Developing a High-Temperature Solvent-Free System for Efficient Biocatalysis of Octyl Ferulate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Primary Experiment
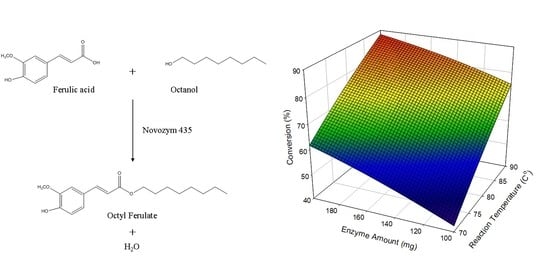
2.2. Model Fitting
2.3. Optimal Synthesis Conditions
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Synthesis of Octyl Ferulate
3.3. Experimental Design
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Wen, X.Y.; Lu, J.F.; Kan, J.; Jin, C.H. Free radical mediated grafting of chitosan with caffeic and ferulicacids: Structures and antioxidant activity. Int. J. Biol. Macromol. 2014, 65, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.S.; Birch, E.J. Radical scavenging activity of lipophilized products from lipase-catalyzed transesterification of triolein with cinnamic and ferulic acids. Lipids 2009, 44, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Karboune, S.; St-Louis, R.; Kermasha, S. Enzymatic synthesis of structured phenolic lipids by acidolysis of flaxseed oil with selected phenolic acids. J. Molec. Catal. B Enzym. 2008, 52–53, 96–105. [Google Scholar] [CrossRef]
- Zheng, Y.; Branford-White, C.; Wu, X.M.; Wu, C.Y.; Xie, J.G.; Quan, J.; Zhu, L.M. Enzymatic synthesis of novel feruloylated lipids and their evaluation as antioxidants. J. Amer. Oil Chem. Soc. 2010, 87, 305–311. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.K.; Ravinder, T.; Kanjilal, S. Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids. Food Chem. 2012, 134, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.D.; Song, F.F.; Bi, Y.L.; Yang, G.L.; Liu, W. Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: Optimized by response surface methodology. J. Biotechnol. 2012, 164, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zhang, L.; Chen, L.; Zheng, Y.; Wu, X.; Xia, C. Lipase-catalyzed synthesis of ferulyl oleins in solvent-free medium. Food Chem. 2009, 112, 640–645. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Xu, X. Enzymatic lipophilisation of phenolic acids through esterification with fatty alcohols in organic solvents. Food Chem. 2012, 132, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mu, Y.; Chen, H.; Xiu, Z.; Yang, T. Enzymatic synthesis of feruloylated lysophospholipid in a selected organic solvent medium. Food Chem. 2013, 141, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Shima, M.; Kadota, M.; Tsuno, T.; Adachi, S. Suppressive effect of alkyl ferulate on the oxidation of linoleic acid. Biosci. Biotechnol. Biochem. 2006, 70, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Z.; Diao, X.-J.; Yang, W.-H.; Li, F.; He, G.-W.; Gong, G.-Q.; Xu, Y.-G. Design, synthesis and antithrombotic evaluation of novel dabigatran prodrugs containing methyl ferulate. Bioorg. Med. Chem. Lett. 2013, 23, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Piana, C.; Setti, L.; Hochkoeppler, A.; Pifferi, P.G.; Williamson, G.; Faulds, C.B. Synthesis of pentylferulate by a feruloyl esterase from Aspergillus niger using water-in-oil microemulsions. Biotechnol. Lett. 2001, 23, 325–330. [Google Scholar] [CrossRef]
- Saija, A.; Tomaino, A.; Cascio, R.L.; Trombetta, D.; Proteggente, A.; Pasquale, A.D.; Uccella, N.; Bonina, F. Ferulic and caffeic acids as potential protective agents against photooxidative skin damage. J. Sci. Food Agric. 1999, 79, 476–480. [Google Scholar] [CrossRef]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Enzymatic synthesis of hydrophilic and hydrophobic derivatives of natural phenolic acids in organic media. J. Mol. Catal. B Enzym. 2001, 11, 323–328. [Google Scholar] [CrossRef]
- Matsuo, T.; Kobayashi, T.; Kimura, Y.; Tsuchiyama, M.; Oh, T.; Sakamoto, T.; Adachi, S. Synthesis of glyceryl ferulate by immobilized ferulic acid esterase. Biotechnol. Lett. 2008, 30, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Laszlo, J.A.; Berhow, M.A. Lipase-catalyzed synthesis of ferulate esters. J. Am. Oil Chem. Soc. 2000, 77, 513–519. [Google Scholar] [CrossRef]
- Katsoura, M.H.; Polydera, A.C.; Tsironis, L.D.; Petraki, M.P.; Rajačić, S.K.; Tselepis, A.D.; Stamatis, H. Efficient enzymatic preparation of hydroxycinnamates in ionic liquids enhances their antioxidant effect on lipoproteins oxidative modification. New Biotechnol. 2009, 26, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Adachi, S.; Matsuno, R. Lipase-catalyzed condensation of p-methoxyphenethyl alcohol and carboxylic acids with different steric and electrical properties in acetonitrile. Biotechnol. Lett. 2003, 25, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Zagozda, M.; Plenkiewicz, J. Biotransformations of 2,3-epoxy-3-arylpropanenitriles by Debaryomyces hansenii and Mortierella isabellina cells. Tetrahedron Asymmetry 2008, 19, 1454–1460. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, S.; Bi, Y. Solvent-free enzymatic synthesis of feruloylated structured lipids by the transesterification of ethyl ferulate with castor oil. Food Chem. 2014, 158, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Kazlauskas, R.J. Hydrolases in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006; pp. 61–183. [Google Scholar]
