Halogen Interactions in 2,4,5-Tribromoimidazolium Salts
Abstract
:1. Introduction
2. Results and Discussion

| Compound | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|
| CCDC no. | 881953 | 881952 | 881955 | 881951 | 881954 | 881956 | 881957 | 881958 |
| Chemical formula | C6H3Br3N2 | C7H8Br3N2·BF4 | C7H6Br3N2·BF4 | C8H10Br3N2·F6P | C8H8Br3N2·F6P | C7H6Br3N2·C2F6NO4S2 | C8H8Br5N2·F6P | C8H8Br5N2·Br |
| Mr | 342.83 | 446.69 | 444.68 | 518.88 | 516.86 | 638.02 | 676.68 | 611.62 |
| Crystal size/mm3 | 0.48 × 0.08 × 0.08 | 0.28 × 0.24 × 0.20 | 0.27 × 0.24 × 0.17 | 0.32 × 0.20 × 0.02 | 0.16 × 0.12 × 0.01 | 0.24 × 0.24 × 0.02 | 0.28 × 0.20 × 0.08 | 0.32 × 0.08 × 0.08 |
| Crystal system | Orthorhombic | Tetragonal | Tetragonal | Monoclinic | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | Pbc21 | I41/a | I41/a | P21/n | P  | P  | P  | P  |
| a/Å | 7.1109(2) | 23.6138(3) | 23.4674(4) | 7.5434(3) | 8.2368(5) | 7.5438(4) | 7.6729(4) | 7.3200(7) |
| b/Å | 7.8014(2) | 23.6138(3) | 23.4674(4) | 15.5493(7) | 13.4522(8) | 8.6311(5) | 7.8858(5) | 11.5867(12) |
| c/Å | 15.6397(5) | 18.8371(4) | 18.6531(4) | 12.7552(4) | 13.6605(6) | 15.9716(7) | 15.8383(7) | 18.1972(16) |
| α/° | 90 | 90 | 90 | 90 | 92.476(4) | 75.020(4) | 90.001(4) | 87.611(8) |
| β/° | 90 | 90 | 90 | 90.198(3) | 100.965(4) | 87.083(4) | 88.104(4) | 81.121(8) |
| γ/° | 90 | 90 | 90 | 90 | 92.884(5) | 66.580(5) | 62.169(8) | 86.375(8) |
| V/Å3 | 867.61(4) | 10503.8(3) | 10272.6(3) | 1496.11(10) | 1481.96(14) | 920.25(8) | 846.88(9) | 1521.0(3) |
| Z | 4 | 32 | 32 | 4 | 4 | 2 | 2 | 4 |
| Dx/g·cm−3 | 2.63 | 2.26 | 2.30 | 2.30 | 2.32 | 2.30 | 2.65 | 2.67 |
| μ/mm−1 | 13.88 | 9.24 | 9.45 | 8.25 | 11.70 | 6.88 | 12.01 | 15.81 |
| F(000)/e | 632 | 6720 | 6656 | 984 | 976 | 608 | 628 | 1120 |
| sin θmax/λ | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.67 | 0.60 |
| h, k, l range | −8 ≤ h ≤ 8 | −28 ≤ h ≤ 26 | −28 ≤ h ≤ 24 | −9 ≤ h ≤ 8 | −9 ≤ h ≤ 9 | −9 ≤ h ≤ 6 | −9 ≤ h ≤ 10 | −8 ≤ h ≤ 8 |
| −9 ≤ k ≤ 9 | −28 ≤ k ≤ 28 | −28 ≤ k ≤ 23 | −18 ≤ k ≤ 15 | −16 ≤ k ≤ 15 | −10 ≤ k ≤ 9 | −10 ≤ k ≤ 10 | −12 ≤ k ≤ 13 | |
| −18 ≤ l ≤ 15 | −14 ≤ l ≤ 22 | −22 ≤ l ≤ 21 | −15 ≤ l ≤ 15 | −16 ≤ l ≤ 12 | −19 ≤ l ≤ 19 | −20 ≤ l ≤ 14 | −21 ≤ l ≤ 18 | |
| Absorption correction | Multi-scan | Multi-scan | Analytical | Multi-scan | Multi-scan | Gaussian | Multi-scan | Multi-scan |
| Measured reflections | 4789 | 32072 | 31973 | 9203 | 16144 | 5550 | 5619 | 12598 |
| Independent reflections | 1276 | 4811 | 4684 | 2715 | 5289 | 3329 | 3506 | 5551 |
| ( Rint) | (0.031) | (0.052) | (0.068) | (0.066) | (0.038) | (0.040) | (0.023) | (0.052) |
