New Compounds with [As7]3– Clusters: Synthesis and Crystal Structures of the Zintl Phases Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14
Abstract
: Three new cluster compounds, Cs2NaAs7, Cs4ZnAs14, and Cs4CdAs14 were obtained from high temperature reactions. Their structures feature heptaarsenide [As7]3− anions, where the clusters in Cs4ZnAs14 and Cs4CdAs14 are dimerized by the linkers Zn and Cd, respectively. The bonding characteristics of these clusters are discussed and compared. Band structure calculation on Cs2NaAs7 suggests that this compound is a semiconductor with an energy gap of circa 1.6 eV, which is in consistent with the dark red color of the crystals.1. Introduction
The typical Zintl phases are compounds formed between the alkali or alkaline-earth metals and the metalloids from group 13, 14, or 15 [1]. The chemical bonding in such systems could be rationalized following the Zintl-Klemm concept [2], which assumes that the electropositive elements act as electron donors (becoming spectator cations), while the electronegative counterparts form covalent bonds to obtain closed-shell electronic configurations. In recent years the field has been expanded to include some d-metals, as well as the nominally divalent Eu and Yb from the lanthanide family. Such materials, e.g., Yb14MnSb11 [3], CaxYb1−xZn2Sb2 [4], and EuZn2Sb2 [5], among others, have received recognition for their potential in thermoelectric energy conversion [6]. There has also been a lasting research interest in cluster-like Zintl ions [7], which is primarily instigated from the fundamental understanding of their bonding characteristics [7,8], as well as the novel properties offered by materials based on cluster assemblies [9-13].
In this article, we report three new Zintl compounds, Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14 all of which feature the nortricyclane-like [As7]3− clusters. In Cs2NaAs7 (formally [Cs+]2[Na+][As7]3−) the [As7]3− polyanions are discrete and “solvated” by Cs+ and Na+ cations, while Cs4ZnAs14, and Cs4CdAs14 (formally [Cs+]4[Zn2+]{[As7]3−}2 and [Cs+]4[Cd2+]{[As7]3−}2) are better viewed as containing larger heteroatomic [As7-Zn-As7]4− and [As7-Cd-As7]4− units, respectively. The latter can be considered as being made of two [As7]3− species, bridged by tetrahedrally coordinated Zn and Cd atoms. The crystal structures and the bonding characteristics of the three compounds are presented, alongside with a discussion on the electronic structure of Cs2NaAs7, calculated using the LMTO method [14-16].
2. Results and Discussion
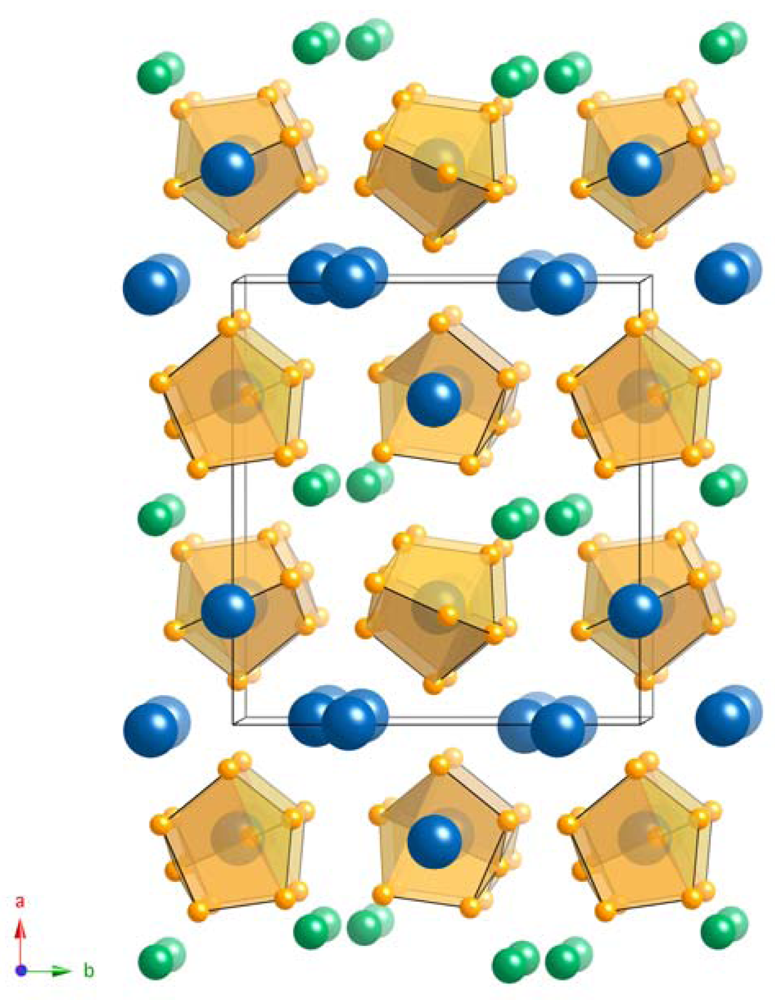
Cs2NaAs7 crystallizes in a monoclinic crystal system with space group P21/c (No. 14, Pearson symbol mP40). Important crystallographic information pertaining to the structure is given in Table 1. Although it has identical formula to A3As7 (A = Li, Na, K, Rb, Cs) [17-19], it is not isostructural to any of the above-mentioned binary compounds. In Cs2NaAs7, which is a true ternary phase, Cs and Na atoms are ordered on different crystallographic sites.
