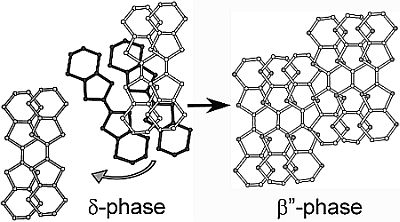
Single-Crystal-to-Single-Crystal Transformation from δ-(BEDT-TTF)4[OsNOCl5]1.33(C6H5NO2)0.67 to β"-(BEDT-TTF)3[OsNOCl5]
1. Introduction
2. Results and Discussion
2.1. Crystal Structures of δ-(BEDT-TTF)4[OsNOCl5]1.33(C6H5NO2)0.67 (1) and β"-(BEDT-TTF)3[OsNOCl5] (2): Different Conducting Layer Packing
| δ (1) | β" (2) | |
|---|---|---|
| Chemical formula | C44H35.33Cl6.67N2O2.67Os1.33S32 | C30H24Cl5NOOsS24 |
| Formula weight | 2150.83 | 1551.39 |
| Crystal system | Monoclinic | Triclinic |
| а, Å | 15.034(3) | 7.6672(8) |
| b, Å | 6.728(2) | 9.8657(11) |
| c, Å | 35.211(6) | 17.9733(12) |
| α, ° | 90 | 91.151(9) |
| β, ° | 92.98(1) | 93.636(7) |
| γ, ° | 90 | 102.434(9) |
| V, Å3 | 3556(1) | 1324.2(2) |
| Space group, Z | I2/a, 2 | Р  , 1 , 1 |

 0] direction. Central C=C bond lengths analysis in the TTF fragments leads to the conclusion that the positive charge is not uniformly distributed along the stack in contrast to the δ-phase which is built of one independent donor BEDT-TTF0.67+. In the β" structure, donor A with a longer C=C bond of 1.373(9) Å is close to a fully charged radical cation BEDT-TTF.+ while molecule B with a shorter C=C bond of 1.345(14) Å is neutral. Calculation of the molecular charge using the empirical formula [29] gives values 0.81+ and 0.15+ for A and B, respectively. Therefore, the molecular sequence in the stack is described as …-A•+-A•+-B0-A•+-A•+-B0-… . The sulfur…sulfur intermolecular interactions in the layer are mainly of side-by-side type. They are formed between the neighbor stacks along the [120] direction and the shortest S…S distance is 3.400(3) Å. Interplane separations in the stack are large, 4.03(8) Å (A-A) and 3.9(2) Å (A-B), that prevents existence of notable S…S van der Waals intrastack interactions. Some slightly shortened S…S contacts ≥3.610(3) Å are found inside the step-chains running along [210].
0] direction. Central C=C bond lengths analysis in the TTF fragments leads to the conclusion that the positive charge is not uniformly distributed along the stack in contrast to the δ-phase which is built of one independent donor BEDT-TTF0.67+. In the β" structure, donor A with a longer C=C bond of 1.373(9) Å is close to a fully charged radical cation BEDT-TTF.+ while molecule B with a shorter C=C bond of 1.345(14) Å is neutral. Calculation of the molecular charge using the empirical formula [29] gives values 0.81+ and 0.15+ for A and B, respectively. Therefore, the molecular sequence in the stack is described as …-A•+-A•+-B0-A•+-A•+-B0-… . The sulfur…sulfur intermolecular interactions in the layer are mainly of side-by-side type. They are formed between the neighbor stacks along the [120] direction and the shortest S…S distance is 3.400(3) Å. Interplane separations in the stack are large, 4.03(8) Å (A-A) and 3.9(2) Å (A-B), that prevents existence of notable S…S van der Waals intrastack interactions. Some slightly shortened S…S contacts ≥3.610(3) Å are found inside the step-chains running along [210]. 