| Observed reflections | 1243 | 3786 | 3838 | 2078 | 4748 | 2302 | 2920 | 4184 |
| [ I ≥ 2 σ ( I )] | ||||||||
| Restraints/parameters | 1/104 | 10/315 | 14/315 | 15/188 | 0/363 | 1/243 | 7/204 | 0/291 |
| R1/wR2 [I ≥ 2σ(I)] | 0.019/0.042 | 0.043/0.097 | 0.044, 0.103 | 0.055/0.121 | 0.039/0.102 | 0.039/0.091 | 0.043/0.102 | 0.040/0.062 |
| R1/wR2 (all data) | 0.021/0.043 | 0.060/0.105 | 0.057, 0.110 | 0.0810.138 | 0.043/0.106 | 0.061/0.095 | 0.057/0.110 | 0.065/0.071 |
| Goodness of fit | 1.09 | 1.04 | 1.07 | 1.04 | 1.03 | 0.94 | 1.07 | 1.00 |
| ∆ ρmax/min/e Å−3 | 0.53/−0.48 | 1.59/−0.76 | 1.96/−1.03 | 1.26/−0.88 | 1.82/−0.79 | 1.01/−0.88 | 1.73/−1.49 | 0.86/−0.77 |
| Compound | Interaction | Distance (d/Å) | d − ∑rvdW (Å) | Angle (°) | Symmetry Operator |
|---|---|---|---|---|---|
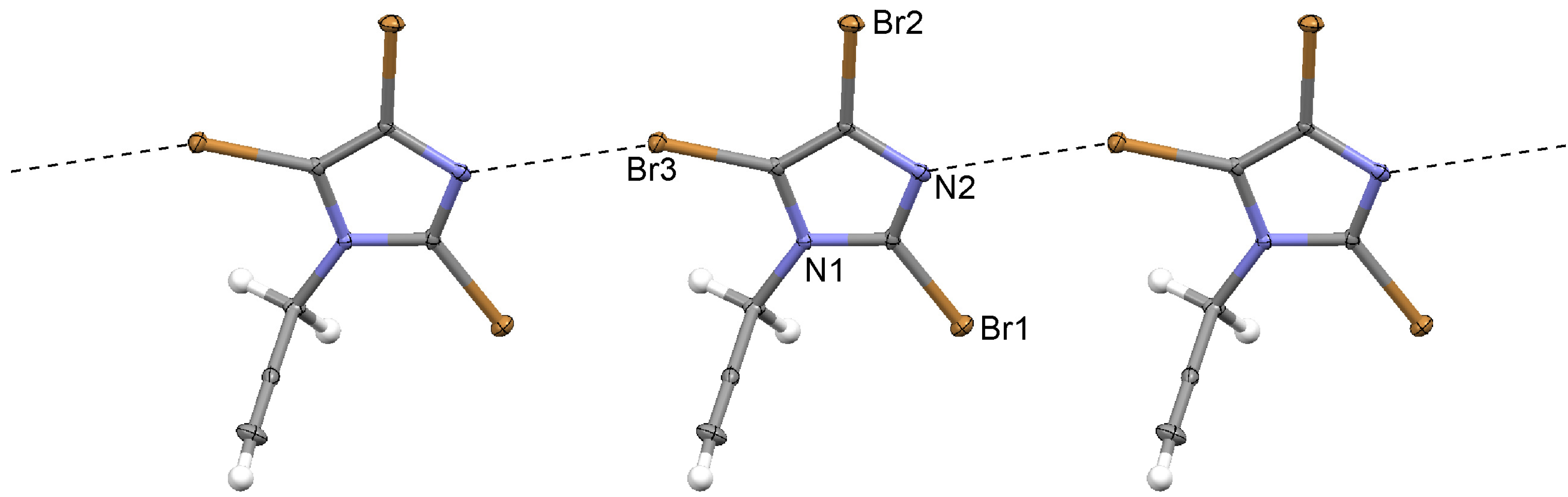
| 2 | C–Br3...N2 | 3.089(4) | −0.311 | 159.8(2) | 1 + x, y, z |
| 3 | C–Br11...F5 | 2.869(5) | −0.451 | 166.1(2) | x, y, z |
| C–Br31...F7 | 2.869(4) | −0.451 | 168.9(2) | −1/2 + x, y, 1/2 − z | |
| C–Br21...F4 | 2.906(5) | −0.414 | 179.5(2) | 3/4 + y, 3/4 − x, −1/4 + z | |
| C–Br12...F3 | 3.041(6) | −0.279 | 158.1(2) | x, y, z | |
| C–Br22...F8 | 3.090(5) | −0.230 | 172.2(2) | 5/4 + y, 5/4 − x, 1/4 + z | |
| C–Br32...F3 | 3.137(6) | −0.183 | 147.4(2) | 5/4 + y, 5/4 − x, 1/4 + z | |
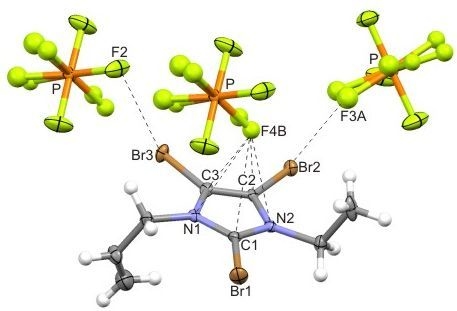
| 4 | C–Br22...F2 | 2.822(5) | −0.498 | 167.8(2) | 1/4 + y, 1/4 − x, 1/4 + z |
| C–Br12...F5 | 2.826(6) | −0.494 | 166.1(2) | −1/2 + x, y, 3/2 − z | |
| C–Br32...F6 | 2.944(5) | −0.376 | 165.3(2) | x, y, z | |
| C–Br11...F3 | 2.972(7) | −0.348 | 161.7(2) | 1 − x, −y, 1 − z | |
| C–Br21...F7 | 3.094(5) | −0.226 | 167.2(2) | 1/4 − y, −1/4 + x, −1/4 + z | |
| C–Br32...F7 | 3.141(7) | −0.179 | 151.6(2) | x, y, z | |
| C–Br31...F3 | 3.244(7) | −0.076 | 152.2(2) | 1/4 + y, 3/4 − x, 3/4 − z | |
| 5 | C–Br2...F3A | 2.927(10) | −0.393 | 161.5(4) | 1 − x, −y, 2 − z |
| C–Br3...F2 | 2.989(5) | −0.331 | 160.0(3) | 3/2 + x, 1/2 − y, 1/2 + z | |
| 6 | C–Br21...F13 | 3.010(3) | −0.310 | 160.9(1) | 1 − x, 1 − y, 1 − z |
| C–Br31...F22 | 3.060(4) | −0.260 | 161.1(2) | x, y, z | |
| C–Br22...F14 | 3.084(3) | −0.236 | 158.9(1) | x, y, z | |
| C–Br11...F23 | 3.157(5) | −0.163 | 154.9(2) | x, y, 1 + z | |
| C–Br12...F21 | 3.209(6) | −0.111 | 127.0(2) | 1 − x, −y, −z | |
| C–Br32...F15 | 3.255(4) | −0.065 | 171.0(1) | 1 − x, 1 − y, 1 − z | |
| 7 | C–Br3...O3 | 2.943(4) | −0.427 | 167.1(2) | −1 + x, 1 + y, z |
| C–Br2...O3 | 3.201(3) | −0.169 | 152.9(2) | 1 − x, 1 − y, 1 − z | |
| C–H6...O1 | 2.32(5) | −0.402 | 160(5) | −x, 1 − y, 2 − z | |
| C–F3...F3 | 2.700(7) | −0.240 | 163.7(4) | 1 − x, 1 − y, 2 − z | |