The packing of the [As7]3− clusters resembles fcc arrangement with Cs2+ cations residing in the octahedral holes left between the clusters, and Cs1+ and Na+ cations in the tetrahedral holes, respectively (Figure 1). Such anion packing (ABCABC) is the same as in the structure of Cs3As7 [19], which is distinct from the double hexagonal close packing (ABAC) in K3As7 [19] and the hexagonal close packing (ABAB) in Rb3As7 [19].
Cs4ZnAs14 and Cs4CdAs14 also crystallize with the same monoclinic space group P21/c (No. 14), but their structures are different (Table 1 and Figure 2). Notably, despite the same formulae, they are not isostructural to each other, with Cs4ZnAs14 having a four-times larger repeating unit (Pearson symbol mP304) than Cs4CdAs14 (Pearson symbol mP76). The detailed descriptions of each structure follow.
As already mentioned, the structure of Cs2NaAs7 features isolated nortricyclane-like (or birdcagelike) [As7]3− clusters, with Cs+ and Na+ cations filling the space between them. The As–As distances (Table 2) could be grouped into three sets based on their length: set A, 2.4799(17)-2.5139(19) Å, which comprises the longest bonds, found between the As atoms of the triangular base (As3, As5, As6); the intermediate set B, which accounts for the bonds between the apical As (As2) and the three As atoms at the “waist” of the cluster (As1, As4, As7), ranging from 2.4042(18) to 2.4237(17) Å; and set C, which encompasses the shortest As–As contacts (2.3411(18)-2.348(2) Å), observed between the three basal As atoms and the three atoms in the waist (Figure 3(a)). Such distribution of bond distances seems to be a signature of all [As7]3− cluster anions, as it is noted in the binary intermetallic compounds A3As7 (A = Li, Na, K, Rb, Cs) [17-19], as well as the compounds [Li(NH3)4]3As7·NH3 [20], [Rb(18-crown-6)]3As7·8NH3 [20], and Cs3As7·6NH3 [20], which are crystallized from liquid ammonia solutions. The bond angles are also characteristic, with α, β, γ, and δ (Figure 3(a)) close to 101°, 99°, 105°, and 60°, respectively. These metric parameters are comparable with the corresponding angles in the above-mentioned compounds [17-20]. Following the Zintl-Klemm concept [2], the formal charges in the [As7]3− clusters can be assigned as (3b-As0)4(2b-As−)3, and the Cs2NaAs7 formula can be readily rationalized as [Cs+]2[Na+][As7]3− Electronic band structure calculations confirm this reasoning.