2.2. Electronic Band Structures of δ and β" Crystals Are very Distinct

| Interaction * | S...S (< 4.0 Å) | tHOMO-HOMO (meV) |
|---|---|---|
| I (A-B) | 3.837, 3.910 | ~0 |
| II (A-A) | 3.948 | −21 |
| III (A-A) | 3.458 (x2), 3.480 (x2), 3.826, 3.856 (x2) | −69 |
| IV (A-B) | 3.400, 3.485, 3.571, 3.629, 3.877 | −27 |
| V (A-A) | 3.610 (x2), 3.644 (x2), 3.658 (x2), 3.939 | +229 |
| VI (A-B) | 3.654, 3.722, 3.765, 3.796, 3.945, 3.960, 3.986 | +78 |
2.3. On the Origin of SCSC Transformation: Huge Structural Changes in both the Donor and Anion Layers



3. Experimental Section
3.1. X-ray Diffraction
3.2. Electronic Band Structure Calculations
4. Conclusions
Acknowledgments
References
- Kushch, L.; Buravov, L.; Tkacheva, V.; Yagubskii, E.; Zorina, L.; Khasanov, S.; Shibaeva, R. Molecular metals based on radical cation salts of ET and some its analogues with the photochromicnitroprusside anion, [Fe(CN)5NO]2−. Synthet. Metal. 1999, 102, 1646–1649. [Google Scholar] [CrossRef]
- Gener, M.; Canadell, E.; Khasanov, S.S.; Zorina, L.V.; Shibaeva, R.P.; Kushch, L.A.; Yagubskii, E.B. Band structure and Fermi surface of the (BEDT-TTF)4M[Fe(CN)5NO]2 (M = Na, K, Rb,...) molecular metals contaning the photochromic nitroprusside anion. Solid State Commun. 1999, 111, 329–333. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-Garcia, C.J.; Fabre, J.M. Molecular conductors based upon TTF-type donors and octahedral magnetic complexes. Synthet. Metal. 1999, 103, 2279–2282. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Galán-Mascarós, J.R.; Gómez-Garcia, C.J.; Canadell, E. Hybrid molecular materials based upon the photochromic nitroprusside complex, [Fe(CN)5NO]2−, and organic π-electron donors. Synthesis, structure, and properties of the radical salt (TTF)7[Fe(CN)5NO]2 (TTF = Tetrathiafulvalene). Inorg. Chem. 2000, 39, 5394–5397. [Google Scholar]
- Zorina, L.V.; Khasanov, S.S.; Shibaeva, R.P.; Gener, M.; Rousseau, R.; Canadell, E.; Kushch, L.A.; Yagubskii, E.B.; Drozdova, O.O.; Yakushi, K. A new stable organic metal based on the BEDO-TTF donor and the doubly charged nitroprusside anion, (BEDO-TTF)4[Fe(CN)5NO]. J. Mater. Chem. 2000, 10, 2017–2023. [Google Scholar] [CrossRef]
- Khasanov, S.S.; Zorina, L.V.; Shibaeva, R.P. Structure of organic metals based on BEDT-TTF with photochromicnitroprusside anion, (BEDT-TTF)4M[Fe(CN)5NO]2 where M = Na+, K+, NH4+, Tl+, Rb+, Cs+. Russ. J. Coord. Chem. 2001, 27, 259–269. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-Garcia, C.J.; Ribera, E.; Vidal-Gancedo, J.; Rovira, C.; Canadell, E.; Laukhin, V. Hybrid molecular materials based upon organic π-electron donors and metal complexes. Radical salts of bis(ethylenethio)tetrathiafulvalene (BET-TTF) with the octahedral anions hexacyanoferrate(III) and nitroprusside. The first kappa phase in the BET-TTF family. Inorg. Chem. 2001, 40, 3526–3533. [Google Scholar]
- Sanchez, M.-E.; Doublet, M.-L.; Faulman, C.; Malfant, I.; Cassoux, P.; Kushch, L.; Yagubskii, E. Anion conformation and physical properties in BETS salts with the nitroprusside anion and its related ruthenium halide (X = Cl, Br) mononitrosyl complexes: θ-(BETS)4[Fe(CN)5NO], (BETS)2[RuBr5NO] and (BETS)2[RuCl5NO]. Eur. J. Inorg. Chem. 2001, 11, 2797–2804. [Google Scholar]
