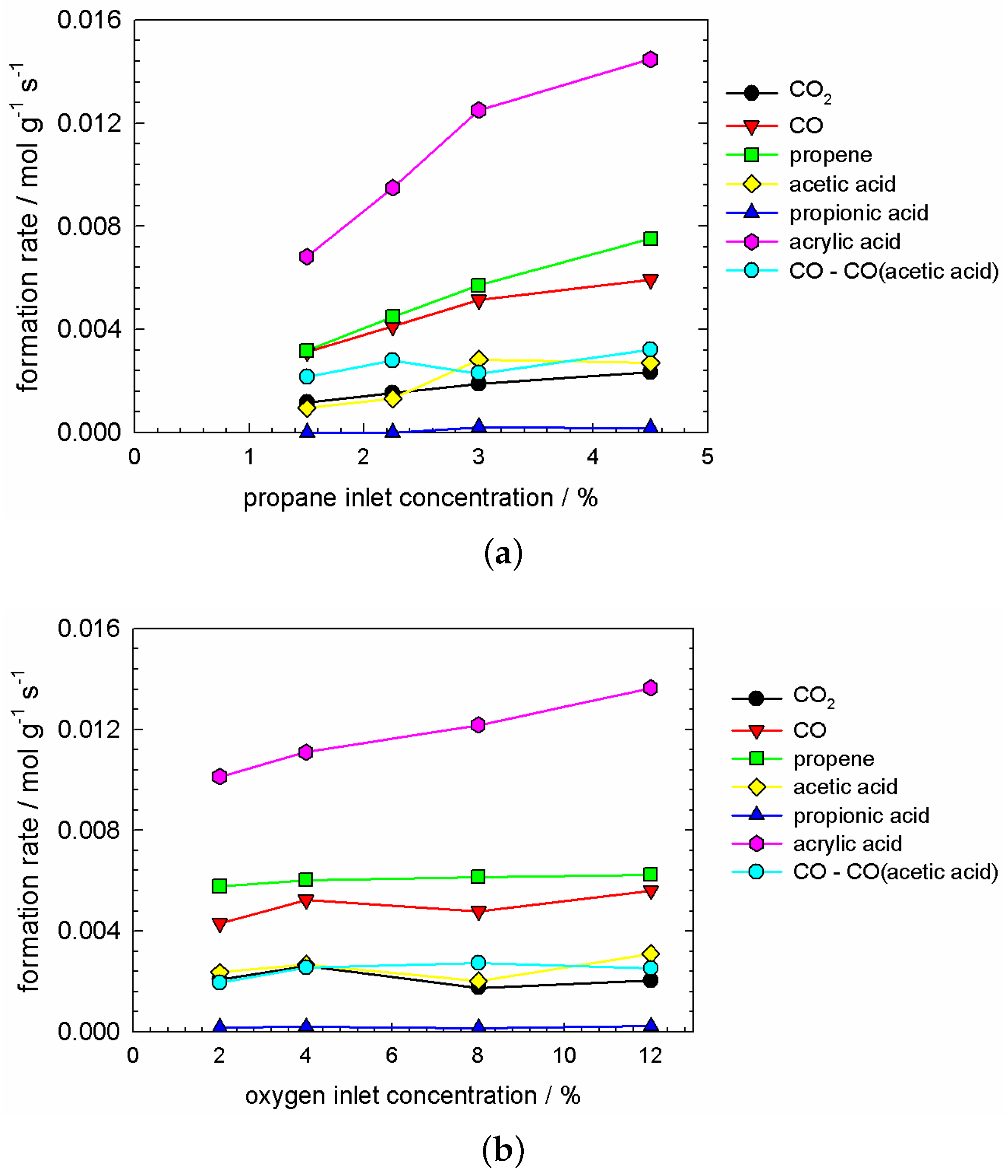
- Kinetic experiments under catalyst steady-state conditions: Partial pressure variations of propane (1.5–4.5%), oxygen (2–12%), and water (5–20%) at 573 K and a constant WHSV of 10 mL g h.