Cs4ZnAs14 crystallizes with an unusually large unit cell for an intermetallic compound (V = 10309.9(14) Å3) and the structure is devoid of any disorder. The structure contains four crystallographically independent [ZnAs14]4− polyanions, which are made up of tetrahedral Zn atoms, bridging two [As7]3− clusters (Figure 3(b)). The structural complexity is apparently caused by the intricate orientation and packing of the [ZnAs14]4− units. Interestingly, all the As atoms bonded to Zn are the 2-bonded As atoms at the waist of the [As7]3– “birdcage”, wherein they could be assigned as negatively charged (the remaining As atoms are 3-bonded, and thus, with a formal charge 0). The same bonding features have also been revealed for the cluster compounds which have the similar structural motif, e.g., [K(2,2,2-crypt)]3[In(P7)2]·3.5py [21], [K(2,2,2-crypt)]4ZnE14(E = P, As) [22], and [K(2,2,2-crypt)]4CdP14·6py [22]. The Zn–As bond distances fall in the range 2.514 to 2.598 Å, comparing well with the sum of their covalent radii [23], as well as with the corresponding distances found in other Zintl compounds with Zn-As bonding such as Ba2ZnAs2 [24], Eu11Zn6As12 [25], and RbZn4As3 [26].
Another interesting observation is the geometric distortion of the [As7]3− moiety of the [ZnAs14]4− anion compared to the isolated [As7]3−. Although the nortricyclane-like topology essentially remains intact, the “bidentate” coordination of the [As7]3− ligand to the Zn atom induces some changes. For example, with a reference to the [Zn(1)As14]4− species, the α angles involving the chelating As atoms significantly decrease (∠As4-As5-As12 = 94.256° and ∠As20-As29-As24 = 94.193°), concomitant with the slight increase of the bond distances (0.02-0.04 Å). On the other hand, the distances between the apical As and the bridging As atoms that are not bonded to Zn decrease (As5−As8 = 2.379 Å, As29−As54 = 2.365 Å) to a degree that they fall in the range of distances for the shortest set C (Table 3). Similar distortion is observed for all the eight [As7]3– subunits, which again, are not symmetry-equivalent to each other. Examples in which the insertion of transition metals leads to more significant changes to the [Pn7]3– cluster are also known from the organometallic literature. For instance, in the [E7M(CO)3]3− clusters (E = P, As, Sb and M = Cr, Mo, W) [27,28], the nortricyclane-like E73− fragments transformed into norbornadiene-like clusters due to the four bonds between the transition metal and two of the bridging and two of the basal pnictogens. More profound change has been noted in [Sb7Ni3(CO)3]3– [29], where the Sb73– is bonded to three Ni atoms, forming a 10-vertex nido cluster.
Cs4CdAs14 crystallizes with the same space group as Cs4ZnAs14, however, with only one [CdAs14]4− polyanion in the asymmetric unit. The Cd atoms are tetrahedrally bonded to the As atoms from two adjacent [As7]3− clusters, with average Cd–As distance of 2.757 Å, which is just slightly larger than the sum of their covalent radii (rCd = 1.38 Å; rAs = 1.21 Å) [23]. In both Cs4ZnAs14 and Cs4CdAs14, the [ZnAs14]4– and [CdAs14]4– anions form a simple cubic array (very distorted in the case of Cs4ZnAs14). Compared with the ZnAs4 tetrahedra in the analogous [ZnAs14]4− motif of Cs4ZnAs14, the CdAs4 tetrahedra are more distorted. This can be seen from an inspection of the As-Cd-As angles, which deviate more from the ideal 109.5° tetrahedral angle. The [As7]3– units experience similar geometric distortion due to the “bidentate” coordination to Cd; however, comparison of the corresponding distances (Table 3) and angles reveals that the distortion in [CdAs14]4− is less pronounced. This is understandable since the longer Cd–As distances would naturally allow more flexibility.
Following the above discussion, the electron count in both Cs4ZnAs14 and Cs4CdAs14 could be assigned in the same way: (Cs+)4(4b-Zn2−)(3b-As0)12(2b-As−)2 and (Cs+)4(4b-Cd2−)(3b-As0)12(2b-As−)2, which is in consistent with the Zintl formalism [2]. Exaggerating the ionicity of the Zn–As and Cd–As interactions, the formula units can also be broken down as [Cs+]4[Zn2+]{[As7]3−}2 and [Cs+]4[Cd2+]{[As7]3−}2, respectively.
The electronic structure of Cs2NaAs7 was calculated using the LMTO method [14-16] and the plot of the total and partial density of states is shown in Figure 4(a). A band-gap in the order of 1.6 eV is noticeable from the plot, suggesting that Cs2NaAs7 should be an intrinsic semiconductor. Note that the LMTO method usually underestimates the band-gap, this value actually agrees very well with the dark red color of the crystals. Due to the low symmetry and very complex structures of Cs4ZnAs14 and Cs4CdAs14, the electronic structures for neither were carried out; the appearance of their crystals also suggested semiconducting behavior with a band-gap in the visible range.