- Shevyakova, I.Y.; Buravov, L.I.; Kushch, L.A.; Yagubskii, E.B.; Khasanov, S.S.; Zorina, L.V.; Shibaeva, R.P.; Drichko, N.V.; Oleinischak, I. Radical cation salts of TTT and TSeT with photochromic anion [FeNO(CN)5]2−. Russ. J. Coord. Chem. 2002, 28, 487–495. [Google Scholar] [CrossRef]
- Zorina, L.V.; Gener, M.; Khasanov, S.S.; Shibaeva, R.P.; Canadell, E.; Kushch, L.A.; Yagubskii, E.B. Crystal and electronic structures of the radical cation salt based on EDT-TTF and the photochromicnitroprusside anion, (EDT-TTF)3[Fe(CN)5NO]. Synthet. Metal. 2002, 128, 325–332. [Google Scholar] [CrossRef]
- Shevyakova, I.Y.; Zorina, L.V.; Khasanov, S.S.; Buravov, L.I.; Tkacheva, V.A.; Shibaeva, R.P.; Yagubskii, E.B.; Canadell, E. The first mixed valence radical cation salts of BEDT-TTF with the photochromic metal mononitrosyl complexes [RuNOX5]2− (X = Br, Cl) as counterions. J. Solid State Chem. 2002, 168, 514–523. [Google Scholar] [CrossRef]
- Shibaeva, R.P.; Yagubskii, E.B.; Canadell, E.; Khasanov, S.S.; Zorina, L.V.; Kushch, L.A.; Prokhorova, T.G.; Shevyakova, I.Y.; Buravov, L.I.; Tkacheva, V.A.; et al. Structure-properties relationships in organic molecular conductors based on radical cation salts with octahedral metal complexes as counterions. Synthet. Metal. 2003, 133-134, 373–375. [Google Scholar] [CrossRef]
- Shevyakova, I.; Buravov, L.; Tkacheva, V.; Zorina, L.; Khasanov, S.; Simonov, S.; Yamada, J.; Canadell, E.; Shibaeva, R.; Yagubskii, E. New organic metals based on BDH-TTP radical cation salts with the photochromicnitroprusside anion [FeNO(CN)5]2−. Adv. Funct. Mater. 2004, 14, 660–668. [Google Scholar] [CrossRef]
- Zorina, L.V.; Khasanov, S.S.; Shibaeva, R.P.; Shevyakova, I.Y.; Kotov, A.I.; Yagubskii, E.B. Crystal structure of the new radical cation salt (DOET)4[Fe(CN)5NO]1.25(C6H5Cl)0.75. Crystallogr. Rep. 2004, 49, 1010–1017. [Google Scholar] [CrossRef]
- Simonov, S.V.; Shevyakova, I.Y.; Zorina, L.V.; Khasanov, S.S.; Buravov, L.I.; Emel’yanov, V.A.; Canadell, E.; Shibaeva, R.P.; Yagubskii, E.B. Variety of molecular conducting layers in the family of radical cation salts based on BEDT-TTF with the metal mononitrosyl complex [OsNOCl5]2−. J. Mater. Chem. 2005, 15, 2476–2488. [Google Scholar]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Kushch, L.A.; Buravov, L.I.; Yagubskii, E.B.; Boudron, S.; Méziere, C.; Batail, P.; et al. Crystalline patterns and band structure dimensionality in a series of conducting hybrids associating amide-functionalized EDT-TTF π-donors with the isosteric octahedral anions [FeNO(CN)5]2− and [M(CN)6]3− (M = Co, Fe)”. Synthet. Metal. 2005, 155, 527–538. [Google Scholar] [CrossRef]
- Simonov, S.V.; Zorina, L.V.; Khasanov, S.S.; Shibaeva, R.P.; Shevyakova, I.Y.; Buravov, L.I.; Yagubskii, E.B.; Canadell, E. Molecular conductors with the common and robust building block (BEDT-TTF)2NP (NP = [FeNO(CN)5]2−) but different band filling. J. Mater. Chem. 2006, 16, 787–794. [Google Scholar]
- Shibaeva, R.P.; Khasanov, S.S.; Zorina, L.V.; Simonov, S.V. Structural Features of Low-dimensional molecular conductors—representatives of new hybrid polyfunctional materials: Review. Crystallogr. Rep. 2006, 51, 949–967. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Khasanov, S.S.; Shibaeva, R.P. Peudopolymorphism, superstructure, and phase transitions in the crystals of (BEDT-TTF)4[MNOX5]2–xGx molecular conductor family, where M = Os, Ru; X = Cl, Br; G is a solvent molecule. J. Struct. Chem. 2009, 50, S152–S159. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Khasanov, S.S.; Shibaeva, R.P.; Suslikova, I.Y.; Kushch, L.A.; Yagubskii, E.B. Crystal structure of the new organic conductor (TSeF)7[FeNO(CN)5]2 with unusual molecular packing of conducting layer. Crystallogr. Rep. 2011, 56, 1111–1115. [Google Scholar]