- Kinetic experiments during catalyst evolution (’slow dynamics’ scale of min and hours): Observation of catalyst evolution with time-on-stream (TOS) under CH/O/HO/N = 3/12/10/75 feed, after treatment in reducing conditions for 12 h in CH/O/HO/N = 3/0/10/87. Both parts of the experiment were carried out at 573 K and a constant WHSV of 10 mL g h.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| PFR | Plug Flow Reactor |
| TAP | Temporal Analysis of Products |
| ER | Eley-Rideal |
| MvK | Mars-van-Krevelen |
| LH | Langmuir-Hinshelwood |
| LHHW | Langmuir-Hinshelwood-Hougen-Watson |
| WHSV | Weight Hourly Space Velocity |
| aca | acetic acid |
| aa | acrylic acid, CHO |
| TOS | Time-On-Stream |
| a.u. | arbitrary units |
| apparent kinetic order | |
| product formation rate | |
| kinetic coefficient | |
| apparent equilibrium constant | |
| is the concentration of component A | |
| the concentration of oxygen in the bulk catalyst phase | |
| dimensionless concentration of surface intermediate j | |
| M1 | phase (ICSD No. 55097) |
| XRF | X-ray Fluorescence |
| BET | nitrogen adsorption (Brunauer–Emmett–Teller method) |
| XRD | X-ray powder Diffractometry |
| LEIS | Low-Energy Ion Scattering |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| NEXAFS | Near-Edge X-ray Absorption Fine Structure |
Appendix A. Supporting Information–Additional Experimental Data
References
- López Nieto, J.M.; Solsona, B. 5–Gas phase heterogeneous partial oxidation reactions. In Metal Oxides in Heterogeneous Catalysis: 5—Gas Phase Heterogeneous Partial Oxidation Reactions; Védrine, J.C., Korotcenkov, G., Eds.; Elsevier Inc.: New York, NY, USA, 2018; pp. 211–286. [Google Scholar]
- Védrine, J.C. Heterogeneous catalytic partial oxidation of lower alkanes (C1–C6) on mixed metal oxides. J. Energy Chem. 2016, 25, 936–946. [Google Scholar] [CrossRef]
- Barrault, J.; Batiot, C.; Magaud, L.; Ganne, M. Selective oxidation of propane into oxygenated compounds over promoted nickel-molybdenum catalysts. Stud. Surf. Sci. Catal. 1997, 110, 375–382. [Google Scholar] [CrossRef]
- Li, W.; Ueda, W. Catalytic selective oxidation of C2–C4 alkanes over reduced heteropolymolybdates. Stud. Surf. Sci. Catal. 1997, 110, 433–442. [Google Scholar] [CrossRef]
- Botella, P.; Lopez Nieto, J.M.; Solsona, B.; Mifsud, A.; Marquez, F. The Preparation, Characterization, and Catalytic Behavior of MoVTeNbO Catalysts Prepared by Hydrothermal Synthesis. J. Catal. 2002, 209, 445–455. [Google Scholar] [CrossRef]
- Novakova, E.K.; Vedrine, J.C.; Derouane, E.G. Propane Oxidation on Mo–V–Sb–Nb Mixed-Oxide Catalysts. J. Catal. 2002, 211, 226–234. [Google Scholar] [CrossRef]
- Vitry, D.; Morikawa, Y.; Dubois, J.L.; Ueda, W. Propane selective oxidation over monophasic Mo–V–Te–O catalysts prepared by hydrothermal synthesis. Top. Catal. 2003, 23, 47–53. [Google Scholar] [CrossRef]
- Widi, R.K.; Hamid, S.B.A.; Schlögl, R. Kinetic investigation of propane oxidation on diluted Mo1V0.3Te0.23Nb0.12Ox mixed-oxide catalyst. React. Kinet. Catal. Lett. 2009, 98, 273–286. [Google Scholar] [CrossRef]
- Fushimi, R.; Shekhtman, S.O.; Gaffney, A.; Han, S.; Yablonsky, G.S.; Gleaves, J.T. TAP Vacuum Pulse-Response and Normal-Pressure Studies of Propane Oxidation over MoVTeNb Oxide Catalysts. Ind. Eng. Chem. Res. 2005, 44, 6310–6319. [Google Scholar] [CrossRef]