Analyzing the electronic structure of Cs2NaAs7, one can readily see that the states below the Fermi level in the range from −5 to 0 eV (set as the Fermi level) are predominately contributed from As p orbitals, with only a small admixture of states from the alkali metals, in agreement with the Zintl formalism. In the vicinity of the Fermi level, however, the contributions of the three different types of As atoms differ very much, as seen from Figure 4(b). The apical As atom has almost negligible partial DOS, while the contribution from the As atoms at the bridging position (waist of the cluster) is significant in this energy window. Such characteristics of the electronic structure should indicate different chemical and physical properties of As atoms at different positions, i.e., chemical reactivity.
3. Experimental Section
All manipulations involving the alkali metals were performed either inside an argon-filled glove box with controlled moisture/oxygen levels or under vacuum. Crystals of Cs2NaAs7, Cs4ZnAs14, and Cs4CdAs14 were all identified from reactions originally aimed at the pnictide clathrates Cs8Zn18As28, Cs8Cd18As28 or the mixed-cation variant Na6Cs2Zn18As28. In the typical reactions, the elements (Na/Cs/Ca/Zn/Cd/As, purchased from either Alfa Aesar or Aldrich with the stated purity higher than 99.9%, used as received) with the desired stoichiometric ratio were loaded into niobium ampoules. The niobium ampoules were arc-welded under high purity Ar and then jacketed within fused silica tubes, which were subsequently flame-sealed under vacuum. The reaction mixtures were heated up to 500 °C in a programmable furnace, and equilibrated for 1 week before being slowly cooled to room temperature. The dark red crystals of Cs2NaAs7 were initially identified as a byproduct of a reaction with starting elements Na, Cs, Zn, and As, carried out as described above. The major product was CsZn4As3 [26], with Zn3As2 [30] and Cs2NaAs7 as side products. Cs2NaAs7 can be made as phase pure material from stoichiometric mixture without Zn.
From an analogous reaction with starting elements Na, Cs, Cd, and As, Cs4CdAs14 was initially identified, together with Cs8Cd18As28 [31], NaCd4As3 [26], and CdAs2 [32]. The differentiation from the other phases was possible due to the red color and transparent habit of the crystals. Cs4ZnAs14 was originally obtained from a reaction loaded as Ca16Cs8Zn58.67As77.33, and subjected to the above-described heat treatment. The dark red crystals in this reaction were identified as Cs4ZnAs14, between the black crystals of CaZn2As2 [33] and the silvery ones of Zn3As2 [30]. After the structures and the compositions were established from single-crystal diffraction work, Cs4CdAs14 and Cs4ZnAs14 were synthesized in high yield from the corresponding stoichiometric reactions.
All three compounds degraded quickly upon exposure to air.
The crystal structures of the title compounds were established using single-crystal X-ray diffraction. The diffraction data were collected on a Bruker SMART CCD-based diffractometer using monochromated Mo Kα1 radiation. Crystals were cut to suitable dimensions (<100 μm) under a microscope and then mounted on glass fiber with Paratone-N oil. The fiber was then quickly transferred to the goniometer of the diffractometer, where a cold nitrogen stream (200(2) K) was used to harden the oil in order to protect the crystals from being oxidized. Full spheres of data were collected in four batch runs with a frame width of 0.3° for ω and θ. Integration of the intensity data was done with SAINT program [34], and semi-empirical absorption correction based on equivalents was applied with the SADABS code [35]. The structures were solved by direct method and refined to convergence by full matrix least squares on F2 using the SHELXTL package [36]. The atomic coordinates were standardized with the aid of the Structure TIDY [37]. In the last refinement cycles, all atoms were treated with anisotropic displacement parameters. Tables with selected refinement parameters and the atomic coordinates and equivalent isotropic displacement parameters are submitted as electronic supplementary information. Selected crystal data and refinement parameters are given in Table 1; important bond distances and angles are listed in Table 2 and Table 3. CIFs have also been deposited with Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany, (fax: (49) 7247-808-666; e-mail: [email protected])—depository numbers CSD-423080 for Cs4NaAs7, CSD-423081 for Cs4CdAs14, and CSD-423082 for Cs4CdAs14.