| 8 | C–Br3...F2 | 3.017(6) | −0.303 | 173.6(2) | x, 1 + y, z |
| C–Br5...Br5 | 3.517(1) | −0.183 | 143.6(2) | 3 − x, −y, 2 − z | |
| C–Br2...Br2 | 3.575(1) | −0.125 | 129.6(2) | 2 − x, 1 − y, 1 − z | |
| C–H6...F6 | 2.38(3) | −0.290 | 164(4) | x, y, z | |
| C–H6...F2 | 2.50(6) | −0.174 | 140(4) | x, y, z | |
| 9 | C–Br1...Br6 | 3.032(1) | −0.668 | 177.7(2) | 1 − x, −y, −z |
| C–Br7...Br12 | 3.092(1) | −0.608 | 177.6(2) | 1 − x, 1 − y, 1 − z | |
| C–Br8...Br6 | 3.201(1) | −0.499 | 176.6(2) | 1 + x, y, z | |
| C–Br9...Br12 | 3.230(1) | −0.470 | 175.2(2) | 1 − x, −y, 1 − z | |
| C–Br3...Br12 | 3.291(1) | −0.409 | 175.6(2) | x, y, z | |
| C–Br4...Br4 | 3.474(1) | −0.226 | 152.9(2) | 2 − x, −1 − y, −z | |
| C–H8...Br6 | 2.756(1) | −0.294 | 148.3(4) | x, y, z | |
| C–H16...Br12 | 2.812(1) | −0.238 | 155.1(4) | x, y, z |
| Compound | Interaction | Distance (d/Å) | d − ∑rvdW (Å) | Symmetry Operator |
|---|---|---|---|---|
| 3 | C11...F5 | 2.900(7) | −0.270 | 3/4 + y, 5/4 − x, 1/4 − z |
| C21...F5 | 3.084(7) | −0.086 | 3/4 + y, 5/4 − x, 1/4 − z | |
| N21...F5 | 2.935(6) | −0.085 | 3/4 + y, 5/4 − x, 1/4 − z | |
| C31...F5 | 3.137(7) | −0.033 | 3/4 + y, 5/4 − x, 1/4 − z | |
| N11...F5 | 3.025(7) | +0.005 | 3/4 + y, 5/4 − x, 1/4 − z | |
| centroid...F5 | 2.790 | |||
| 4 | C32...F5 | 3.162(9) | −0.008 | 1/4 − y, −1/4 + x, −1/4 + z |
| N22...F5 | 2.957(7) | −0.063 | 1/4 − y, −1/4 + x, −1/4 + z | |
| C12...F5 | 2.940(8) | −0.230 | 1/4 − y, −1/4 + x, −1/4 + z | |
| C22...F5 | 3.109(8) | −0.061 | 1/4 − y, −1/4 + x, −1/4 + z | |
| N12...F5 | 3.073(8) | +0.053 | 1/4 − y, −1/4 + x, −1/4 + z | |
| centroid...F5 | 2.824 | |||
| 5 | C3...F4B | 3.10(1) | –0.068 | 1/2 + x, 1/2 − y, 1/2 + z |
| C1...F4B | 3.11(1) | –0.055 | 1/2 + x, 1/2 − y, 1/2 + z | |
| C2...F4B | 3.13(1) | –0.044 | 1/2 + x, 1/2 − y, 1/2 + z | |
| N1...F4B | 3.10(1) | +0.080 | 1/2 + x, 1/2 − y, 1/2 + z | |
| N2...F4B | 3.16(1) | +0.140 | 1/2 + x, 1/2 − y, 1/2 + z | |
| centroid...F4B | 2.901 | |||
| 7 | C3...O2 | 2.901(6) | −0.319 | x, y, z |
| C2...O2 | 3.054(7) | −0.166 | x, y, z | |
| N1...O2 | 3.041(7) | −0.029 | x, y, z | |
| C1...O2 | 3.255(8) | +0.035 | x, y, z | |
| N2...O2 | 3.278(7) | +0.208 | x, y, z | |
| centroid...O2 | 2.887 |
| Interaction | Compound | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3A | 3B | 4A | 4B | 5 | 6A | 6B | 7 | 8 | 9A | 9B | |
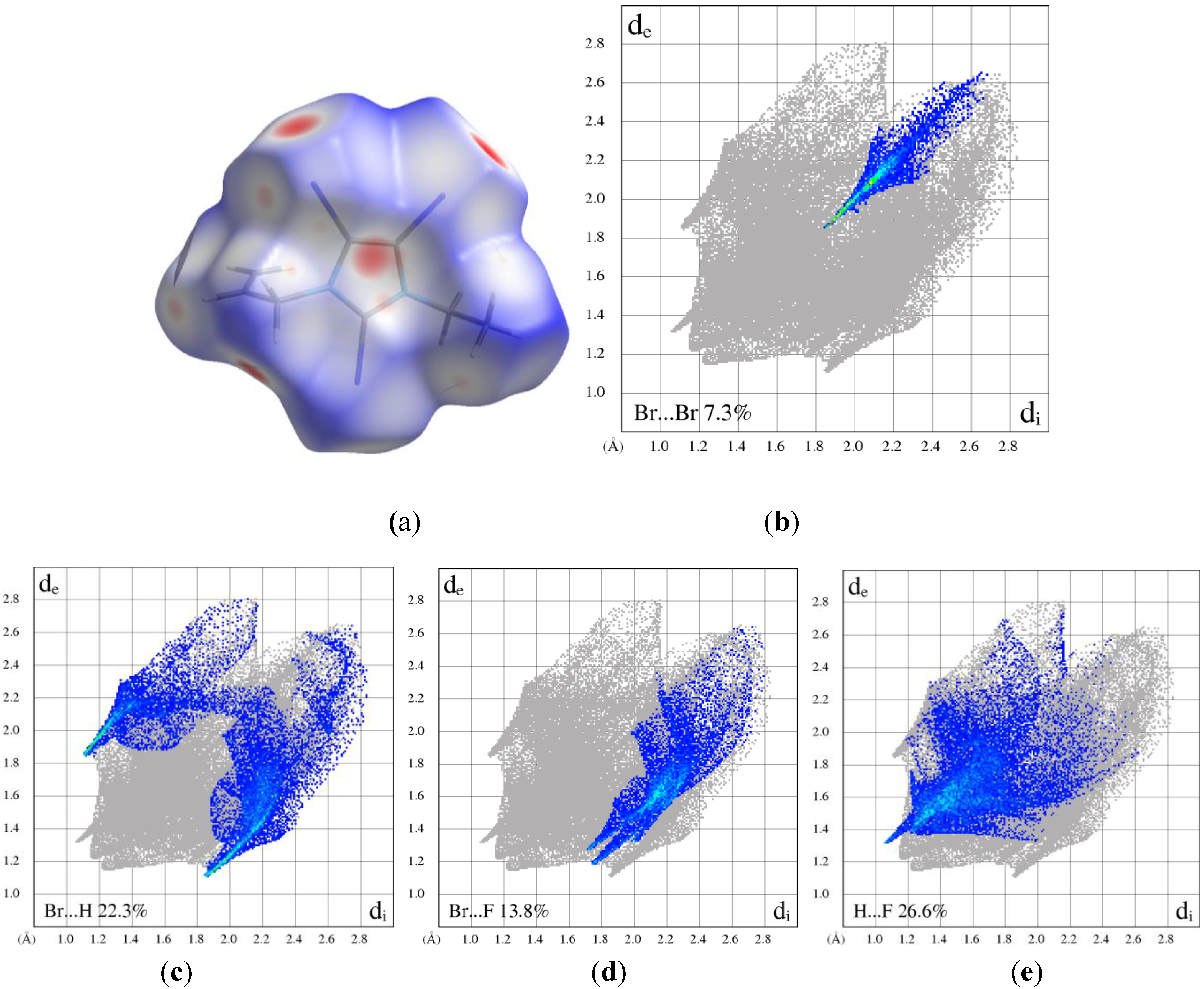
| Br...Br | 12.0 | 9.4 | 9.5 | 11.1 | 10.1 | 7.3 | 5.8 | 5.2 | 5.6 | 19.0 | 22.4 | 22.7 |
| Br...H/H...Br | 36.3 | 24.4 | 27.0 | 23.1 | 21.8 | 22.3 | 20.5 | 18.5 | 13.5 | 29.2 | 54.7 | 55.4 |
| Br...C/C...Br | 18.8 | 1.6 | 0.9 | 1.8 | 4.2 | 1.1 | 3.2 | 3.5 | 4.0 | 5.9 | 8.9 | 6.9 |
