APA2[Zn3(HPO4)4(H2O)2], a Layered Zincophosphate Featuring Template-to-Framework N–H⋯O and “Synergic” Framework-to-Template O–H⋯N Hydrogen Bonds and C–H⋯O Interactions (APA = 2-Amino-1-phenyleneammonium, C6H9N2+)
Abstract
:1. Introduction
2. Results and Discussion
Crystal Structure of 1
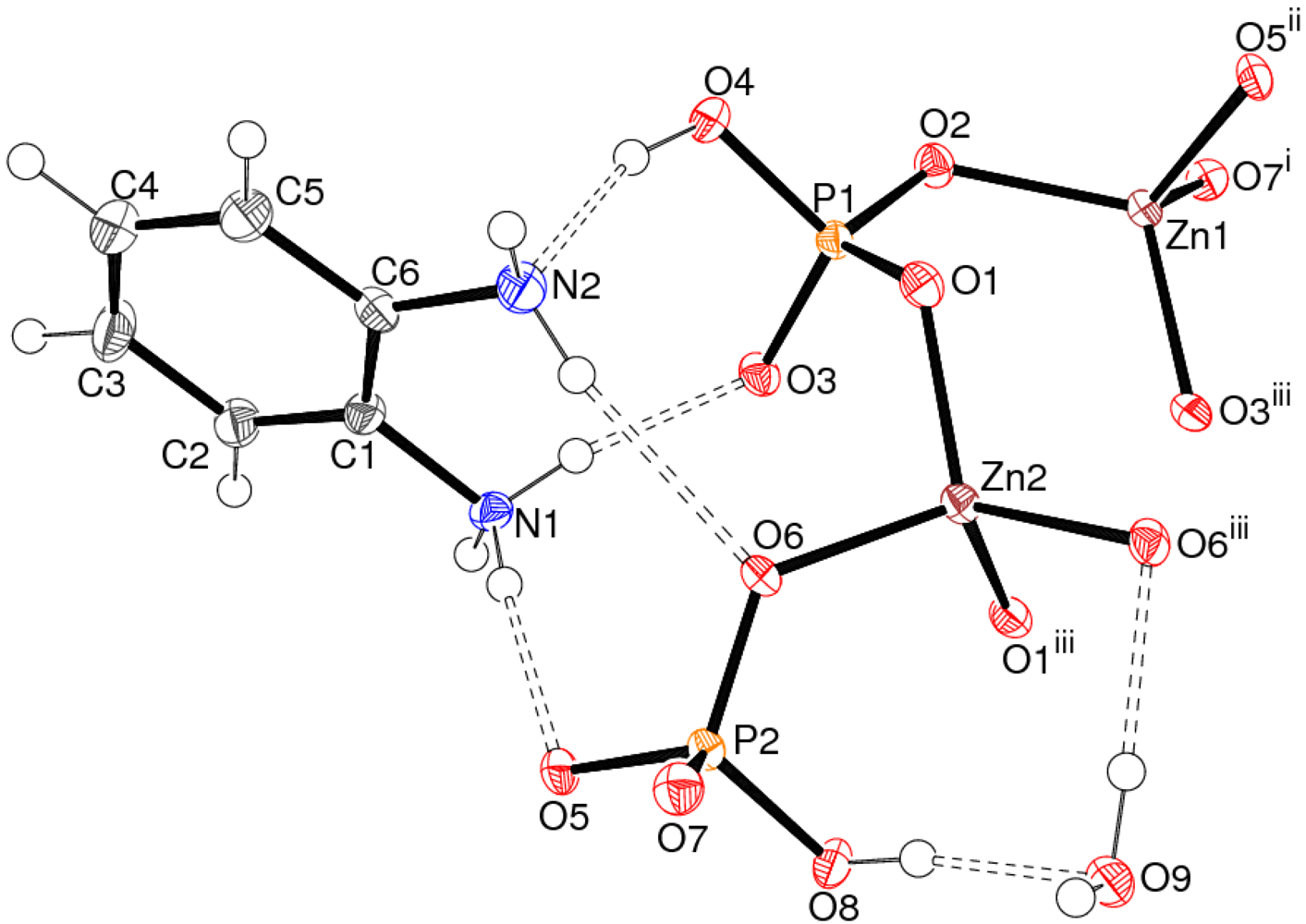
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Zn1–O7 i | 1.916 (3) | Zn1–O5 ii | 1.952 (3) |
| Zn1–O3 iii | 1.962 (3) | Zn1–O2 | 1.965 (3) |
| Zn2–O1 iii | 1.935 (3) | Zn2–O1 | 1.935 (3) |
| Zn2–O6 | 1.959 (3) | Zn2–O6 iii | 1.959 (3) |
| P1–O1 | 1.514 (3) | P1–O2 | 1.527 (3) |
| P1–O3 | 1.535 (3) | P1–O4 | 1.578 (4) |
| P2–O7 | 1.506 (4) | P2–O6 | 1.530 (3) |
| P2–O5 | 1.531 (3) | P2–O8 | 1.577 (3) |
| Bond | Angle | Bond | Angle |
| P1–O1–Zn2 | 119.6 (2) | P1–O2–Zn1 | 119.3 (2) |
| P1–O3–Zn1 iii | 126.6 (2) | P2–O5–Zn1 iv | 125.7 (2) |
| P2–O6–Zn2 | 127.6 (2) | P2–O7–Zn1 v | 145.2 (2) |
| Bond | D-H | H... A | D...A | D-H...A |
|---|---|---|---|---|
| O4-H1p...N2 | 1.00 | 1.86 | 2.826 (5) | 161 |
| O8-H2p...O9 | 0.83 | 1.74 | 2.558 (5) | 170 |
| O9-H1w...O6 iii | 0.91 | 1.92 | 2.819 (5) | 170 |
| O9-H2w...O2 v | 0.88 | 1.87 | 2.723 (5) | 162 |
| N1-H1a...O3 | 0.91 | 1.91 | 2.803 (5) | 166 |
| N1-H1b...O8 vi | 0.91 | 1.97 | 2.817 (5) | 154 |
| N1-H1c...O5 | 0.91 | 1.93 | 2.825 (5) | 166 |
| N2-H2b...O6 | 0.88 | 2.30 | 3.165 (5) | 169 |
| C4-H4...O7 vii | 0.95 | 2.50 | 3.394 (6) | 157 |