- Naraschewski, F.N.; Jentys, A.; Lercher, J.A. On the Role of the Vanadium Distribution in MoVTeNbO x Mixed Oxides for the Selective Catalytic Oxidation of Propane. Top. Catal. 2011, 54, 639. [Google Scholar] [CrossRef]
- Mazloom, G.; Alavi, S.M. Kinetic study of selective propane oxidation to acrylic acid over Mo1V0.3Te0.23Nb0.12Ox using the genetic algorithm. React. Kinet. Mech. Catal. 2013, 110, 387–403. [Google Scholar] [CrossRef]
- Naumann d’Alnoncourt, R.; Csepei, L.I.; Hävecker, M.; Girgsdies, F.; Schuster, M.E.; Schlögl, R.; Trunschke, A. The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts. J. Catal. 2014, 311, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Bell, A.T.; Iglesia, E. Kinetics and Mechanism of Oxidative Dehydrogenation of Propane on Vanadium, Molybdenum, and Tungsten Oxides. J. Phys. Chem. B 2000, 104, 1292–1299. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, R.K.; Buttery, D.J.; Burrington, J.D.; Andersson, A.; Holmberg, J.; Ueda, W.; Kubo, J.; Lugmair, C.G.; Volpe, A.F., Jr. Active centers, catalytic behavior, symbiosis and redox properties of MoV(Nb,Ta)TeO ammoxidation catalysts. Top. Catal. 2006, 38, 7–16. [Google Scholar] [CrossRef]
- Novakova, J.; Dolejsek, Z.; Habersberger, K. A Note on the Influence of Water Vapour in the Oxidation of Propene to Acrylic Acid on a Mixed Oxide Catalyst. React. Kinet. Catal. Lett. 1976, 4, 389–395. [Google Scholar] [CrossRef]
- Chen, K.; Iglesia, E.; Bell, A.T. Kinetic Isotopic Effects in Oxidative Dehydrogenation of Propane on Vanadium Oxide Catalysts. J. Catal. 2000, 192, 197–203. [Google Scholar] [CrossRef]
- Chen, K.; Bell, A.T.; Iglesia, E. Isotopic Tracer Studies of Reaction Pathways for Propane Oxidative Dehydrogenation on Molybdenum Oxide Catalysts. J. Phys. Chem. B 2001, 105, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Mestl, G. MoVW mixed metal oxides catalysts for acrylic acid production: from industrial catalysts to model studies. Top. Catal. 2006, 38, 69–82. [Google Scholar] [CrossRef]
- Grasselli, R.K. Genesis of site isolation and phase cooperation in selective oxidation catalysis. Top. Catal. 2001, 15, 93–101. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Andersson, A.; Buttery, D.J.; Burrington, J.D.; Lugmair, C.G.; Volpe, A.F., Jr. Proceedings of the Abstract of papers 228th ACS National Meeting, Philadelphia, PA, USA, 22–26 August 2004.
- Grasselli, R.K. Site isolation and phase cooperation: Two important concepts in selective oxidation catalysis: A retrospective. Catal. Today 2014, 238, 10–27. [Google Scholar] [CrossRef]
- Schlögl, R. Active Sites for Propane Oxidation: Some Generic Considerations. Top. Catal. 2011, 54, 627. [Google Scholar] [CrossRef]
- Haber, J. Catalysis by Transition Metal Oxides. In Solid State Chemistry in Catalysis: Catalysis by Transition Metal Oxides; Grasselli, R.K., Brazdil, J.F., Eds.; American Chemical Society: Washington, DC, USA, 1985; pp. 3–21. [Google Scholar]
- Somorjai, G.A. The 13th International Symposium on Relations Between Homogeneous and Heterogeneous Catalysis—An Introduction. Top. Catal. 2008, 48, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hävecker, M.; Wrabetz, S.; Kröhnert, J.; Csepei, L.I.; Naumann d’Alnoncourt, R.; Kolen’ko, Y.V.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid. J. Catal. 2012, 285, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Wachs, I.E.; Routray, K. Catalysis Science of Bulk Mixed Oxides. ACS Catal. 2012, 2, 1235–1246. [Google Scholar] [CrossRef]