| Br...N/N...Br | 10.5 | 0.2 | 0.2 | 0.2 | 0.4 | 0 | 0 | 0 | 0.2 | 0 | 1.3 | 1.3 |
| Br...O | - | - | - | - | - | - | - | - | 8.9 | - | - | - |
| Br...F | - | 13.3 | 13.2 | 16.5 | 13.7 | 13.8 | 17.5 | 15.8 | 14.4 | 13.7 | - | - |
| C...H/H...C | 12.9 | 4.0 | 5.1 | 16.6 | 9.9 | 1.7 | 15.2 | 12.4 | 0.7 | 0 | 3.2 | 4.5 |
| C...O | - | - | - | - | - | - | - | - | 6.3 | - | - | - |
| C...F | - | 4.9 | 4.5 | 7.6 | 9.2 | 5.1 | 5.3 | 5.8 | 6.8 | 4.4 | - | - |
| H...F | - | 20.9 | 16.8 | 7.6 | 16.3 | 26.6 | 18.7 | 24.7 | 17.2 | 19.6 | - | - |
| H...H | 1.1 | 19.1 | 20.6 | 5.5 | 11.8 | 20.4 | 11.4 | 11.7 | 4.2 | 6.5 | 9.6 | 9.1 |
| H...O | - | - | - | - | - | - | - | - | 11.9 | - | - | - |
2.1. 2,4,5-Tribromo-1-(prop-2-ynyl)imidazole (2)
2.2. 2,4,5-Tribromo-3-methyl-1-(prop-2-enyl)imidazolium Tetrafluoroborate (3)
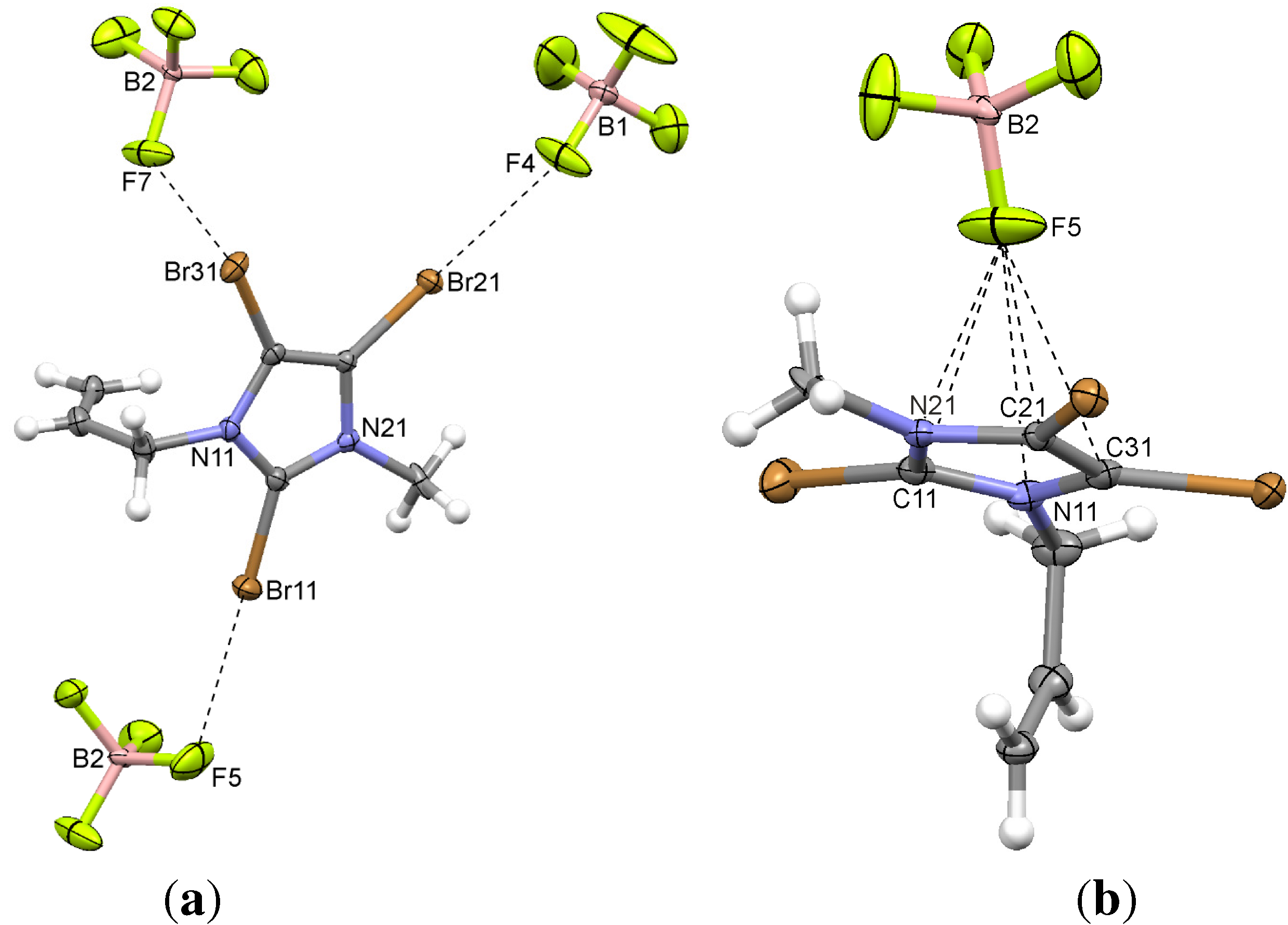
2.3. 2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium Tetrafluoroborate (4)

2.4. 2,4,5-Tribromo-3-ethyl-1-(prop-2-enyl)imidazolium Hexafluorophosphate (5)
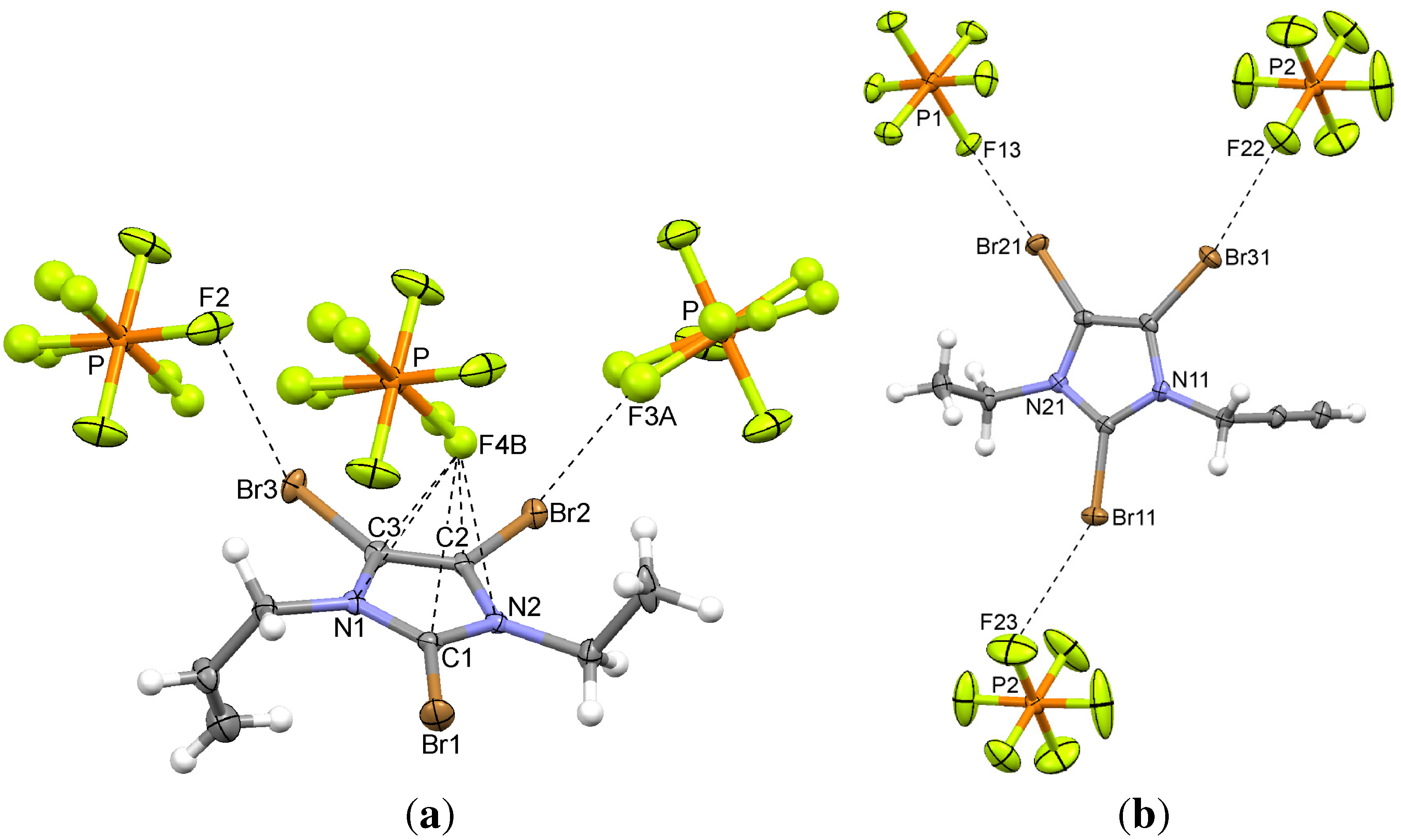
2.5. 2,4,5-Tribromo-3-ethyl-1-(prop-2-ynyl)imidazolium Hexafluorophosphate (6)
2.6. 2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium Triflimide (7)
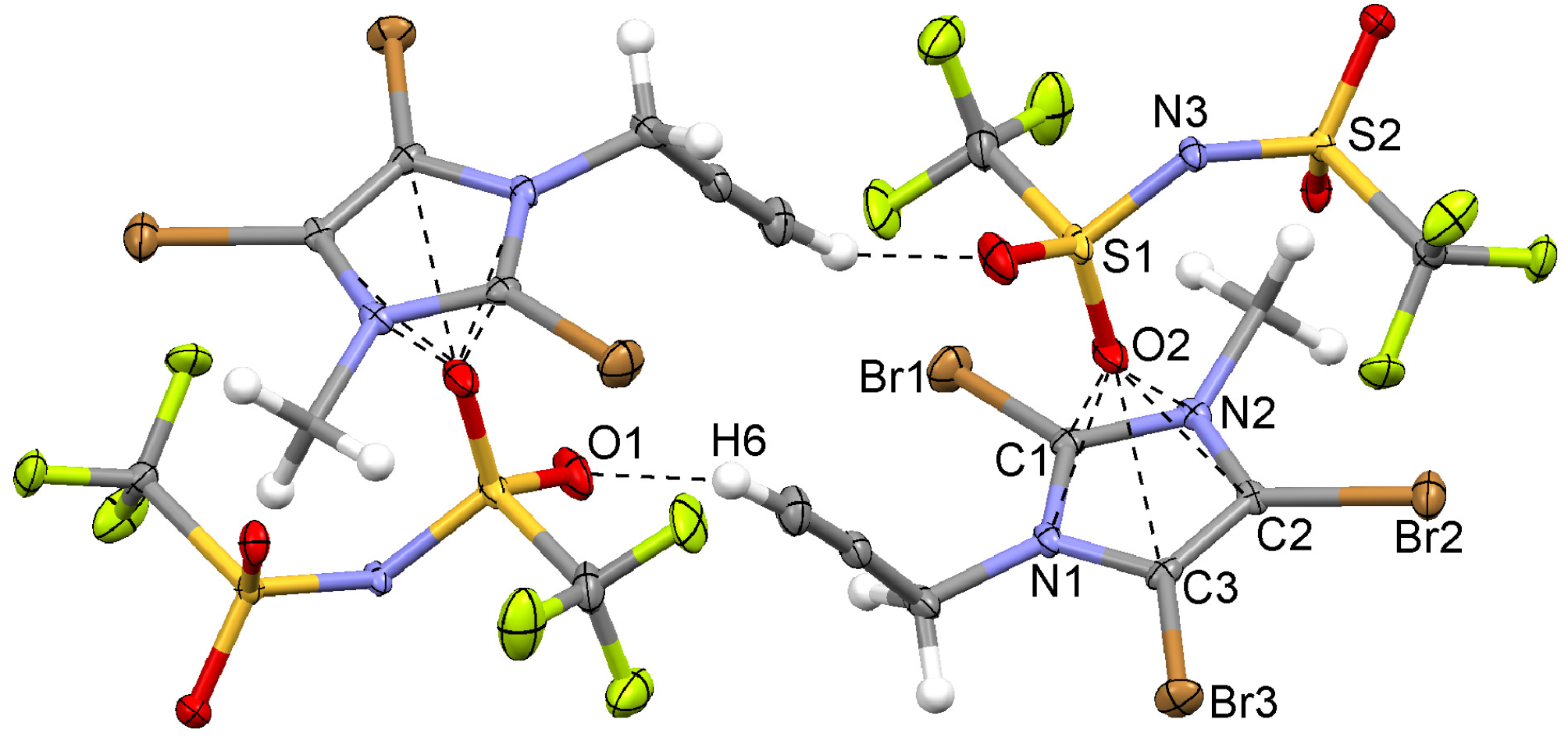
2.7. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Hexafluorophosphate (8)
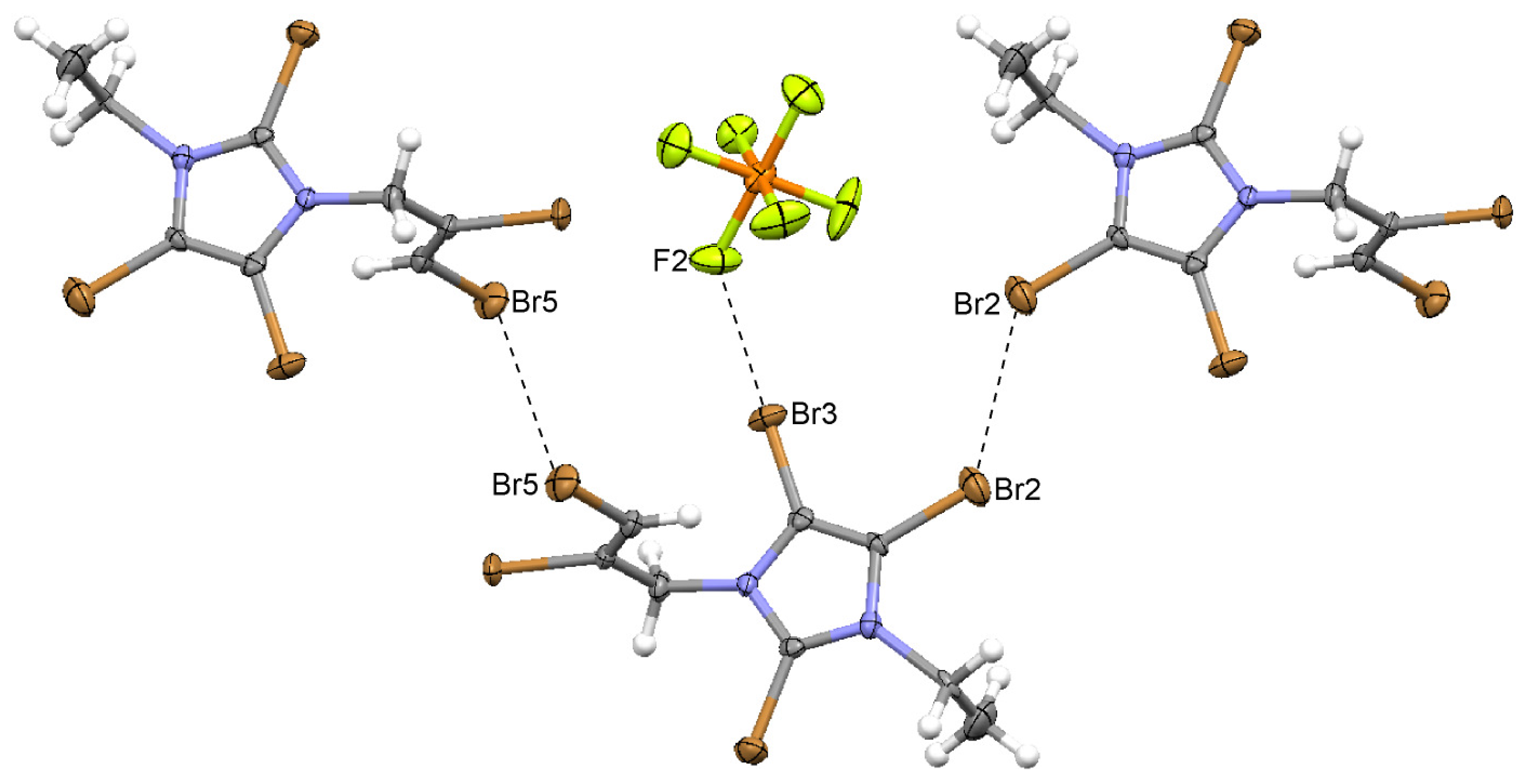
2.8. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Bromide (9)
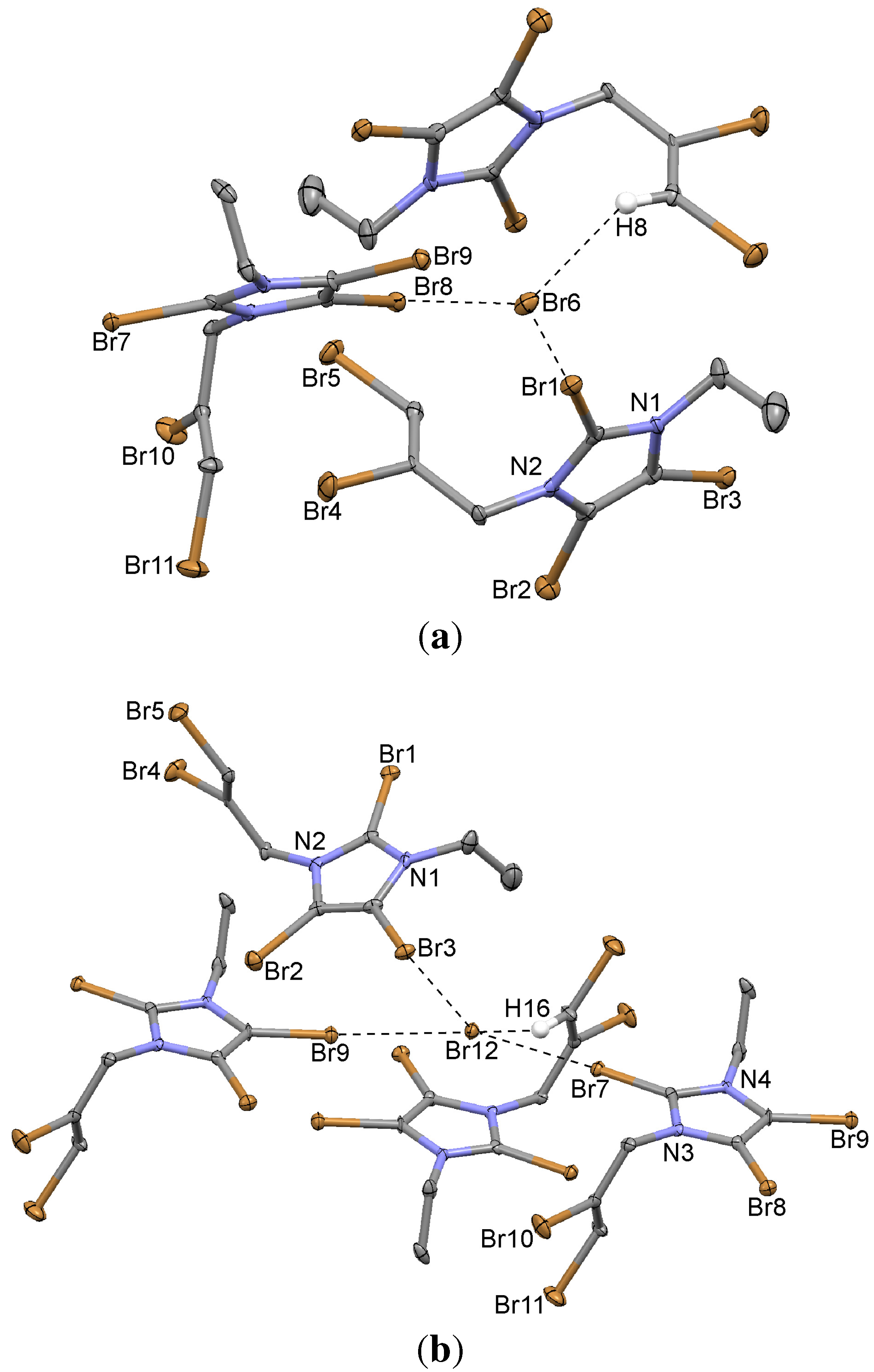
3. Experimental Section
3.1. Data of 2,4,5-Tribromo-1-(Prop-2-Ynyl)Imidazole (2)
3.2. General Procedure for the Alkylation of 1-Alkylimidazoles (3–6)
3.3. 2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium Triflimide (7)
3.4. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Hexafluorophosphate (8)
3.5. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Bromide (9)
3.6. X-ray Crystal Structure Determination
4. Conclusions
Acknowledgments
References
- Wyss, G. Zur Kenntnis des Glyoxalins. Chem. Ber. 1877, 10, 1365–1375. [Google Scholar]
- Balaban, I.E.; Pyman, F.L. Bromo-Derivatives of Glyoxaline. J. Chem. Soc. Trans. 1922, 121, 947–958. [Google Scholar]
- Chaudhuri, M.K.; Khan, A.T.; Patel, B.K.; Dey, D.; Kharmawophlang, W.; Lakshmiprabha, T.R.; Mandal, G.C. An environmentally benign synthesis of organic ammonium tribromides (OATB) and bromination of selected organic substrates by tetrabutylammonium tribromide (TBATB). Tetrahedron Lett. 1998, 39, 8163–8166. [Google Scholar]
- Bahnous, M.; Mouats, C.; Fort, Y.; Gros, P.C. Convenient multi-gram scale synthesis of polybrominated imidazoles building blocks. Tetrahedron Lett. 2006, 47, 1949–1951. [Google Scholar]