| C5-H5...O2 viii | 0.95 | 2.53 | 3.421 (6) | 157 |
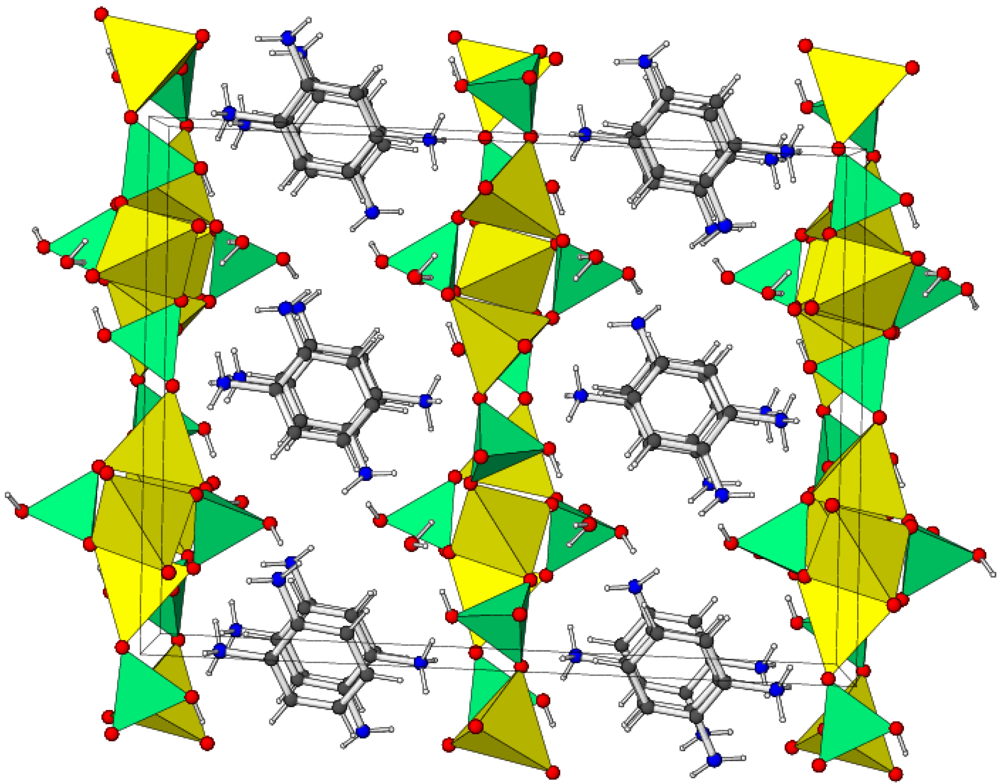

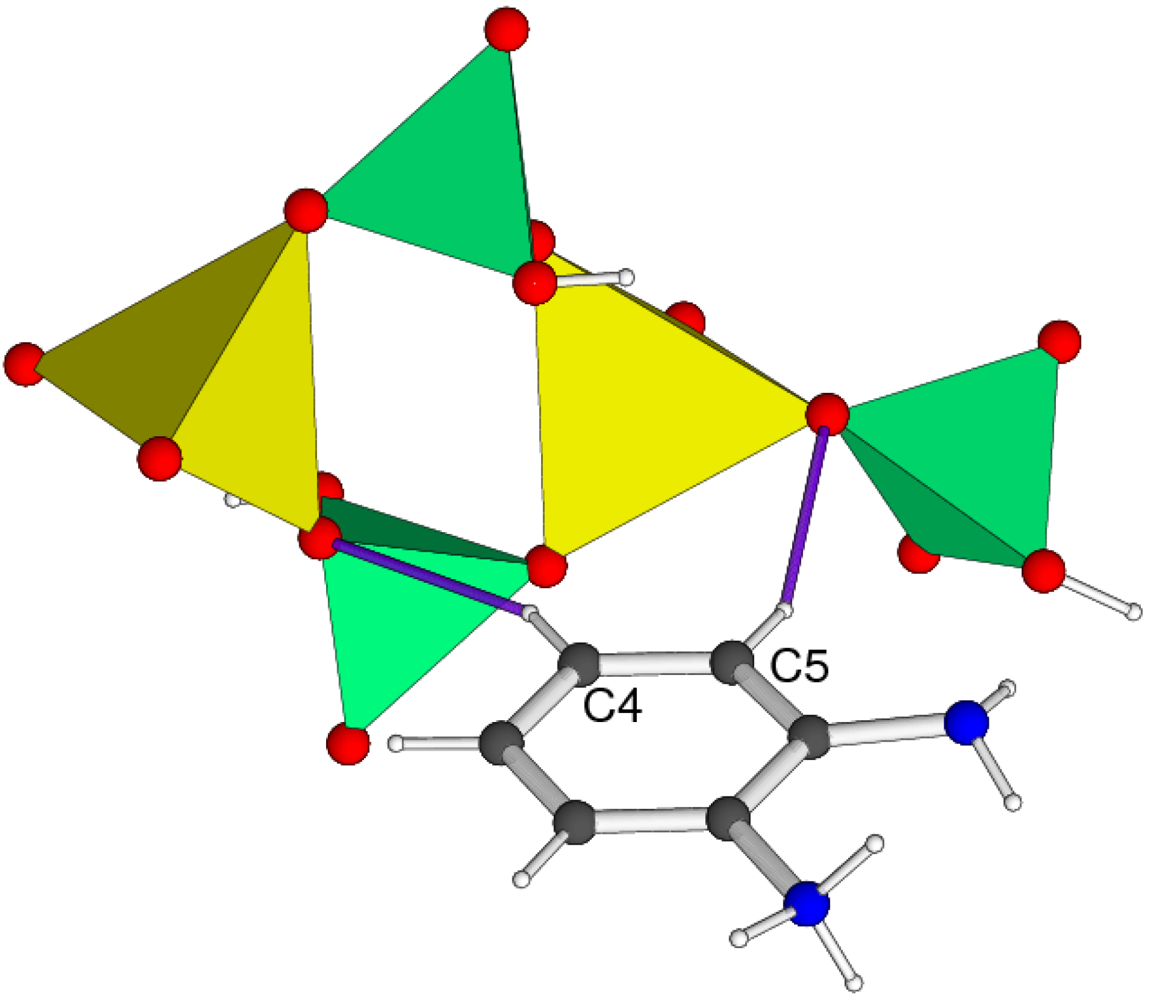
3. Experimental Section
| Empirical formula | C12H26N4O18P4Zn3 |
|---|---|
| Mr | 834.36 |
| Temperature (K) | 93 (2) |
| λ (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | C2/c (No. 15) |
| a (Å) | 20.194 (8) |
| b (Å) | 8.682 (3) |
| c (Å) | 15.123 (6) |
| β (°) | 91.510 (11) |
| V (Å3) | 2650.5 (17) |
| Z | 4 |
| ρcalc (g cm−3) | 2.091 |
| µ (mm−1) | 3.024 |
| F(000) | 1680 |
| Data/restraints/parameters | 2401/0/187 |
| Goodness-of-fit on F2 | 1.065 |
| R(F) [I > 2σ(I)] | 0.048 |
| wR(F2) (all data) | 0.112 |
| Min., max. Δρ ( e Å−3) | −0.88, +1.29 |
| CSD Deposition number | CCDC-876420 |
4. Conclusions
References
- Harrison, W.T.A.; Martin, T.E.; Gier, T.E.; Stucky, G.D. Tetrahedral-Atom zincophosphate structures–synthesis and structural characterization of two novel anionic 8-ring frameworks containing cationic 1,4-diazabicyclo[2.2.2]octane guests. J. Mater. Chem. 1992, 2, 175–181. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Nenoff, T.M.; Eddy, M.M.; Martin, T.E.; Stucky, G.D. Organic templates in zincophosphate synthesis–Zn5(PO4)2(HPO4)4·H2O·2H2N2C6H12 containing two distinct 8-ring channels and Zn3(PO4)(HPO4)2·HN2C6H12 containing tetrahedral 3-rings and Zn–N bonds. J. Mater. Chem. 1992, 2, 1127–1134. [Google Scholar] [CrossRef]
