Novel Composite Electrode of the Reduced Graphene Oxide Nanosheets with Gold Nanoparticles Modified by Glucose Oxidase for Electrochemical Reactions
Abstract
:1. Introduction
2. Results and Discussion
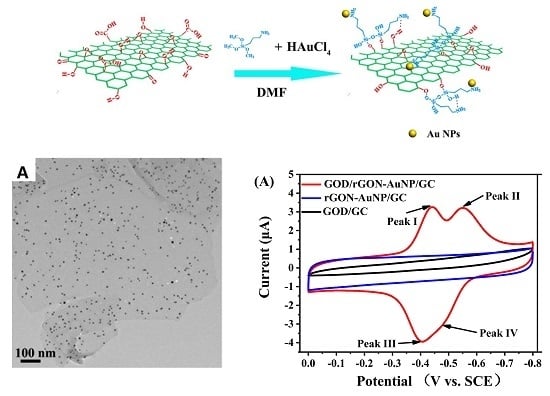
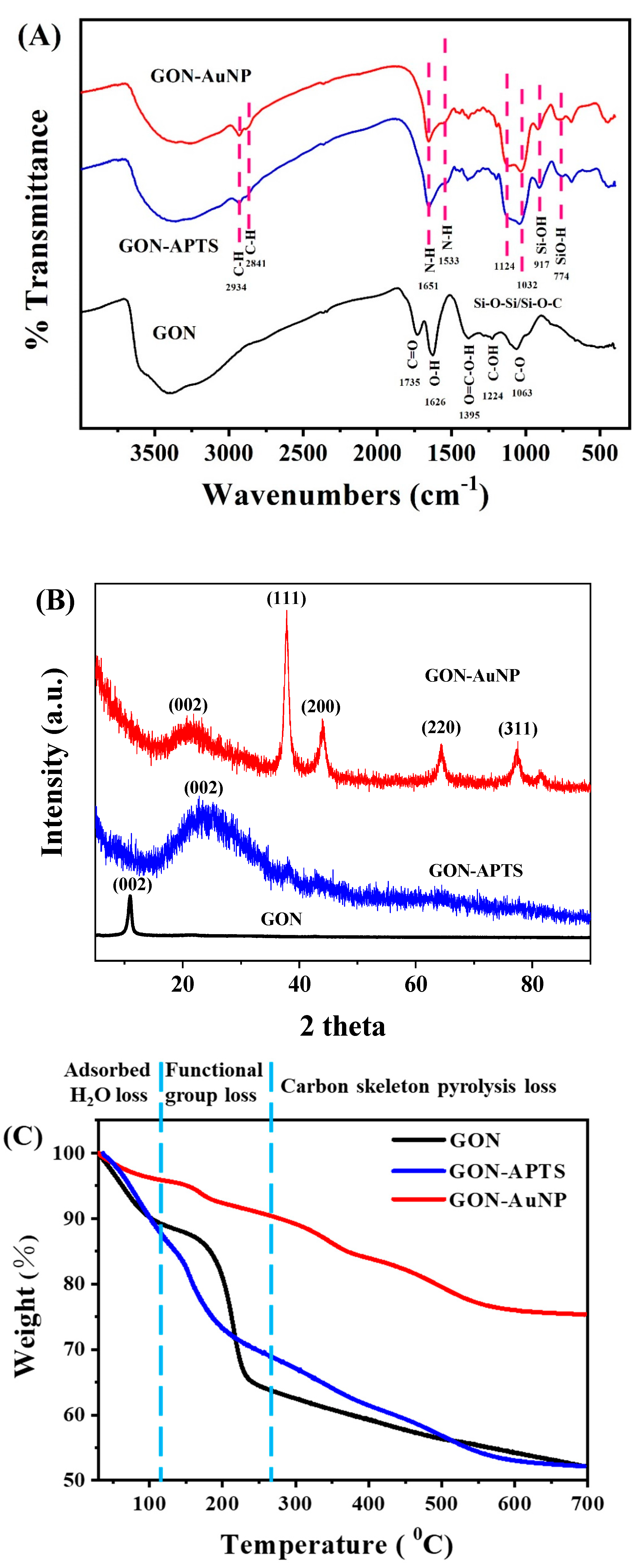
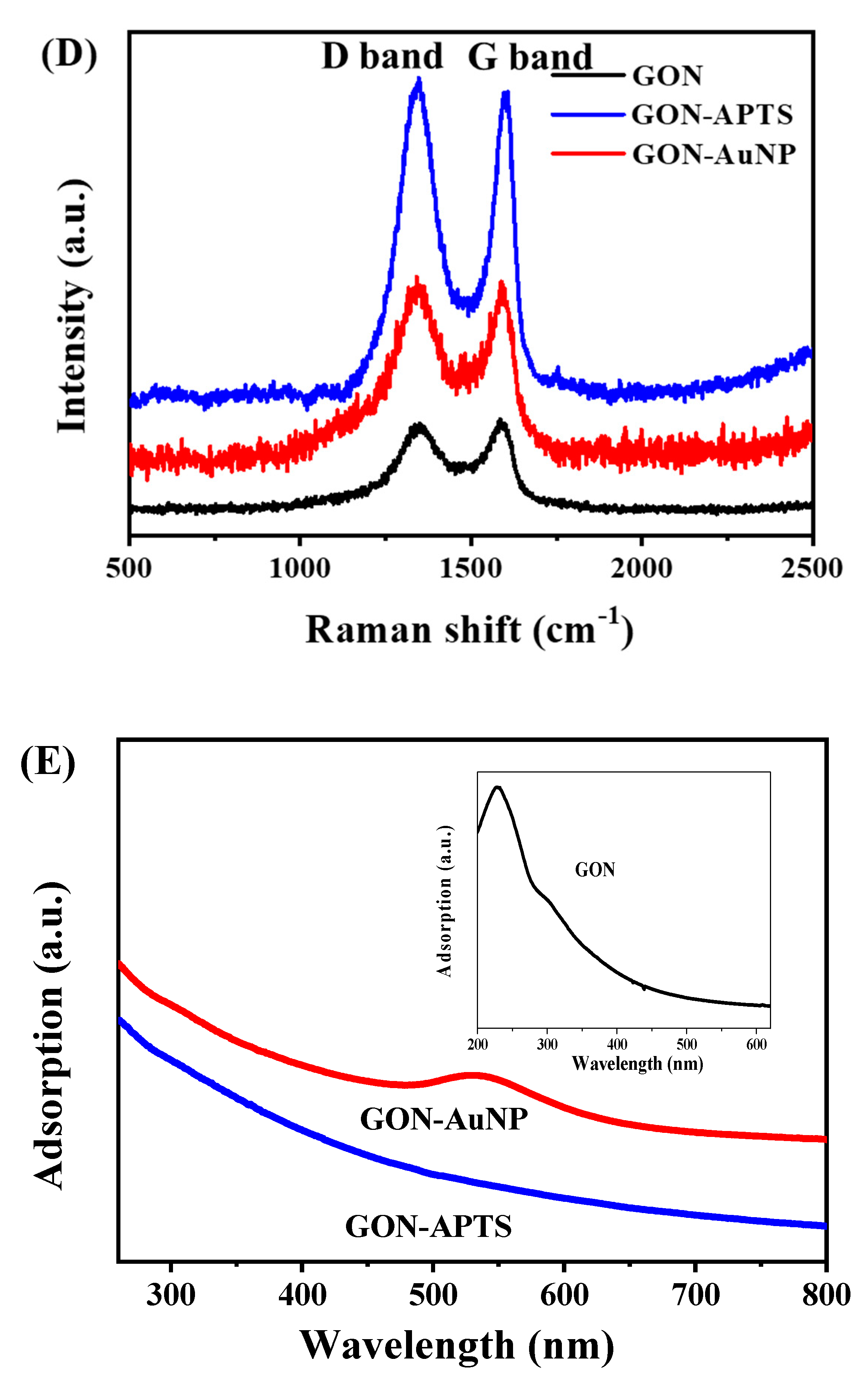
2.1. Morphology and Composition of GON-AuNP Composite
2.2. Potentiostatic Reduction of GON-AuNP
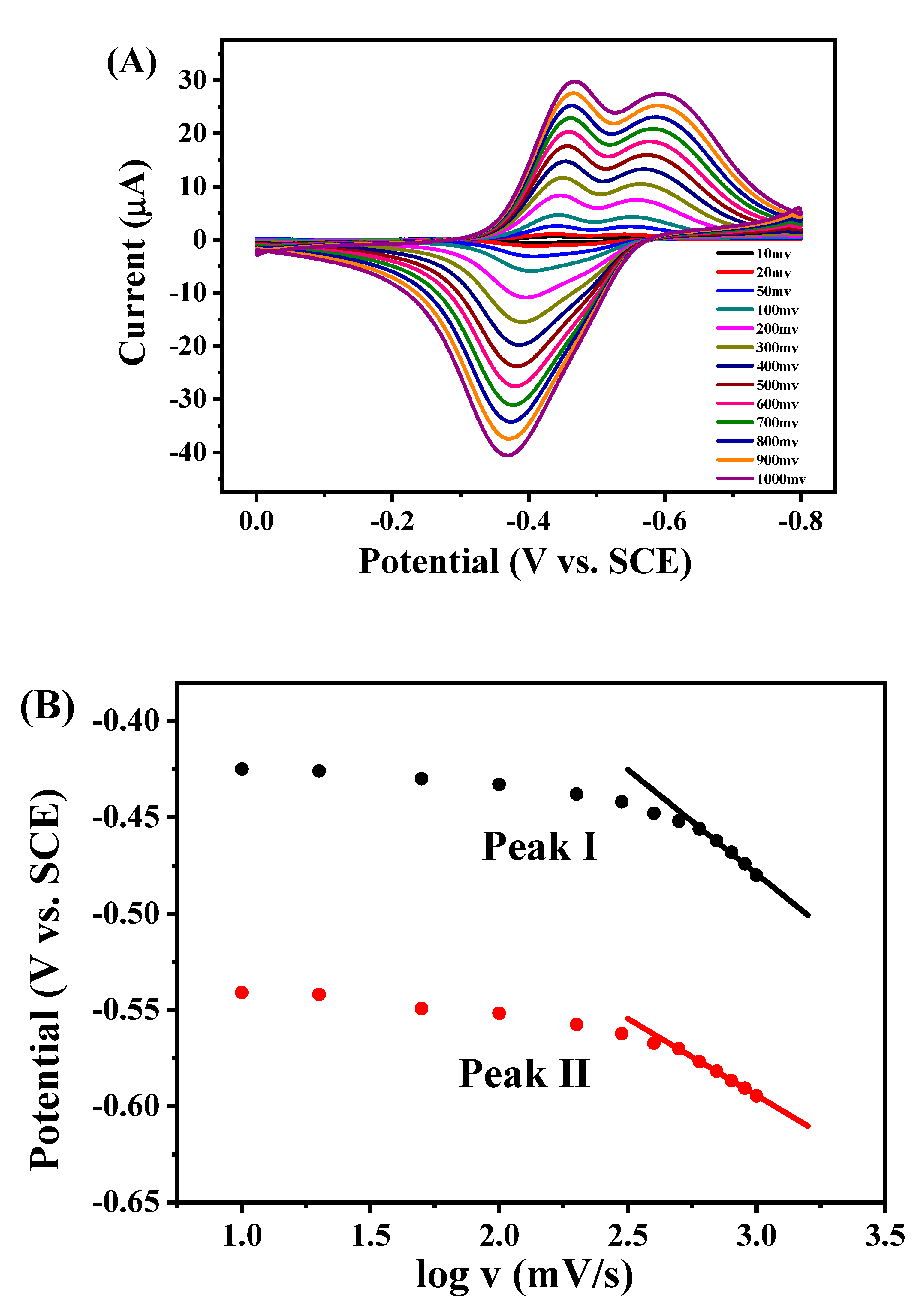
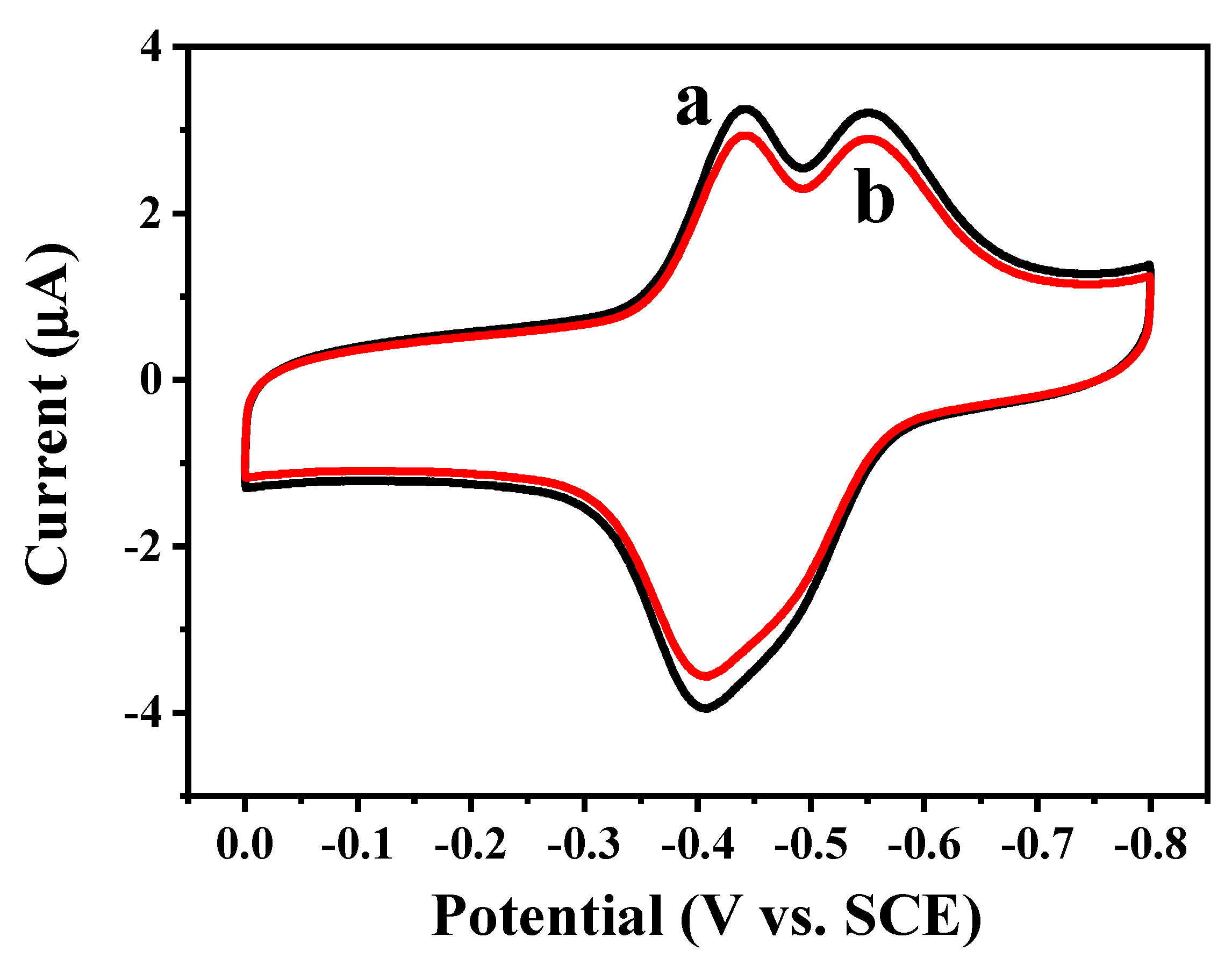
2.3. Direct Electron Transfer between GOD and rGON-AuNP/GC Electrode
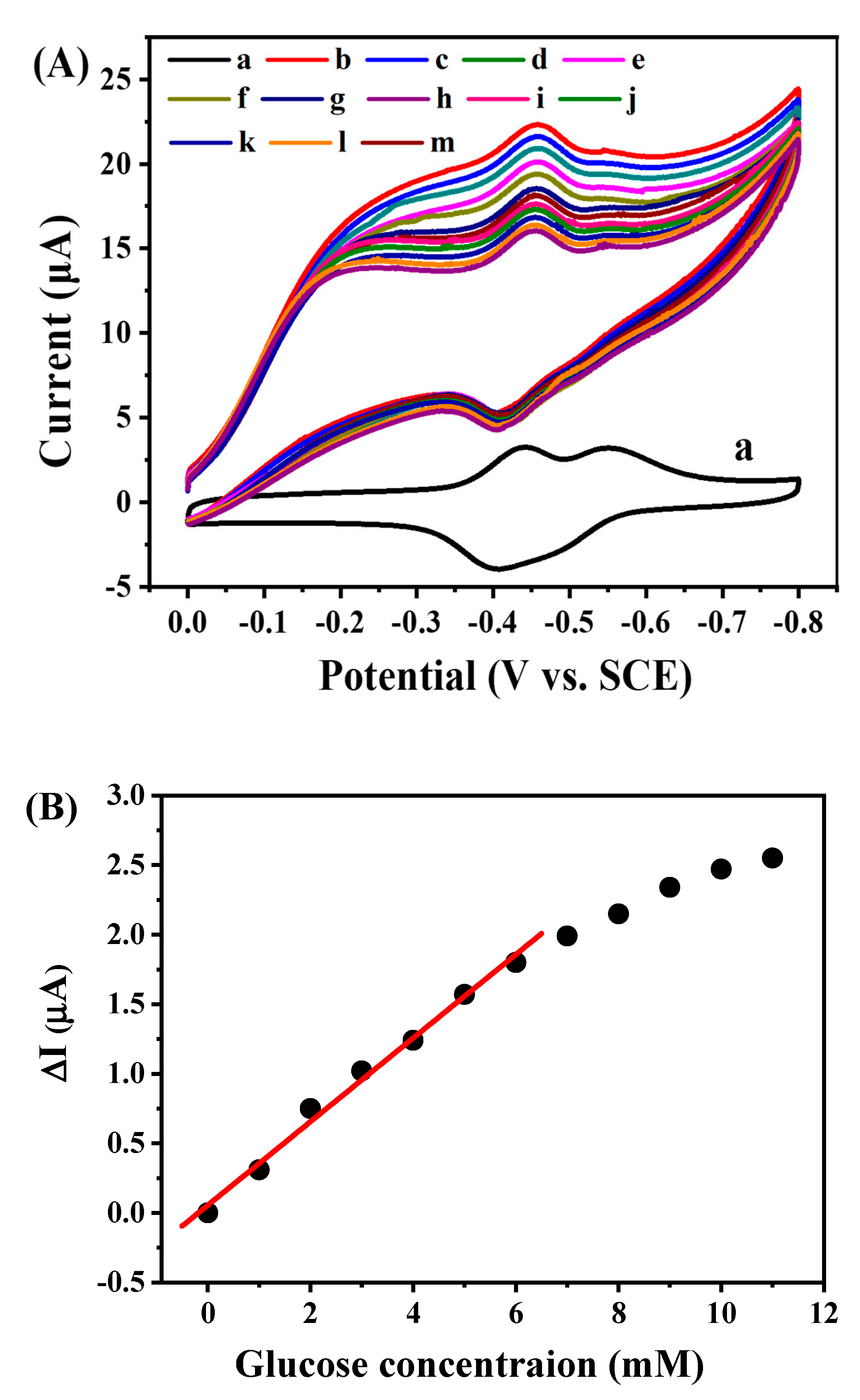
2.4. Electrocatalytic Behavior of GOD/rGON-AuNP/GC Electrode toward Glucose Oxidation
3. Experimental
3.1. Chemicals and Reagents
3.2. Preparation of rGON-AuNP
3.3. Construction of GOD Modified Electrodes
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tian, N.; Lu, B.-A.; Yang, X.-D.; Huang, R.; Jiang, Y.-X.; Zhou, Z.-Y.; Sun, S.-G. Rational Design and Synthesis of Low-Temperature Fuel Cell Electrocatalysts. Electrochem. Energy Rev. 2018, 1, 54–83. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Qiao, Y.; Li, C.M. Boosting Microbial Electrocatalytic Kinetics for High Power Density: Insights into Synthetic Biology and Advanced Nanoscience. Electrochem. Energy Rev. 2018, 1, 567–598. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Du, W.; Bai, Y.-L.; Xu, J.; Zhao, H.; Zhang, L.; Li, X.; Zhang, J. Advanced metal-organic frameworks (MOFs) and their derived electrode materials for supercapacitors. J. Power Sources 2018, 402, 281–295. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of Graphene and Related Materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors (Basel) 2017, 17, 2161. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-Gold Nanoparticles Hybrid-Synthesis, Functionalization, and Application in a Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials (Basel) 2016, 9, 406. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Luo, Z.; Somers, L.A.; Dan, Y.; Ly, T.; Kybert, N.J.; Mele, E.J.; Johnson, A.T.C. Size-Selective Nanoparticle Growth on Few-Layer Graphene Films. Nano Lett. 2010, 10, 777–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindhan, M.; Amiri, M.; Chen, A. Au nanoparticle/graphene nanocomposite as a platform for the sensitive detection of NADH in human urine. Biosens. Bioelectron. 2015, 66, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.; Ma, Y.; Gao, J.; Zhang, P.; Xia, Y.; Tian, Y.; Sun, X.; Zhong, J.; Sun, X. Facile Synthesis of Graphene/Metal Nanoparticle Composites via Self-Catalysis Reduction at Room Temperature. Inorg. Chem. 2013, 52, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Mecheri, B.; De Porcellinis, D.; Campana, P.T.; Rainer, A.; Trombetta, M.; Marletta, A.; Oliveira, O.N.; Licoccia, S. Tuning Structural Changes in Glucose Oxidase for Enzyme Fuel Cell Applications. ACS Appl. Mater. Interfaces 2015, 7, 28311–28318. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Z.; He, S.; Zhang, B.; Li, X.; Yao, M. Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode. Biosens. Bioelectron. 2014, 52, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.; Pan, L.; Zhai, D.; Wang, Y.; Li, L.; Cheng, W.; Yin, W.; Wang, X.; Xu, J.-B.; et al. ZnO-nanorods/graphene heterostructure: A direct electron transfer glucose biosensor. Sci. Rep. 2016, 6, 32327. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.-M.; Yang, C.-Y.; Periakaruppan, P.; Lou, B.-S. Direct electrochemistry of glucose oxidase and sensing of glucose at a glassy carbon electrode modified with a reduced graphene oxide/fullerene-C60 composite. RSC Adv. 2015, 5, 77651–77657. [Google Scholar] [CrossRef]
- Terse-Thakoor, T.; Komori, K.; Ramnani, P.; Lee, I.; Mulchandani, A. Electrochemically Functionalized Seamless Three-Dimensional Graphene-Carbon Nanotube Hybrid for Direct Electron Transfer of Glucose Oxidase and Bioelectrocatalysis. Langmuir 2015, 31, 13054–13061. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of direct electron transfer and enzyme activity of glucose oxidase on graphene surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Seehuber, A.; Dahint, R. Conformation and Activity of Glucose Oxidase on Homogeneously Coated and Nanostructured Surfaces. J. Phys. Chem. B 2013, 117, 6980–6989. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ma, W.-S.; Li, J.; Deng, B.-J.; Zhao, X.-S. Preparation and characterization of long-chain alkyl silane-functionalized graphene film. J. Mater. Sci. 2013, 48, 156–161. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, X.; Zhou, X.; Qian, G.; Zhou, J.; Yuan, W. Controlling and Formation Mechanism of Oxygen-Containing Groups on Graphite Oxide. Ind. Eng. Chem. Res. 2014, 53, 253–258. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Jin, M.H.; An, K.H.; Lee, Y.H. Structural Stability and Variable Dielectric Constant in Poly Sodium 4-Styrensulfonate Intercalated Graphite Oxide. J. Phys. Chem. C 2009, 113, 13060–13064. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- White, L.D.; Tripp, C.P. Reaction of (3-Aminopropyl)dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interface Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Yan, W.; Yu, W.-J.; Wang, L.; Zhang, D.; Ge, X.-Q.; Hang, J.-Z.; Deng, W.; Shi, L.-Y. Preparation of Partially Reduced Graphene Oxide Nanosheets/Poly(Sodium 4-Styrenesulfonate) Composite with High Capacitance. Electrochim. Acta 2014, 147, 257–264. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation of PVP-Protected Metal Nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar] [CrossRef]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au−ZnO Hybrid Nanopyramids and Their Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2010, 22, 055705. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, F.; Shan, C.; Han, D.; Zhang, Q.; Niu, L.; Ivaska, A. Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J. Mater. Chem. 2009, 19, 4632–4638. [Google Scholar] [CrossRef]
- Bhowmik, K.; Pramanik, S.; Medda, S.K.; De, G. Covalently functionalized reduced graphene oxide by organically modified silica: A facile synthesis of electrically conducting black coatings on glass. J. Mater. Chem. 2012, 22, 24690–24697. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, Z.; Yuwen, L.; Tian, J.; Zhu, X.; Wang, L. Synthesis of silver nanoparticles on reduced graphene oxide under microwave irradiation with starch as an ideal reductant and stabilizer. Appl. Surf. Sci. 2013, 266, 188–193. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, J.; Sim, D.H.; Tay, Y.Y.; Lu, Z.; Zhang, X.; Sharma, Y.; Srinivasan, M.; Zhang, H.; Hng, H.H.; et al. Achieving high specific charge capacitances in Fe3O4/reduced graphene oxide nanocomposites. J. Mater. Chem. 2011, 21, 3422–3427. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Militaru, A.; Shah, A.; Qureshi, R.; Tugulea, L.; Brett, A.M.O. Redox mechanism of lumazine at a glassy carbon electrode. J. Electroanal. Chem. 2010, 647, 1–7. [Google Scholar] [CrossRef]
- Filip, J.; Tkac, J. The pH dependence of the cathodic peak potential of the active sites in bilirubin oxidase. Bioelectrochemistry 2014, 96, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Nasri, Z.; Shams, E. A glucose biosensor based on direct electron transfer of glucose oxidase immobilized onto glassy carbon electrode modified with nitrophenyl diazonium salt. Electrochim. Acta 2013, 112, 640–647. [Google Scholar] [CrossRef]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Immobilization of glucose oxidase on electrodeposited nickel oxide nanoparticles: Direct electron transfer and electrocatalytic activity. Biosens. Bioelectron. 2007, 22, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.; Ju, H. Amperometric glucose sensor based on catalytic reduction of dissolved oxygen at soluble carbon nanofiber. Biosens. Bioelectron. 