- Muathen, H.A. 1,8-Diazabicyclo[5.4.0]undec-7-ene hydrobromide perbromide: A new mild stable brominating agent for aromatic compounds. J. Org. Chem. 1992, 57, 2740–2741. [Google Scholar] [CrossRef]
- Nath, J.; Chaudhuri, M.K. Boric acid catalyzed bromination of a variety of organic substrates: An eco-friendly and practical protocol. Green Chem. Lett. Rev. 2008, 1, 223–230. [Google Scholar] [CrossRef]
- Stensiö, K.-E.; Wahlberg, K.; Wahren, R. Synthesis of brominated imidazoles. Acta Chem. Scand. 1973, 27, 2179–2183. [Google Scholar]
- Benkendorff, K.; Pillai, R.; Bremner, J.B. 2,4,5-Tribromo-1H-imidazole in the egg masses of three muricid molluscs. Nat. Prod. Res. 2004, 18, 427–431. [Google Scholar]
- Schulze, H.; Klein, H.P. Fire-retardant polyurethane composition. US Patent 4,032,484, 28 June 1977. [Google Scholar]
- Ye, C.; Shreeve, J.M. Syntheses of very dense halogenated liquids. J. Org. Chem. 2004, 69, 6511–6513. [Google Scholar]
- Yano, T.; Tomioka, H.; Takada, Y.; Takeda, H.; Hirata, N. New trihaloimidazole derivatives possessing a high insecticidal activity against the pyrethroid-resistant colony of the German cockroach, Blattella germanica (Dictyoptera: Blattellidae). Appl. Ent. Zool. 1991, 26, 469–475. [Google Scholar]
- Martin, H.; Pissiotas, G. Ectoparasiticidal 1-alkynyl-2,4,5-trihaloimidazoles. German Patent DE 1950991A.
- Pissiotas, G. Acaricidal 2,4,5-trihaloimidazoles. German Patent DE 2031400 A.
- Draber, W.; Falbe, J.F.; Buechel, K.H.; Korte, F.W.A.G.K. Plant Growth Control. U.S. Patent 3,501,286, 17 March 1970. [Google Scholar]
- Noland, W.E.; Cole, K.P.; Britton, D. 4,5-Dibromo-1-methyl-1H-imidazole. Acta Crystallogr. 2003, E59, o458–o460. [Google Scholar]
- Mukai, T.; Nishikawa, K. Halogen-Bonded and hydrogen-bonded network structures in crystals of 1-propyl- and 1-butyl-4,5-dibromo-3-methylimidazolium bromides. Chem. Lett. 2009, 38, 402–403. [Google Scholar] [CrossRef]
- Mukai, T.; Nishikawa, K. Syntheses and crystal structures of two ionic liquids with halogen-bonding groups: 4,5-dibromo- and 4,5-diiodo-1-butyl-3-methylimidazolium trifluoromethane-sulfonates. Solid State Sci. 2010, 12, 783–788. [Google Scholar] [CrossRef]
- Serpell, C.J.; Kilah, N.L.; Costa, P.J.; Felix, V.; Beer, P.D. Halogen bond anion templated assembly of an imidazolium pseudorotaxane. Angew. Chem. Int. Ed. 2010, 49, 5322–5326. [Google Scholar]
- Blackman, A.G.; Buckingham, D.A.; Clark, C.R.; Simpson, J. Bromination of imidazole complexes of pentaammine-cobalt(III). Synthesis, structure and reactivity. J. Chem. Soc. Dalton Trans. 1991, 3031–3041. [Google Scholar]