- Chidambaram, D.; Neeraj, S.; Natarajan, S.; Rao, C.N.R. Open-Framework zinc phosphates synthesized in the presence of structure-directing organic amines. J. Solid State Chem. 1999, 147, 154–169. [Google Scholar] [CrossRef]
- Gale, P.A.; Steed, J.W. Supramolecular Chemistry: From Molecules to Nanomaterials; John Wiley: Weinheim, Germany, 2012. [Google Scholar]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation–quantitative measure of distortion in coordination polyhedra. Science (Washington, DC) 1971, 172, 567–570. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-Valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst. 1985, B41, 244–247. [Google Scholar]
- Popović, L.; de Waal, D.; Boeyens, J.C.A. Correlation between Raman wavenumbers and P–O bond lengths in crystalline inorganic phosphates. J. Raman Spectrosc. 2005, 36, 2–11. [Google Scholar] [CrossRef]
- Ferraris, G.; Ivaldi, G. X–OH and O–H...O bond lengths in protonated oxoanions. Acta Cryst. 1984, B40, 1–6. [Google Scholar]
- Czapik, A.; Gdaniec, M. A new polymorph of benzene-1,2-diamine: Isomorphism with 2-aminophenol and two-dimensional isostructurality of polymorphs. Acta Cryst. 2010, C66, o198–o201. [Google Scholar]
- Chen, L.; Bu, X.-H. (3,4)-Connected zincophosphites as structural analogues of zinc hydrogen phosphate. Inorg. Chem. 2006, 45, 4654–4660. [Google Scholar] [CrossRef]
