Metallic One-Dimensional Conductors Composed of Axially Ligated (Phthalocyanato)CoIII with Supramolecular Cations of A(EtOH)4 (A = Na and K)
Abstract
:1. Introduction
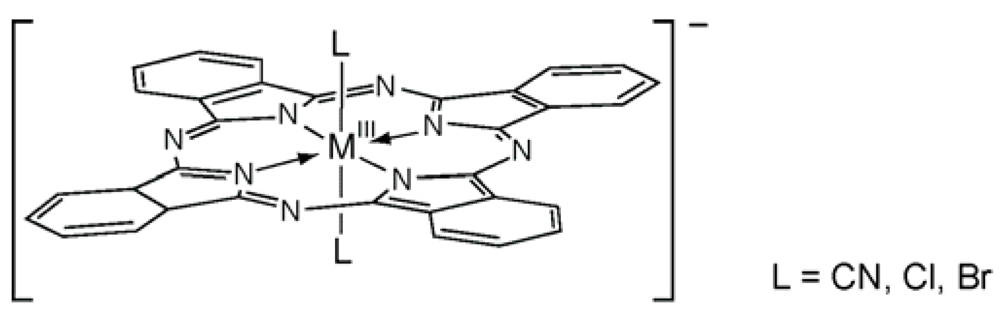
2. Results and Discussion
2.1. Crystal Structures
| Na[Co(Pc)(CN)2]2.4(EtOH) | K[Co(Pc)(CN)2]2.4(EtOH) | TPP[Co(Pc)(CN)2]2 | |
|---|---|---|---|
| space group | P42/n | P42/n | P42/n |
| a/Å | 21.163(4) | 21.370(2) | 21.676(8) |
| c/Å | 7.414(1) | 7.461(1) | 7.474(4) |
| V/Å3 | 3320(1) | 3407.2(6) | 3511(3) |
| temperature/K | 273 | 298 | 296 |
| overlap integral | 7.4 × 10−3 | 7.9 × 10−3 | 8.5 × 10−3 |
 axes in P42/n.
axes in P42/n.
 axes in P42/n.
axes in P42/n.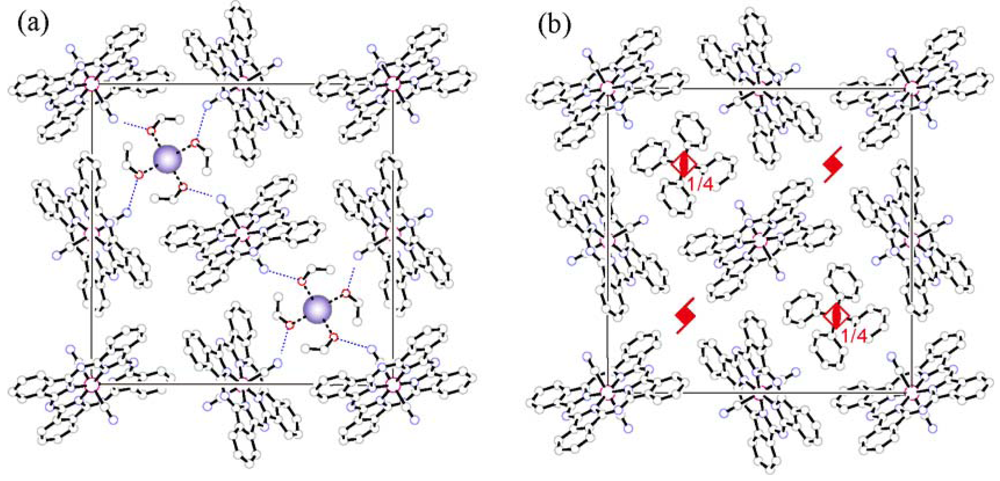
 position. In Na[Co(Pc)(CN)2]2.4EtOH and K[Co(Pc)(CN)2]2.4EtOH, this position is occupied by Na and K, respectively. Four EtOH molecules coordinate to the alkaline metal ion so as to satisfy this symmetry element. The Na...O and K...O distances are 2.315 Å and 2.633 Å, respectively, which are shorter than the sum of the ionic radius (Na+; 0.95 Å, K+; 1.33 Å) and van der Waals radius of oxygen (1.52 Å). A distinct feature of these supramolecular cationic units is the formation of hydrogen bonds with the axial CN ligand of the Pc unit. The OH...N hydrogen-bond distance is 2.889 Å in the Na salt and 2.858 Å in the K salt, which are substantially shorter than the O...N van der Waals contact (3.07 Å [13]). These hydrogen bonds tightly bind the Pc units and the cationic units in the ab plane, which is expected to lead to anisotropic thermal contraction of the lattice.