- Hassel, O. Structural aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar]
- Awwadi, F.F.; Willett, R.D.; Peterson, K.A.; Twamley, B. The nature of halogen...halogen synthons: Crystallographic and theoretical studies. Chem. Eur. J. 2006, 12, 8952–8960. [Google Scholar] [CrossRef]
- Dikundwar, A.G.; Guru Row, T.N. Evidence for the “amphoteric” nature of fluorine in halogen bonds: An instance of Cl...F contact. Cryst. Growth Des. 2012, 12, 1713–1716. [Google Scholar]
- Kubicki, M. Structural phase transitions, and Br...N and Br...Br interactions in 1-phenyl-2-methyl-4-nitro-5-bromoimidazole. Acta Crystallogr. 2004, B60, 333–342. [Google Scholar]
- Valkonen, J.; Pitkanen, I.; Pajunen, A. Molecular and crystal structure and IR spectrum of 3,5-Dibromo-1,2,4-triazole. Acta Chem. Scand. 1985, A39, 711–716. [Google Scholar]
- Ansell, G.B. Tetrazole studies. Part II. Crystal structure of 5-bromotetrazole. J. Chem. Soc. Perkin Trans. 2 1973, 2036–2038. [Google Scholar] [CrossRef]
- Eißmann, F.; Schindler, D.; Weber, E. X-ray crystal structures of halogen containing nucleobase derivatives in unsolvated and DMSO solvated forms. Struct. Chem. 2010, 21, 245–254. [Google Scholar] [CrossRef]
- Chopra, D.; Guru Row, T.N. Role of organic fluorine in crystal engineering. CrystEngComm. 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- D’Oria, E.; Novoa, J.J. On the hydrogen bond nature of the C-H...F interactions in molecular crystals. An exhaustive investigation combining a crystallographic database search and ab initio theoretical calculations. CrystEngComm 2008, 10, 423–436. [Google Scholar]
- Cole, M.L.; Jones, C.; Junk, P.C. Studies of the reactivity of N-heterocyclic carbenes with halogen and halide sources. New J. Chem. 2002, 26, 1296–1303. [Google Scholar] [CrossRef]
- Laus, G.; Schwärzler, A.; Schuster, P.; Bentivoglio, G.; Hummel, M.; Wurst, K.; Kahlenberg, V.; Lörting, T.; Schütz, J.; Peringer, P.; et al. N,N'-Di(alkyloxy)imidazolium salts: New patent-free ionic liquids and NHC precatalysts. Z. Naturforsch. 2007, B62, 295–308. [Google Scholar]
- Kuhn, N.; Abu-Rayyan, A.; Eichele, K.; Schwarz, S.; Steimann, M. Weak interionic interactions in 2-bromoimidazolium derivatives. Inorg. Chim. Acta 2004, 357, 1799–1804. [Google Scholar] [CrossRef]
- Sünkel, K.; Bernhartzeder, S. Coordination chemistry of perhalogenated cyclopentadienes and alkynes. XXVIII new high-yield synthesis of monobromoferrocene and simplified procedure for the synthesis of pentabromoferrocene. Molecular structures of 1,2,3-tribromoferrocene and 1,2,3,4,5-pentabromoferrocene. J. Organomet. Chem. 2011, 696, 1536–1540. [Google Scholar]