- Brunner, G.O. Which frameworks will form SiO2 analogues: The significance of loop configurations. Zeolites 1993, 13, 592–593. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Nenoff, T.E.; Gier, T.E.; Stucky, G.D. Preparation and crystal-structure of Na2Zn(HPO4)2·4H2O, a new layered sodium zinc phosphate containing bifurcated tetrahedral 12-rings. J. Solid State Chem. 1994, 113, 168–175. [Google Scholar] [CrossRef]
- Natarajan, S.; Mandal, S. Open-Framework structures of transition-metal compounds. Angew. Chem. Int. Ed. 2008, 47, 4798–4828. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph-set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Rodgers, J.A.; Phillips, M.L.F.; Nenoff, T.E. In situ template generation for zincophosphate synthesis leading to guanylurea zinc phosphate, C2H7N4O·ZnPO4, containing template-to-template N–H· ·O hydrogen bonds. Solid State Sci. 2002, 4, 969–972. [Google Scholar] [CrossRef]
- Rodgers, J.A.; Harrison, W.T.A. H3N(CH2)6NH3·Zn4(PO4)2(HPO4)2·3H2O, a novel three-dimensional zinc phosphate framework containing 5-rings and 20-rings. J. Mater. Chem. 2000, 10, 2853–2856. [Google Scholar] [CrossRef]
- Steiner, T. C–H...O hydrogen bonding in crystals. Crystallogr. Rev. 2006, 6, 1–57. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar]
- Kirkpatrick, A.; Harrison, W.T.A. Two zincophosphite networks templated by 1,4-dianonobenzene: Syntheses and structures of C6N2H10·Zn(HPO3)2 and (C6N2H8)0.5·ZnHPO3. Solid State Sci. 2004, 6, 593–598. [Google Scholar] [CrossRef]