position. In Na[Co(Pc)(CN)2]2.4EtOH and K[Co(Pc)(CN)2]2.4EtOH, this position is occupied by Na and K, respectively. Four EtOH molecules coordinate to the alkaline metal ion so as to satisfy this symmetry element. The Na...O and K...O distances are 2.315 Å and 2.633 Å, respectively, which are shorter than the sum of the ionic radius (Na+; 0.95 Å, K+; 1.33 Å) and van der Waals radius of oxygen (1.52 Å). A distinct feature of these supramolecular cationic units is the formation of hydrogen bonds with the axial CN ligand of the Pc unit. The OH...N hydrogen-bond distance is 2.889 Å in the Na salt and 2.858 Å in the K salt, which are substantially shorter than the O...N van der Waals contact (3.07 Å [13]). These hydrogen bonds tightly bind the Pc units and the cationic units in the ab plane, which is expected to lead to anisotropic thermal contraction of the lattice.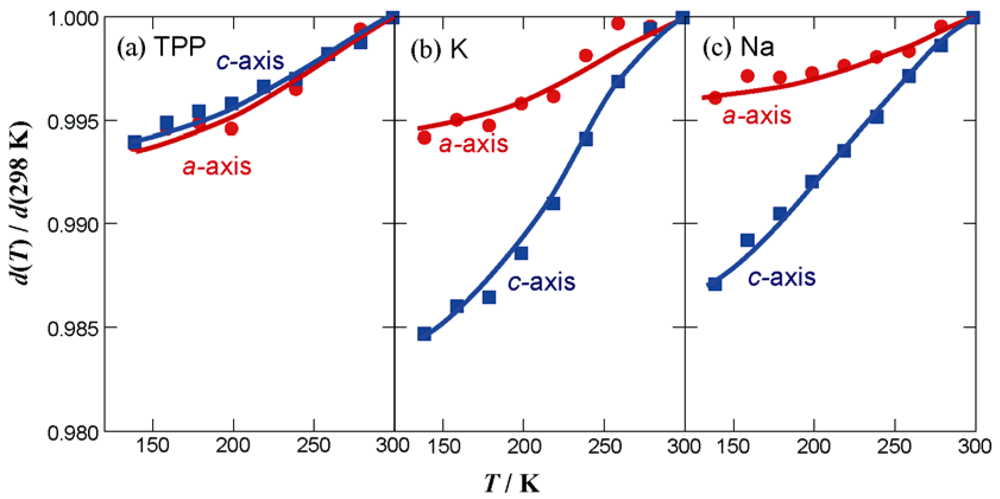
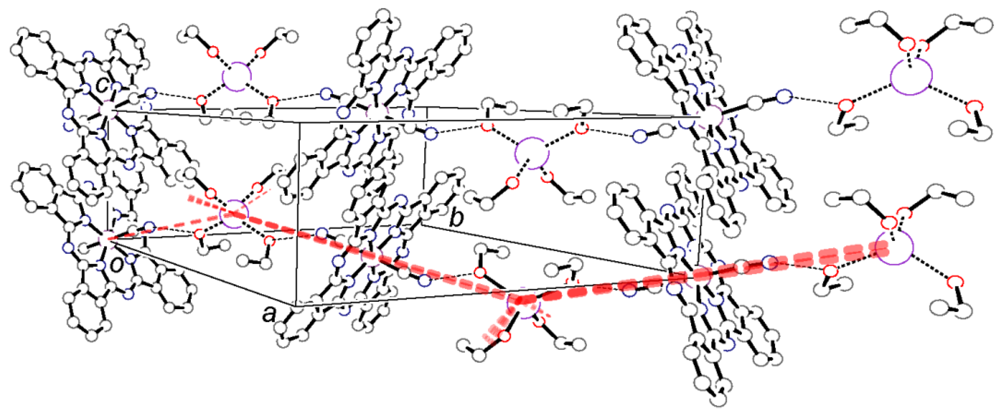
 axis in (a) K[Co(Pc)(CN)2]2.4(EtOH) and (b) TPP[Co(Pc)(CN)2]2 with space filling drawing.
axis in (a) K[Co(Pc)(CN)2]2.4(EtOH) and (b) TPP[Co(Pc)(CN)2]2 with space filling drawing.
 axis in (a) K[Co(Pc)(CN)2]2.4(EtOH) and (b) TPP[Co(Pc)(CN)2]2 with space filling drawing.