- Estarellas, C.; Bauza, A.; Frontera, A.; Quiñonero, D.; Deya, P.M. On the directionality of anion–π interactions. Phys. Chem. Chem. Phys. 2011, 13, 5696–5702. [Google Scholar]
- Robertazzi, A.; Krull, F.; Knapp, E.-W.; Gamez, P. Recent advances in anion-πinteractions. CrystEngComm 2011, 13, 3293–3300. [Google Scholar] [CrossRef]
- Pointer, D.J.; Wilford, J.B. Crystal and molecular structure of 3,3'-thio-bis-(2-methyl-1-phenylimidazo-[1,5-a]pyridinium) bistetrafluoroborate; a potent new sulphur-containing curariform agent. J. Chem. Soc. Chem. Commun. 1978, 816–817. [Google Scholar]
- Frank, B.; Beckert, R.; Rau, S.; Görls, H. 2-Azaanthraquinones: Building blocks for new ring-fused imidazoles and their transformation into benzo[f]isoindole-4,9-diones. Z. Naturforsch. 2005, B60, 771–779. [Google Scholar]
- Kunz, D.; Deißler, C.; Gierz, V.; Rominger, F.; Oeser, T. Reduction of dipyrido ureas via 6-alkyloxydipyrido[1,2-c;2',1'-e]imidazolium salts. Z. Naturforsch. 2010, B65, 861–872. [Google Scholar]
- Brown, D.H.; Skelton, B.W. Nickel complexes of a bis(benzimidazolin-2-ylidene)pyridine pincer ligand with four- and five-coordinate geometries. Dalton Trans. 2011, 40, 8849–8858. [Google Scholar] [CrossRef]
- Laus, G.; Wurst, K.; Kahlenberg, V.; Kopacka, H.; Kreutz, C.; Schottenberger, H. N-Heterocyclic carbene (NHC) derivatives of 1,3-di(benzyloxy)imidazolium salts. Z. Naturforsch. 2010, B65, 776–782. [Google Scholar]
- Scheele, U.J.; Dechert, S.; Meyer, F. Bridged dinucleating N-heterocyclic carbene ligands and their double helical mercury(II) complexes. Inorg. Chim. Acta 2006, 359, 4891–4900. [Google Scholar] [CrossRef]
- Weigand, J.J.; Feldmann, K.-O.; Henne, F.D. arbene-Stabilized phosphorus(III)-centered cations [LPX2]+ and [L2PX]2+ (L = NHC; X = Cl, CN, N3). J. Am. Chem. Soc. 2010, 132, 16321–16323. [Google Scholar]
- Maas, G.; Stang, P.J. Structures of dication ethers. Crystal and molecular structures of bis(1,3-dimethyl-2-imidazoliniumyl) ether ditriflate and bis[1,2-bis(dimethylamino)-3-cyclopropenyl-iumyl] ether ditriflate. J. Org. Chem. 1983, 48, 3038–3043. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. Cryst. Eng. Comm. 2008, 10, 377–388. [Google Scholar]
- Dean, P.M.; Pringle, J.M.; Forsyth, C.M.; Scott, J.L.; MacFarlane, D.R. Interactions in bisamide ionic liquids—insights from a Hirshfeld surface analysis of their crystalline states. New J. Chem. 2008, 32, 2121–2126. [Google Scholar] [CrossRef]
- Albrecht, M.; Müller, M.; Mergel, O.; Rissanen, K.; Valkonen, A. CH-Directed anion–π interactions in the crystals of pentafluorobenzyl-substituted ammonium and pyridinium salts. Chem. Eur. J. 2010, 16, 5062–5069. [Google Scholar] [CrossRef]