- Xu, J.N.; Yuan, H.-M.; Mao, Y.-G.; Shi, S.-H.; Lee, Y.-K.; Chang, T.-S.; Song, T.-Y.; Feng, S.-H.; Xu, R.-R. Nonaqueous synthesis and characterization of a zincophosphate with 12-membered ring channel open framework. Chem. J. Chin. Univ. 2000, 21, 509–512. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aughey, J.C.; Harrison, W.T.A.; Slawin, A.M.Z. APA2[Zn3(HPO4)4(H2O)2], a Layered Zincophosphate Featuring Template-to-Framework N–H⋯O and “Synergic” Framework-to-Template O–H⋯N Hydrogen Bonds and C–H⋯O Interactions (APA = 2-Amino-1-phenyleneammonium, C6H9N2+). Crystals 2012, 2, 1146-1154. https://doi.org/10.3390/cryst2031146
Aughey JC, Harrison WTA, Slawin AMZ. APA2[Zn3(HPO4)4(H2O)2], a Layered Zincophosphate Featuring Template-to-Framework N–H⋯O and “Synergic” Framework-to-Template O–H⋯N Hydrogen Bonds and C–H⋯O Interactions (APA = 2-Amino-1-phenyleneammonium, C6H9N2+). Crystals. 2012; 2(3):1146-1154. https://doi.org/10.3390/cryst2031146
Chicago/Turabian StyleAughey, Joel C., William T. A. Harrison, and Alexandra M. Z. Slawin. 2012. "APA2[Zn3(HPO4)4(H2O)2], a Layered Zincophosphate Featuring Template-to-Framework N–H⋯O and “Synergic” Framework-to-Template O–H⋯N Hydrogen Bonds and C–H⋯O Interactions (APA = 2-Amino-1-phenyleneammonium, C6H9N2+)" Crystals 2, no. 3: 1146-1154. https://doi.org/10.3390/cryst2031146