- Hao, Z.M.; Zhang, X.M. Solvent induced molecular magnetic changes observed in single-crystal-to-single-crystal transformation. Dalton Trans. 2011, 40, 2092–2098. [Google Scholar]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Magnetic nanoporous coordination polymers. J. Mater. Chem. 2004, 14, 2713–2723. [Google Scholar] [CrossRef]
- Song, Y.; Luo, F.; Luo, M.; Liao, Z.; Sun, G.; Tian, X.; Zhu, Y.; Yuan, Z.J.; Liu, S.; Xu, W.; et al. The application of single-crystal-to-single-crystal transformation towards adjustable SMM properties. Chem. Commun. 2012, 48, 1006–1008. [Google Scholar]
- Shibaeva, R.P.; Yagubskii, E.B. Molecular conductors and superconductors based on trihalides of BEDT-TTF and some of its analogues. Chem. Rev. 2004, 104, 5347–5378. [Google Scholar] [CrossRef]
- Zvarykina, A.V.; Kononovich, P.A.; Laukhin, V.N.; Molchanov, V.N.; Pesotskii, S.I.; Simonov, V.I.; Shibaeva, R.P.; Schegolev, I.F.; Yagubskii, E.B. Nature of the high-temperature superconducting state with Tc =7–8 K in β-(BEDT-TTF)2I3. JETP Lett. 1986, 43, 329–332. [Google Scholar]
- Shibaeva, R.P.; Yagubskii, E.B.; Laukhina, E.E.; Laukhin, V.N. The Physics and Chemistry of Organic Superconductors; Saito, G., Kagoshima, S., Eds.; Springer-Verlag: Berlin, Germany, 1990; p. 342. [Google Scholar]
- Duisenberg, A.J.M.; Kroon-Batenburg, L.; Schreurs, A.M.M. Intensity evaluation method. J. Appl. Cryst. 2003, 36, 220–229. [Google Scholar]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synthet. Metal. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. ActaCrystallogr. Sect. A 2008, 64, 112–122. [Google Scholar]
- Whangbo, M.-H.; Hoffmann, R. The band structure of the tetracyanoplatinate chain. J. Am. Chem. Soc. 1978, 100, 6093–6098. [Google Scholar]
- Ammeter, J.H.; Bürgi, H.B.; Thibeault, J.C.; Hoffmann, R. Counterintuitive orbital mixing in semiempirical and ab initio molecular orbital calculations. J. Am. Chem. Soc. 1978, 100, 3686–3692. [Google Scholar]
- Pénicaud, A.; Boubekeur, K.; Batail, P.; Canadell, E.; Auban-Senzier, P.; Jérome, D. Hydrogen-bond tuning of macroscopic transport properties from the neutral molecular component site along the series of metallic organic-inorganic solvates (BEDT-TTF)4Re6Se5Cl9.[guest], [guest = DMF, THF, dioxane]. J. Am. Chem. Soc. 1993, 115, 4101–4112. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zorina, L.; Simonov, S.; Canadell, E.; Shibaeva, R. Single-Crystal-to-Single-Crystal Transformation from δ-(BEDT-TTF)4[OsNOCl5]1.33(C6H5NO2)0.67 to β"-(BEDT-TTF)3[OsNOCl5]. Crystals 2012, 2, 627-642. https://doi.org/10.3390/cryst2020627
Zorina L, Simonov S, Canadell E, Shibaeva R. Single-Crystal-to-Single-Crystal Transformation from δ-(BEDT-TTF)4[OsNOCl5]1.33(C6H5NO2)0.67 to β"-(BEDT-TTF)3[OsNOCl5]. Crystals. 2012; 2(2):627-642. https://doi.org/10.3390/cryst2020627
Chicago/Turabian StyleZorina, Leokadiya, Sergey Simonov, Enric Canadell, and Rimma Shibaeva. 2012. "Single-Crystal-to-Single-Crystal Transformation from δ-(BEDT-TTF)4[OsNOCl5]1.33(C6H5NO2)0.67 to β"-(BEDT-TTF)3[OsNOCl5]" Crystals 2, no. 2: 627-642. https://doi.org/10.3390/cryst2020627