- Xu, D.; Li, Z.; Ma, S. Novozym-435-catalyzed enzymatic separation of racemic propargylic alcohols. A facile route to optically active terminal aryl propargylic alcohols. Tetrahedron Lett. 2003, 44, 6343–6346. [Google Scholar] [CrossRef]
- Jacobsen, E.E.; van Hellemond, E.; Moen, A.R.; Prado, L.C.V.; Anthonsen, T. Enhanced selectivity in Novozym 435 catalyzed kinetic resolution of secondary alcohols and butanoates caused by the (R)-alcohols. Tetrahedron Lett. 2003, 44, 8453–8455. [Google Scholar] [CrossRef]
- Rajendran, A.; Palanisamy, A.; Thangavelu, V. Lipase catalyzed ester synthesis for food processing industries. Braz. Arch. Biol. Technol. 2009, 52, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Uppenberg, J.; Hansen, M.T.; Patkar, S.; Jones, T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida Antarctica. Structure 1994, 2, 293–308. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Mei, Y.; Miller, L.; Gao, W.; Gross, R.A. Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy. Biomacromolecules 2003, 4, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, Z.; Fernandez-Lorente, G.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Novozym 435 displays very different selectivity compared to lipase from Candida Antarctica B adsorbed on other hydrophobic supports. J. Mol. Catal. B Enzym. 2009, 57, 171–176. [Google Scholar] [CrossRef]
- Burke, P.A.; Griffin, R.G.; Klibanov, A.M. Solid-state nuclear magnetic resonance investigation of solvent dependence of tyrosyl ring motion in an enzyme. Biotechnol. Bioeng. 1993, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Affleck, R.; Xu, Z.F.; Suzawa, V.; Focht, K.; Clark, D.S.; Dordick, J.S. Enzymatic catalysis and dynamics in low-water environments. Proc. Natl. Acad. Sci. USA 1992, 89, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Okumura, S.; Tsujisaka, Y. Synthesis of terpene alcohol esters by lipase. Agric. Biol. Chem. 1980, 44, 2731–2732. [Google Scholar]
- Langrand, G.; Rondot, N.; Triantaphylides, C.; Baratti, J. Short chain flavour esters synthesis by microbial lipases. Biotechnol. Lett. 1990, 12, 581–586. [Google Scholar] [CrossRef]
- Huang, K.-C.; Li, Y.; Kuo, C.-H.; Twu, Y.-K.; Shieh, C.-J. Highly efficient Synthesis of an emerging lipophilic antioxidant: 2-ethylhexyl ferulate. Molecules 2016, 21, 478. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.Y.; Ko, S.-J.; Won, K.; Kang, H.-Y.; Kim, B.T.; Lee, Y.S.; Lee, H. Synthesis of alkyl (R)-lactates and alkyl (S,S)-O-lactyllactates by alcoholysis of rac-lactide using Novozym 435. Tetrahedron Lett. 2006, 47, 6517–6520. [Google Scholar] [CrossRef]





| Treatment No. a | Experimental Factors b | Molar Conversion c (%) | ||
|---|---|---|---|---|
| X1 (°C) | X2 (PLU) | X3 (rpm) | ||
| 1 | –1(70) | –1(1000) | 0(100) | 42.4 ± 5.7 |
| 2 | 1(90) | –1(1000) | 0(100) | 75.2 ± 0.7 |
| 3 | –1(70) | 1(2000) | 0(100) | 61.7 ± 3.6 |
| 4 | 1(90) | 1(2000) | 0(100) | 88.3 ± 0.5 |
| 5 | –1(70) | 0(1500) | –1(50) | 49.4 ± 1.1 |
| 6 | 1(90) | 0(1500) | –1(50) | 88.3 ± 0.1 |
| 7 | –1(70) | 0(1500) | 1(150) | 56.5 ± 0.7 |
| 8 | 1(90) | 0(1500) | 1(150) | 88.4 ± 3.1 |
| 9 | 0(80) | –1(1000) | –1(50) | 56.4 ± 0.1 |
| 10 | 0(80) | 1(2000) | –1(50) | 80.2 ± 3.8 |
| 11 | 0(80) | –1(1000) | 1(150) | 64.7 ± 4.9 |
| 12 | 0(80) | 1(2000) | 1(150) | 83.5 ± 1.1 |
| 13 | 0(80) | 0(1500) | 0(100) | 71.1 ± 1.4 |
| 14 | 0(80) | 0(1500) | 0(100) | 70.2 ± 2.8 |
| 15 | 0(80) | 0(1500) | 0(100) | 70.2 ± 3.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Wu, P.-Y.; Chen, J.-H.; Kuo, C.-H.; Shieh, C.-J. Developing a High-Temperature Solvent-Free System for Efficient Biocatalysis of Octyl Ferulate. Catalysts 2018, 8, 338. https://doi.org/10.3390/catal8080338
Huang S-M, Wu P-Y, Chen J-H, Kuo C-H, Shieh C-J. Developing a High-Temperature Solvent-Free System for Efficient Biocatalysis of Octyl Ferulate. Catalysts. 2018; 8(8):338. https://doi.org/10.3390/catal8080338
Chicago/Turabian StyleHuang, Shang-Ming, Ping-Yu Wu, Jiann-Hwa Chen, Chia-Hung Kuo, and Chwen-Jen Shieh. 2018. "Developing a High-Temperature Solvent-Free System for Efficient Biocatalysis of Octyl Ferulate" Catalysts 8, no. 8: 338. https://doi.org/10.3390/catal8080338