The Stuttgart TB-LMTO 4.7 code [38], which employs the tight-binding linear muffin-tin orbital (TB-LMTO) method [14-16], was used to calculate the band structures of Cs2NaAs7. In this program, local density approximation (LDA) was used to treat exchange and correlation [39]. All relativistic effects except for spin-orbital coupling were taken into account by scalar relativistic approximation [40]. Due to the limited computing resources, Cs4CdAs14 and Cs4ZnAs14 (very complex and large structures) could not be treated. For the calculation of Cs2NaAs7, the basis set included the 6s, 6p, 5d and 4f orbitals for Cs, 3s, 3p and 3d orbitals for Na, and 4s, 4p and 4d orbitals for As. The 6p, 5d and 4f orbitals of Cs, 3p and 3d orbitals of Na, and 4d orbital of As were treated with the downfolding technique [41]. The total and partial density of states were plotted with the Fermi level set as a reference at 0 eV.
4. Conclusions
Three new Zintl phases Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14 have been synthesized via high temperature reactions. The structure of Cs2NaAs7 features the nortricyclane-like cluster [As7]3−, while the structures of the latter two contain very large [ZnAs14]4− and [CdAs14]4− anions, which are composed of two [As7]3− units, linked by 4-coordinated Zn or Cd. Band structure calculations confirm the intrinsic semiconductor with a size of band-gap consistent with the dark red color of the crystals.



| Empirical formula | Cs2NaAs7 | Cs4ZnAs14 | Cs4CdAs14 | |
| Formula weight | 813.25 | 1645.89 | 1692.92 | |
| Space group, Z | P21/c, 4 | P21/c, 16 | P21/c, 4 | |
| Temperature | 200(2) K | |||
| Wavelength | Mo Kα, 0.71073 Å | |||
| Cell parameters | a (Å) | 11.7671(18) | 29.067(2) | 10.608(3) |
| b (Å) | 10.8528(17) | 10.4714(8) | 16.761(5) | |
| c (Å) | 10.2115(15) | 43.039(3) | 14.363(4) | |
| β (°) | 90.213(3) | 128.0920(10) | 91.785(5) | |
| V (Å3) | 1304.1(3) | 10309.9(14) | 2552.6(12) | |
| Calculated density (g/cm3) | 4.142 | 4.241 | 4.405 | |
| Absorption coefficient (cm−1) | 231.94 | 243.43 | 244.74 | |
| Crystal size (mm3) | 0.044 × 0.030 × 0.028 | 0.068 × 0.049 × 0.023 | 0.080 × 0.060 × 0.040 | |
| Goodness-of-fit on F2 | 1.001 | 0.979 | 0.981 | |
| R (I > 2σI)a | 0.0441 | 0.0436 | 0.0320 | |
| wR2 (I > 2σI)a | 0.0678 | 0.0737 | 0.0554 | |
| Largest diff. peak/hole (e−/Å3) | 1.172/−1.055 | 1.260/−1.463 | 1.012/−0.822 | |
| Weight coefficient, A/Ba | 0.0189/0 | 0/17.2682 | 0.0208/0 | |
aR1 = Σ| |Fo|−|Fc| |/Σ|Fo|, wR2 = {Σ[w(Fo2−Fc2)2]/ Σ[w(Fo2)2]}1/2, where w = 1/[σ2Fo2+(AP)2+BP], and P = (Fo2+2Fc2)/3.A and B are weight coefficients.