- Védrine, J.C. Revisiting active sites in heterogeneous catalysis: Their structure and their dynamic behaviour. Appl. Catal. A Gen. 2014, 474, 40–50. [Google Scholar] [CrossRef]
- Boreskov, G.K. Heterogeneous Catalysis; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2003. [Google Scholar]
- Yablonskii, G.S.; Bykov, V.I.; Elokhin, V.I.; Gorban, A.N. Comprehensive Chemical Kinetics. In Kinetic Models of Catalytic Reactions: Comprehensive Chemical Kinetics; Elsevier Inc.: New York, NY, USA, 1991; Volume 32, p. 492. [Google Scholar]
- Haber, J.; Serwicka, E.M. The Role of Oxygen in Catalysis. React. Kinet. Catal. Lett. 1987, 35, 369–379. [Google Scholar] [CrossRef]
- Sanfiz, A.C.; Hansen, T.W.; Sakthivel, A.; Trunschke, A.; Schlögl, R.; Knoester, A.; Brongersma, H.H.; Looi, M.H.; Hamid, S.B.A. How important is the (001) plane of M 1 for selective oxidation of propane to acrylic acid? J. Catal. 2008, 258, 35–43. [Google Scholar] [CrossRef]
- Sanfiz, A.C.; Hanen, T.W.; Teschner, D.; Schnörch, P.; Girgsdies, F.; Trunschke, A.; Schlögl, R.; Looi, M.H.; Hamid, S.B.A. Dynamics of the MoVTeNb Oxide M1 Phase in Propane Oxidation. J. Phys. Chem. C 2010, 114, 1912–1921. [Google Scholar] [CrossRef]
- Heine, C.; Hävecker, M.; Trunschke, A.; Schlögl, R.; Eichelbaum, M. The impact of steam on the electronic structure of the selective propane oxidation catalyst MoVTeNb oxide (orthorhombic M1 phase). Phys. Chem. Chem. Phys. 2015, 17, 8983–8993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centi, G.; Perathoner, S. Reaction Mechanism and Control of Selectivity in Catalysis by Oxides: Some Challenges and Open Questions. Int. J. Mol. Sci. 2001, 2, 183–196. [Google Scholar] [CrossRef]
- Naraschewski, F.N.; Kumar, C.P.; Jentys, A.; Lercher, J.A. Phase formation and selective oxidation of propane over MoVTeNbOx catalysts with varying compositions. Appl. Catal. A Gen. 2011, 391, 63–69. [Google Scholar] [CrossRef]
- Védrine, J.C. 9—Concluding remarks and 9 challenges of heterogeneous catalysis on metal oxides. In Metal Oxides in Heterogeneous Catalysis: 9—Concluding Remarks and Challenges of Heterogeneous Catalysis on Metal Oxides; Védrine, J.C., Korotcenkov, G., Eds.; Elsevier Inc.: New York, NY, USA, 2018; p. 551. [Google Scholar]
- Andrushkevich, T.V.; Chesalov, Y.A. Mechanism of heterogeneous catalytic oxidation of organic compounds to carboxylic acids. Russ. Chem. Rev. 2018, 87, 586. [Google Scholar] [CrossRef]
- Keulks, G.W.; Lo, M.Y. Catalytic Oxidation of Propylene. 11. An Investigation of the Kinetics and Mechanism over Iron-Antimony Oxide. J. Phys. Chem. 1986, 90, 4768–4775. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Burrington, J.D.; Brazdil, J.F. Mechanistic Features of Selective Oxidation and Ammoxidation Catalysis. Faraday Discuss. Chem. Soc. 1981, 72, 203–223. [Google Scholar] [CrossRef]
- Burrington, J.D.; Kartisek, C.T.; Grasselli, R.K. Aspects of Selective Oxidation and Ammoxidation Mechanisms over Bismuth Molybdate Catalysts. J. Catal. 1980, 63, 235. [Google Scholar] [CrossRef]