- Laus, G.; Hummel, M.; Többens, D.M.; Gelbrich, T.; Kahlenberg, V.; Wurst, K.; Griesser, U.J.; Schottenberger, H. The 1:1 and 1:2 salts of 1,4-diazabicyclo[2.2.2]octane and bis(trifluoro-methylsulfonyl)amine: Thermal behaviour and polymorphism. CrystEngComm 2011, 13, 5439–5446. [Google Scholar] [CrossRef]
- Lin, B.-L.; Kang, P.; Stack, T.D.P. Unexpected Ccarbene-X (X: I, Br, Cl) reductive elimination from N-heterocyclic carbene copper halide complexes under oxidative conditions. Organometallics 2010, 29, 3683–3685. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sproll, S.M. Nitrogen-Rich polymers based on 5-bromo-1-vinyl-1H-tetrazole. Eur. J. Org. Chem. 2010, 1169–1175. [Google Scholar]
- Linninger, C.S.; Herdtweck, E.; Hoffmann, S.D.; Herrmann, W.A.; Kühn, F.E. A new palladium(II) complex of a functionalized N-heterocyclic carbene: Synthesis, characterization and application in Suzuki-Miyaura cross-coupling reactions. J. Mol. Struct. 2008, 890, 192–197. [Google Scholar] [CrossRef]
- Domasevitch, K.V.; Boldog, I. Polar hydrogen-bonded organic chains in 4,4'-bipyrazolium bromide and perchlorate monohydrates. Acta Cryst. 2005, C61, o373–o376. [Google Scholar]
- Peters, K.; Peters, E.M.; Reinhardt, R.; Quast, H. Crystal structure of (1R*,7S*,9S*,15R*)-2,6, 10,14-tetraazatricyclo[13.1.0.07,9]hexadeca-2,4,10,12-tetraene bis(hydrobromide). Z. Krist. New Cryst. Struct. 1998, 213, 609–610. [Google Scholar]
- Gelders, Y.G.; De Ranter, C.J.; Van Rooijen-Reiss, C. 7,8-Didehydro-4,5-epoxy-17-(2-propenyl)morphinan-3,6-diol hydrobromide [nalorphine hydrobromide]. Cryst. Struct. Commun. 1979, 8, 995–1003. [Google Scholar]
- Seley, K.L.; Januszczyk, P.; Hagos, A.; Zhang, L.; Dransfield, D.T. Synthesis and antitumor activity of thieno-separated tricyclic purines. J. Med. Chem. 2000, 43, 4877–4883. [Google Scholar] [CrossRef]
- Burla, M.C.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G. More power for direct methods: SIR2002. Z. Kristallogr. 2002, 217, 629–635. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Froschauer, C.; Kahlenberg, V.; Laus, G.; Schottenberger, H. Halogen Interactions in 2,4,5-Tribromoimidazolium Salts. Crystals 2012, 2, 1017-1033. https://doi.org/10.3390/cryst2031017
Froschauer C, Kahlenberg V, Laus G, Schottenberger H. Halogen Interactions in 2,4,5-Tribromoimidazolium Salts. Crystals. 2012; 2(3):1017-1033. https://doi.org/10.3390/cryst2031017
Chicago/Turabian StyleFroschauer, Carmen, Volker Kahlenberg, Gerhard Laus, and Herwig Schottenberger. 2012. "Halogen Interactions in 2,4,5-Tribromoimidazolium Salts" Crystals 2, no. 3: 1017-1033. https://doi.org/10.3390/cryst2031017