| Atom pair | Distance | Angle label | Angle |
|---|---|---|---|
| As2–As1 | 2.4042(18) | As1-As2-As4 | 100.76(6) |
| As2–As4 | 2.4237(17) | As1-As2-As7 | 102.56(6) |
| As2–As7 | 2.4050(18) | As4-As2-As7 | 101.38(6) |
| As1–As3 | 2.348(2) | As2-As1-As3 | 98.97(6) |
| As4–As5 | 2.3411(18) | As2-As4-As5 | 98.90(6) |
| As7–As6 | 2.3465(19) | As2-As7-As6 | 98.45(6) |
| As3–As5 | 2.5139(19) | As3-As5-As6 | 59.98(5) |
| As3–As6 | 2.4964(19) | As5-As6-As3 | 60.68(5) |
| As5–As6 | 2.4799(17) | As6-As3-As5 | 59.33(5) |
| Atom pair | Distance | Atom pair | Distance |
|---|---|---|---|
| Cs4ZnAs14 | Cs4CdAs14 | ||
| Zn1-tetrahedron | Cd-tetrahedron | ||
| Zn1–As4 | 2.534(2) | Cd–As2 | 2.7364(10) |
| Zn1–As12 | 2.590(2) | Cd–As6 | 2.7736(10) |
| Zn1–As20 | 2.560(2) | Cd–As1 | 2.6957(9) |
| Zn1–As24 | 2.560(2) | Cd–As14 | 2.8241(11) |
| “birdcage” 1 | “birdcage” 1 | ||
| As5–As4 | 2.441(2) | As3–As2 | 2.4339(11) |
| As5–As8 | 2.379(2) | As3–As6 | 2.4281(11) |
| As5–As12 | 2.425(2) | As3–As7 | 2.3741(11) |
| As4–As1 | 2.381(2) | As2–As8 | 2.3801(11) |
| As8–As7 | 2.363(3) | As6–As11 | 2.3775(12) |
| As12–As9 | 2.373(2) | As7–As12 | 2.3547(11) |
| As1–As7 | 2.479(2) | As8–As11 | 2.5025(11) |
| As1–As9 | 2.498(2) | As8–As12 | 2.5021(11) |
| As7–As9 | 2.463(2) | As11–As12 | 2.4545(12) |
| “birdcage” 2 | “birdcage” 2 | ||
| As29–As20 | 2.4395(19) | As5–As1 | 2.4236(10) |
| As29–As24 | 2.425(2) | As5–As10 | 2.3873(11) |
| As29–As54 | 2.365(2) | As5–As14 | 2.4332(11) |
| As20–As16 | 2.368(2) | As1–As4 | 2.3951(11) |
| As24–As22 | 2.386(2) | As10–As13 | 2.3656(12) |
| As54–As26 | 2.361(2) | As14–As9 | 2.3760(11) |
| As16–As22 | 2.503(2) | As4–As13 | 2.4721(12) |
| As16–As26 | 2.489(2) | As4–As9 | 2.5090(11) |
| As22–As26 | 2.453(2) | As13–As9 | 2.4694(11) |
Acknowledgments
Svilen Bobev acknowledges financial support from the US Department of Energy through a grant (DE-SC0001360).
References
- Zintl, E. Intermetallische Verbindungen. Angew. Chem. 1939, 52, 1–6. [Google Scholar]
- Schäfer, H.; Eisenman., B.; Müller, W. Zintl Phases-Transitions between Metallic and Ionic Bonding. Angew. Chem. Int. Ed. 1973, 12, 694–712. [Google Scholar]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl Phases for Thermoelectric Devices. Dalton Trans. 2007, 2099–2107. [Google Scholar]
- Brown, S.R.; Kauzlarich, S.M.; Gascoin, F.; Snyder, G.J. Yb14MnSb11: New High Efficiency Thermoelectric Material for Power Generation. Chem. Mater. 2006, 18, 1873–1877. [Google Scholar]
- Gascoin, F.; Ottensmann, S.; Stark, D.; Haile, S.M.; Snyder, G.J. Zintl Phases as Thermoelectric Materials: Tuned Transport Properties of the Compounds CaxYb1-xZn2Sb2. Adv. Funct. Mater. 2005, 15, 1860–1864. [Google Scholar]
- Zhang, H.; Zhao, J.-T.; Grin, Y.; Wang, X.-J.; Tang, M.-B.; Man, Z.-Y.; Chen, H.-H.; Yang, X.-X. A New Type of Thermoelectric Material, EuZn2Sb2. J. Chem. Phys. 2008, 129, 164713. [Google Scholar]
- Kolis, J.W.; Young, D.M. Molecular Transition Metal Complexes of Zintl Ions. In Chemistry, Structure, and Bonding of Zintl Phases and Ions; Kauzlarich, S.M., Ed.; VCH: New York, NY, USA, 1996; pp. 225–244. [Google Scholar]
- Corbett, J.D. Polyatomic Zintl Anions of the Post-Transition Elements. Chem. Rev. 1985, 85, 383–397. [Google Scholar]