- Burrington, J.D.; Kartisek, C.T.; Grasselli, R.K. Surface Intermediates in Selective Propylene Oxidation and Ammoxidation over Heterogeneous Molybdate and Antimonate Catalysts. J. Catal. 1984, 87, 363–380. [Google Scholar] [CrossRef]
- Saleh-Alhamed, Y.A.; Hudgins, R.R.; Silveston, P.L. Investigations of catalytic mechanisms for selective propene oxidation in the presence of steam. Appl. Catal. A Gen. 1995, 127, 177–199. [Google Scholar] [CrossRef]
- Saleh-Alhamed, Y.A.; Hudgins, R.R.; Silveston, P.L. Role of Water Vapor in the Partial Oxidation of Propene. J. Catal. 1996, 161, 430–440. [Google Scholar] [CrossRef]
- Lin, M.M. Selective oxidation of propane to acrylic acid with molecular oxygen. Appl. Catal. A Gen. 2001, 207, 1–16. [Google Scholar] [CrossRef]
- Lin, M.H.; Desai, T.B.; Kaiser, f.W.; Klugherz, P.D. Reaction pathways in the selective oxidation of propane over a mixed metal oxide catalyst. Catal. Today 2000, 61, 223–229. [Google Scholar] [CrossRef]
- Luo, L.; Labinger, J.A.; Davis, M.E. Comparison of Reaction Pathways for the Partial Oxidation of Propane over Vanadyl Ion-Exchanged Zeolite Beta and Mo1V0.3Te0.23Nb0.12Ox. J. Catal. 2001, 200, 222–231. [Google Scholar] [CrossRef]
- Balcells, E.; Borgmeier, F.; Griesstede, I.; Lintz, H.G.; Rosowski, F. Partial oxidation of propane to acrylic acid at a Mo–V–Te–Nb-oxide catalyst. Appl. Catal. A Gen. 2004, 266, 211–221. [Google Scholar] [CrossRef]
- Grabowski, R. Kinetics of the oxidative dehydrogenation of propane on vanadia/titania catalysts, pure and doped with rubidium. Appl. Catal. A Gen. 2004, 270, 37–47. [Google Scholar] [CrossRef]
- Grabowski, R. Kinetics of Oxidative Dehydrogenation of C2 -C3 Alkanes on Oxide Catalysts. Catal. Rev. 2006, 48, 199–268. [Google Scholar] [CrossRef]
- Naumann d’Alnoncourt, R.; Kolenko, Y.V.; Schlögl, R.; Trunschke, A. A New Way of Probing Reaction Networks: Analyzing Multidimensional Parameter Space. Comb. Chem. High Throughput Screen. 2012, 15, 161. [Google Scholar] [CrossRef]
- Kube, P.; Frank, B.; Schlögl, R.; Trunschke, A. Synthesis of MoVTeNb Oxide Catalysts with Tunable Particle Dimens ions. ChemCatChem 2017, 9, 3446. [Google Scholar] [CrossRef]
- Holmberg, J.; Grasselli, R.K.; Andersson, A. A study of the functionalities of the phases in Mo–V–Nb–Te oxides for propane ammoxidation. Top. Catal. 2003, 23, 55–63. [Google Scholar] [CrossRef]
- Vitry, D.; Morikawa, Y.; Dubois, J.L.; Ueda, W. Mo-V-Te-(Nb)-O mixed metal oxides prepared by hydrothermal synthesis for catalytic selective oxidations of propane and propene to acrylic acid. Appl. Catal. A Gen. 2003, 251, 411–424. [Google Scholar] [CrossRef]
- Marin, G.; Yablonsky, G.S. Kinetics of Chemical Reactions—Decoding Complexity; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Ishikawa, S.; Kobayashi, D.; Konya, T.; Ohmura, S.; Murayama, T.; Yasuda, N.; Sadakane, M.; Ueda, W. Redox Treatment of Orthorhombic Mo 29V11O112 and Relationships between Crystal Structure, Microporosity and Catalytic Performance for Selective Oxidation of Ethane. J. Phys. Chem. C 2015, 119, 7195–7206. [Google Scholar] [CrossRef]
- Ueda, W. Personal communication at NGSC 11. Tromsø, Norway, 8 June 2016. [Google Scholar]