2007, 23, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, W.; Chen, X.; Evans, D.G. Direct electrochemistry of glucose oxidase on a graphite nanosheet–Nafion composite film modified electrode. Electrochem. Commun. 2009, 11, 997–1000. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Wu, P.; Cai, C. Detection of glucose based on direct electron transfer reaction of glucose oxidase immobilized on highly ordered polyaniline nanotubes. Anal. Chem. 2009, 81, 1638–1645. [Google Scholar] [CrossRef] [PubMed]





| Electrode Materials | Synthesis Pathway | Structural Properties | Electrochemical Sensor Behaviors (Linear Range, Low Detection Limit, Sensitivity etc.) | Catalytic Activity (Active GOx Surface Coverage, Heterogeneous Electron Transfer Rate Constant (ks), Michaelis–Menten Constant (KM) of Immobilized GOx) | Reference |
|---|---|---|---|---|---|
| GOx-modified GCE | Immobilization of GOx onto GCE electrografted with 4-aminophenyl (AP) by diazonium chemistry | 0.05–4.5 mM 10 μM | 1.23 × 10−12 mol cm−2 4.25 s−1 2.95 mM | [42] | |
| GOx/NiO/GCE | Electrodepositing, electrodissolution and immobilization of glucose oxidase | - 24 μM 446.2 nA/mM | 9.45 × 10−13 mol cm−2 25.2 ± 0.5 s−1 2.7 mM | [43] | |
| GOx–CNF | CNF carboxylation and mixing with glucose oxidase | 0.01–0.35 mM 2.5 μM 36.3 μA mM−1 cm−2 | - - | [44] | |