- Sun, D.; Riley, A.E.; Cadby, A.J.; Richman, E.K.; Korlann, S.D.; Tolbert, S.H. Hexagonal Nanoporous Germanium through Surfactant-Driven Self-Assembly of Zintl Clusters. Nature 2006, 441, 1126–1130. [Google Scholar]
- Korlann, S.D.; Riley, A.E.; Mun, B.S.; Tolbert, S.H. Chemical Tuning of the Electronic Properties of Nanostructured Semiconductor Films Formed through Surfactant Templating of Zintl Cluster. J. Phys. Chem. C 2009, 113, 7697–7705. [Google Scholar]
- Moses, M.J.; Fettinger, J.C.; Eichhorn, B.W. Interpenetrating As20 Fullerene and Ni12 Icosahedra in the Onion-Skin [As@Ni12@As20]3− Ion. Science 2003, 300, 778–780. [Google Scholar]
- Esenturk, E.N.; Fettinger, J.C.; Eichhorn, B.W. The Pb122− and Pb102− Zintl Ions and the M@ Pb122− and M@ Pb102− Cluster Series Where M = Ni, Pd, Pt. J. Am. Chem. Soc. 2006, 128, 9178–9186. [Google Scholar]
- Hull, M.W.; Sevov, S.C. Functionalization of Nine-Atom Deltahedral Zintl Ions with Organic Substituents: Detailed Studies of the Reactions. J. Am. Chem. Soc. 2009, 131, 9026–9037. [Google Scholar]
- Andersen, O.K. Linear Methods in Band Theory. Phys. Rev. B 1975, 12, 3060–3083. [Google Scholar]
- Andersen, O.K.; Jepsen, O. Explicit, First-Principles Tight-Binding Theory. Phys. Rev. Lett. 1984, 53, 2571–2574. [Google Scholar]
- Andersen, O.K.; Pawlowska, Z.; Jepsen, O. Illustration of the Linear Muffin-Tin Orbital Tight-Binding Representation: Compact Orbitals and Charge Density in Si. Phys. Rev. B 1986, 34, 5253–5269. [Google Scholar]
- Hönle, W.; Buresch, J.; Peters, K.; Chang, J.-H.; von Schnering, H.G. Crystal structure of the low-temperature modification of trilithium heptaarsenide, LT-Li3As7. Z. Kristallogr. 2002, 217, 485–486. [Google Scholar]
- Hönle, W.; Buresch, J.; Peters, K.; Chang, J.-H.; von Schnering, H.G. Crystal structure of the low- temperature modification of trisodium heptaarsenide, LT-Na3As7. Z. Kristallogr. 2002, 217, 487–488. [Google Scholar]
- Emmerling, F.; Röhr, C. Alkali Metal Arsenides A3As7 and AAs (A = K, Rb, Cs). Preparation, Crystal Structure, Vibrational Spectroscopy. Z. Naturforsch. 2002, 57b, 963–975. [Google Scholar]
- Hanauer, T.; Grothe, M.; Reil, M.; Korber, N. Syntheses and Crystal Structures of Four New Ammoniates with Heptaarsenide (As73−) Anioins. Helv. Chim. Acta 2005, 88, 950–961. [Google Scholar]
- Knapp, C.M.; Large, J.S.; Rees, N.H.; Goicoechea, J.M. A Versatile Salt-Metathesis Route to Heteroatomic Clusters Derived from Phosphorus and Arsenic Zintl Anions. Dalton Trans. 2011, 40, 735–745. [Google Scholar]
- Knapp, C.M.; Zhou, B.; Denning, M.S.; Rees, N.H.; Goicoechea, J.M. Reactivity Studies of Group 15 Zintl Ions towards Homoleptic Post-Transition Metal Organometallics: a ‘Bottom-up’ Approach to Bimetallic Molecular. Dalton Trans. 2010, 39, 426–436. [Google Scholar]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960; pp. 400–404. [Google Scholar]
- Saparov, B.; Bobev, S. Isolated Chains in the Zintl Phases Ba2ZnPn2 (Pn = As, Sb, Bi) – Synthesis, Structure, and Bonding. Inorg. Chem. 2010, 49, 5173–5179. [Google Scholar]
- Saparov, B.; Bobev, S. Undecaeuropium hexazinc dodecaarsenide. Acta Crystallogr. 2010, E66, i24. [Google Scholar]
- He, H.; Tyson, C.; Bobev, S. 8-coordinated Arsenic in the Zintl Phases RbCd4As3 and RbZn4As3. Synthesis and Structural Characterization. Inorg. Chem. 2011. submitted for publication. [Google Scholar]