- Kolen’ko, Y.V.; Zhang, W.; Naumann d’Alnoncourt, R.; Girgsdies, F.; Hansen, T.W.; Wolfram, T.; Schlögl, R.; Trunschke, A. Synthesis of MoVTeNb Oxide Catalysts with Tunable Particle Dimens ions. ChemCatChem 2011, 3, 1597–1606. [Google Scholar] [CrossRef]












| Kinetic Tool | Experimental Details | Temperature/K | Gas Phase Composition | Reference | ||
|---|---|---|---|---|---|---|
| CH | O | HO | ||||
| PFR | reduced and oxidised Mo-species | 648–698 | 60 | 20 | 20 | [3] |
| MoO/NiMoO | ||||||
| PFR | importance of reduced states | 613 | 3.3 | 3.3 | 33.3 | [4] |
| HPMoO(Py) | ||||||
| PFR | catalyst composition change | 623–693 | 4 | 8 | 30 | [5] |
| MoVTeNbO | ||||||
| PFR | propane/propene oxidation, three route network | 653–713 | 2–6 | 5–60 | 0–60 | [6] |
| MoVSbNbO | ||||||
| PFR | discrimination of active bulk structure | 613–653 | 8 (2.5–20) | 10 (2.5–20) | 44 | [7] |
| MoVTeO | ||||||
| PFR | multi-route mechanism, partial pressure variation | 653–683 | ≤10% | ≤10% | ≤10% | [8] |
| MoVTeNbO | ||||||
| TAP | influence of reactants and products tested | 293–623 | 6.2–40 | 15–50 | 25 | [9] |
| MoVTeNbO | ||||||
| PFR | first order network, structural analysis | 653 | 5 | 10 | 20 | [10] |
| MoVTeNbO M1 | ||||||
| PFR | O/CH and HO/CH change | 653–763 | 1 | 1, 2, 3 | 5, 7.5 | [11] |
| MoVTeNbO | ||||||
| PFR | extended characterisation and testing | 623–663 | 3 | 4.5–12 | 0–40 | [12] |
| MoVTeNbO M1 | ||||||
| PFR | steady-state and non-steady-state kinetics | 573 | 1.5–4.5 | 2–12 | 5–20 | |
| MoVTeNbO M1 | ||||||
| PFR | ODH | 703 | 5–15 | 1–4 | 0–5 | [13] |
| MoO, VO, WO/ZrO | ||||||
| PFR | ammoxidation of propane | 693 | 1 | 1.2 | (NH) 3 | [14] |
| MoVNbTeO & MoVTaTeO | ||||||
| PFR | isotopic labelling HO | 703 | CH 1 | 6.1 | 7 | [15] |
| MoWSnTeO | ||||||
| PFR | ODH; isotopic labelling | 593 | 14.2 | 1.7 | – | [16] |
| 10 wt% VO/ZrO | ||||||
| PFR | ODH; isotopic labelling C, O and H/D | 688–703 | 5–15 | 1–4 | – | [17] |
| MoO/ZrO | ||||||
| PFR | acrolein oxidation | 533–573 | CHO 4–8 | 6–20 | 20 | [18] |
| (MoVW)O | ||||||
| Product | p(CH) | p(O) | p(HO) | ∑ Kin. Order | |||
|---|---|---|---|---|---|---|---|
| log k | log k | log k | |||||
| CO | 0.59 | 0.11 | 0.36 | 1.06 | |||
| CO-aca | 0.29 | 0.15 | 0.21 | 0.65 | |||
| CO | 0.63 | 0.2 | 0.77 | ||||
| CH | 0.78 | 0.04 | 0.04 | 0.86 | |||
| acetic acid | 1.06 | 0.07 | 0.51 | 1.64 | |||
| acrylic acid | 0.7 | 0.16 | 0.46 | 1.32 | |||
| Product | [] | [] | [] |
|---|---|---|---|
| CO | + | 0 | + + |
| CO-aca | 0 | ||
| CO | 0 | ||
| CH | 0 | ||
| acetic acid | + | + | + |
| acrylic acid | + | + | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprung, C.; Yablonsky, G.S.; Schlögl, R.; Trunschke, A. Constructing A Rational Kinetic Model of the Selective Propane Oxidation Over A Mixed Metal Oxide Catalyst. Catalysts 2018, 8, 330. https://doi.org/10.3390/catal8080330
Sprung C, Yablonsky GS, Schlögl R, Trunschke A. Constructing A Rational Kinetic Model of the Selective Propane Oxidation Over A Mixed Metal Oxide Catalyst. Catalysts. 2018; 8(8):330. https://doi.org/10.3390/catal8080330
Chicago/Turabian StyleSprung, Christoph, Gregory S. Yablonsky, Robert Schlögl, and Annette Trunschke. 2018. "Constructing A Rational Kinetic Model of the Selective Propane Oxidation Over A Mixed Metal Oxide Catalyst" Catalysts 8, no. 8: 330. https://doi.org/10.3390/catal8080330