| Nafion–GOx–GNSs | Casting GNSs-Nafion-GOD suspension onto GCEs | GNSs: 0.2–2 μm in diameter, 10–50 nm in thickness | 0.2–1.4 mM - 3.4 μA mM−1 cm−2 | - - | [45] |
| GOx-nanoPANi/Pt | AAO template method | nanoPANi tubes with 250−300 nm in outer diameter | 0.01 to 5.5 mM- - 97.18 ± 4.62μA mM−1 cm−2 | - 1.53 ± 0.45 s−1 2.37 ± 0.5 mM | [46] |
| GOD/rGON-AuNP/GC | Wet chemical and incubating method | AuNPs of 6.2 nm in diameter | 0–6 mM - - | 4.29 × 10–10 mol/cm2 - | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Yu, W.; Liu, M.; Liu, Y.; Shao, Q.; Li, A.; Yan, W.; Zhang, J. Novel Composite Electrode of the Reduced Graphene Oxide Nanosheets with Gold Nanoparticles Modified by Glucose Oxidase for Electrochemical Reactions. Catalysts 2019, 9, 764. https://doi.org/10.3390/catal9090764
Dong L, Yu W, Liu M, Liu Y, Shao Q, Li A, Yan W, Zhang J. Novel Composite Electrode of the Reduced Graphene Oxide Nanosheets with Gold Nanoparticles Modified by Glucose Oxidase for Electrochemical Reactions. Catalysts. 2019; 9(9):764. https://doi.org/10.3390/catal9090764
Chicago/Turabian StyleDong, Li, Wenjuan Yu, Minmin Liu, Yang Liu, Qinsi Shao, Aijun Li, Wei Yan, and Jiujun Zhang. 2019. "Novel Composite Electrode of the Reduced Graphene Oxide Nanosheets with Gold Nanoparticles Modified by Glucose Oxidase for Electrochemical Reactions" Catalysts 9, no. 9: 764. https://doi.org/10.3390/catal9090764