- Eichhorn, B.W.; Haushalter, R.C.; Huffman, J.C. Insertion of Cr(CO)3 into As73– to Form [As7Cr(CO)3]3–: An Inorganic Nortricyclane-to-Norbornadiene Conversion. Angew. Chem. Int. Ed. 1989, 28, 1032–1033. [Google Scholar]
- Charles, S.; Eichhorn, B.W.; Rheingold, A.L.; Bott, S.G. Synthesis, Structure, and Properties of the [E7M(CO)3]3− Complexes Where E = P, As, Sb and M = Cr, Mo, W. J. Am. Chem. Soc. 1994, 116, 8077–8086. [Google Scholar]
- Charles, S.; Eichhorn, B.W.; Bott, S.G. Synthesis and Structure of [Sb7Ni3(CO)3]3−: A New Structural Type for nido 10-Vertex Polyhedral Clusters. J. Am. Chem. Soc. 1993, 115, 5837–5838. [Google Scholar]
- Weglowski, S.; Lukaszewicz, K. The Crystal Structure of Zinc Arsenide polymorphic modifications α-Zn3As2 and α'-Zn3As2. Bull. Acad. Polon. Sci. Ser. Sci. Chim. 1968, 16, 177–182. [Google Scholar]
- He, H.; Bobev, S. type-I clathrate Cs8Cd18As28, space group Pm3̄n, a = 11.3281(5) Å. Unpublished results. 2011. [Google Scholar]
- Cervinka, L.; Hruby, A. The Crystal Structure of CdAs2. Acta Crystallogr. 1970, 26B, 457–458. [Google Scholar]
- Klüfers, P.; Mewis, A. AB2X2 – Verbindungen im CaAl2Si2-typ. III. Zur Struktur der Verbindungen CaZn2P2, CaCd2P2, CaZn2As2, und CaCd2As2. Z. Naturforsch. 1977, 32b, 753–756. [Google Scholar]
- SAINT NT; Version 6.45; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXTL; University of Göttingen: Göttingen, Germany, 2001. [Google Scholar]
- Gelato, L.M.; Parthe, E. Structure Tidy - a Computer Program to Standardize Crystal Structure Data. J. Appl. Crystallogr. 1987, 20, 139–146. [Google Scholar]
- Jepsen, O.; Andersen, O.K. TB-LMTO-ASA Program; version 4.7; Max-Planck-Institut für Festkörperforschung: Stuttgart, Germany, 1998. [Google Scholar]
- von Barth, U.; Hedin, L. Local Exchange-Correlation Potential for Spin Polarized Case 1. J. Phys. C: Solid State Phys. 1972, 5, 1629–1642. [Google Scholar]
- Koelling, D.D.; Harmon, B.N. Technique for Relativistic Spin-Polarized Calculations. J. Phys. C Solid State Phys. 1977, 10, 3107–3114. [Google Scholar]
- Lambrecht, W.R.L.; Andersen, O.K. Minimal Basis-Sets in the Linear Muffin-Tin Orbital Method – Application to the Diamond-Structure Crystals C, Si, and Ge. Phys. Rev. B: Condens. Matter 1986, 34, 2439–2449. [Google Scholar]
- Supplementary Material: Supplementary data associated with this article can be found in the online version at doi: 10.3390/cryst1030087.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, H.; Tyson, C.; Bobev, S. New Compounds with [As7]3– Clusters: Synthesis and Crystal Structures of the Zintl Phases Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14. Crystals 2011, 1, 87-98. https://doi.org/10.3390/cryst1030087
He H, Tyson C, Bobev S. New Compounds with [As7]3– Clusters: Synthesis and Crystal Structures of the Zintl Phases Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14. Crystals. 2011; 1(3):87-98. https://doi.org/10.3390/cryst1030087
Chicago/Turabian StyleHe, Hua, Chauntae Tyson, and Svilen Bobev. 2011. "New Compounds with [As7]3– Clusters: Synthesis and Crystal Structures of the Zintl Phases Cs2NaAs7, Cs4ZnAs14 and Cs4CdAs14" Crystals 1, no. 3: 87-98. https://doi.org/10.3390/cryst1030087