axis in (a) K[Co(Pc)(CN)2]2.4(EtOH) and (b) TPP[Co(Pc)(CN)2]2 with space filling drawing.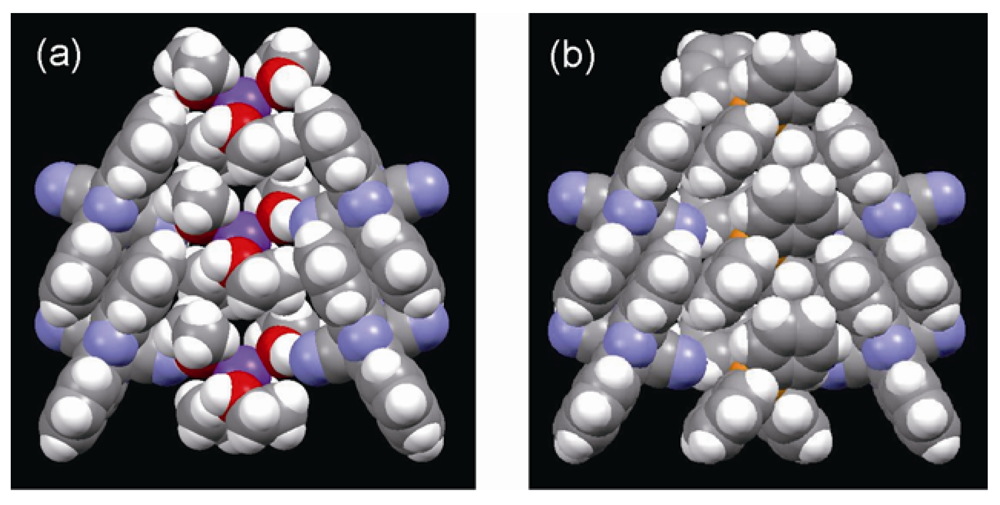
2.2. Physical Properties
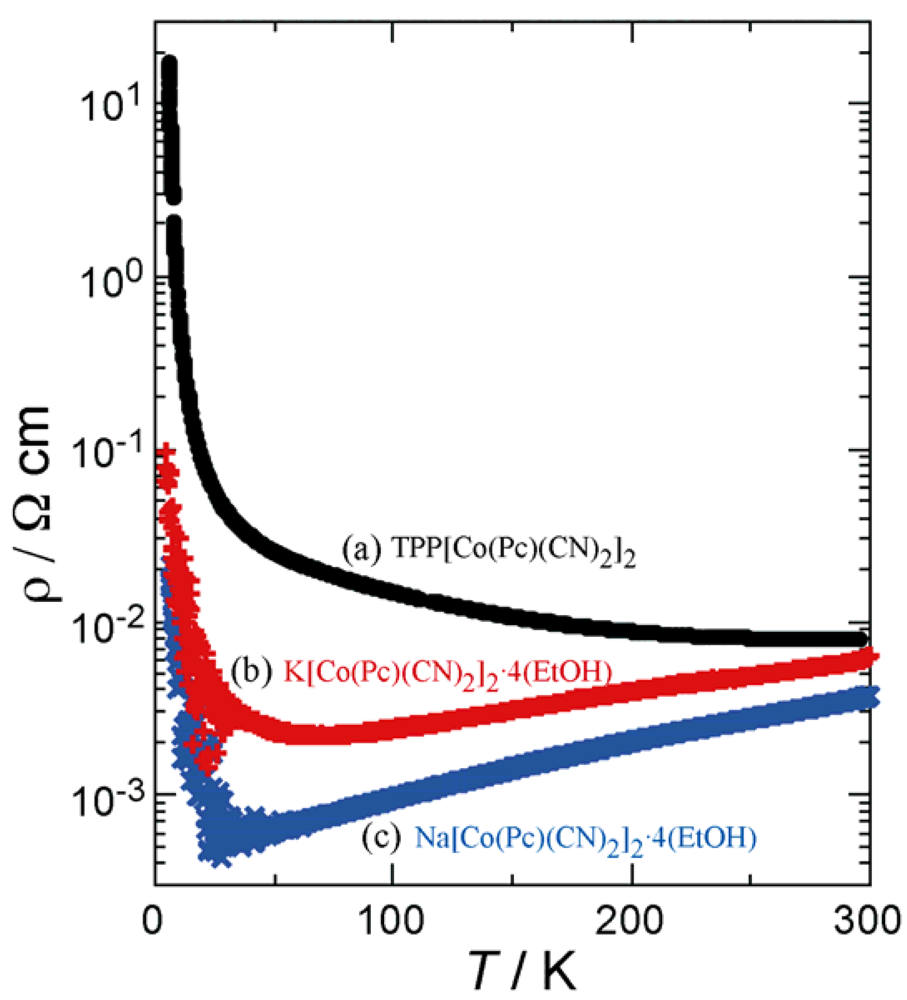
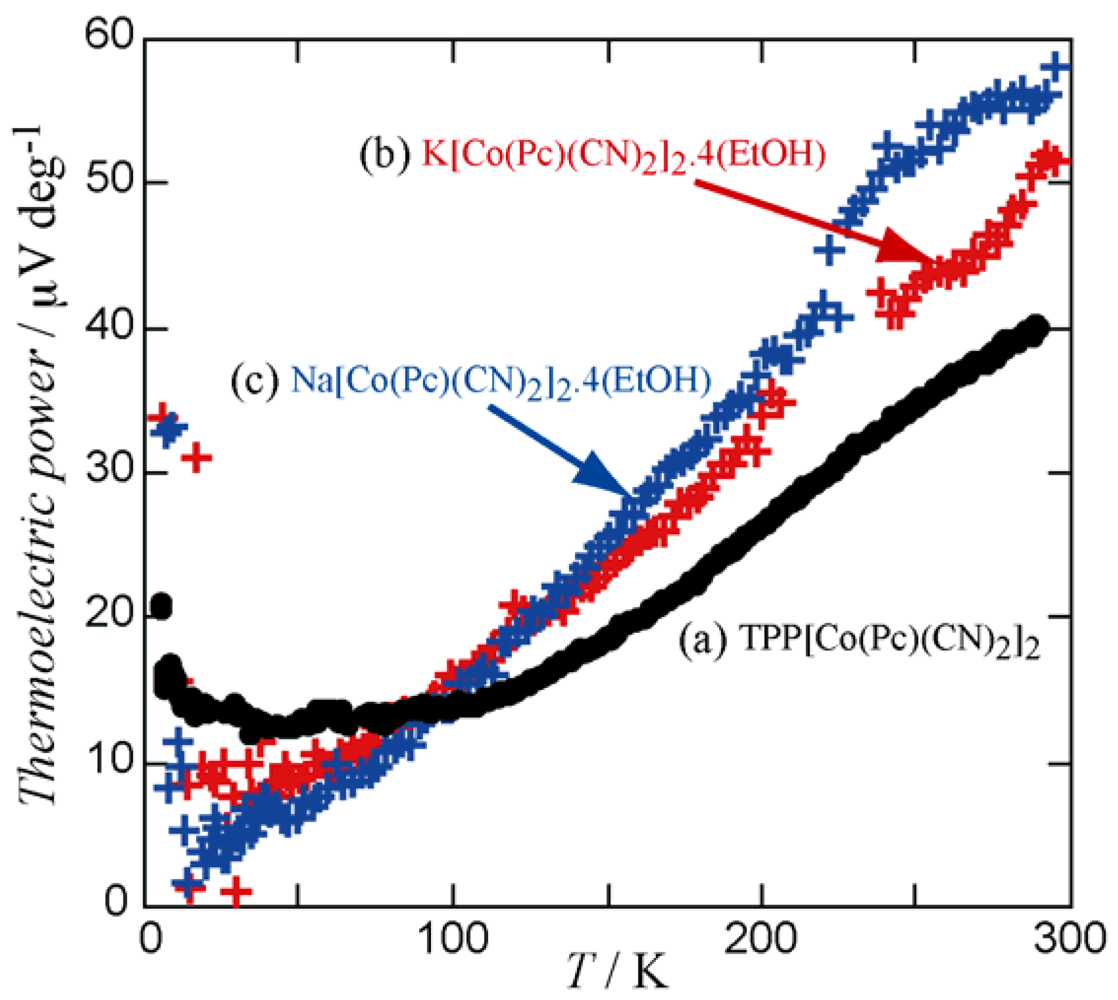
 position will expand. In order to maintain an isomorphous structure, the Pc units around the 42 axis have to move apart at the same time, which is accompanied by the expansion of the void space in the crystal. Since there is a limit to this void expansion, the maximum ab plane area available, while maintaining the tetragonal lattice, can be attained by the TPP salt. Contraction of the ab plane area is expected to induce an increase in interaction between 1-D Pc chains. As for the π-π interactions, overlap integral values along any direction perpendicular to the c axis are negligibly small.
position will expand. In order to maintain an isomorphous structure, the Pc units around the 42 axis have to move apart at the same time, which is accompanied by the expansion of the void space in the crystal. Since there is a limit to this void expansion, the maximum ab plane area available, while maintaining the tetragonal lattice, can be attained by the TPP salt. Contraction of the ab plane area is expected to induce an increase in interaction between 1-D Pc chains. As for the π-π interactions, overlap integral values along any direction perpendicular to the c axis are negligibly small.3. Experimental Section
3.1. Materials and Crystal Growth
3.2. X-ray Structure Determination
3.3. Measurements
4. Conclusions
Acknowledgments
References
- Inabe, T.; Tajima, H. Phthalocyanines–versatile components of molecular conductors. Chem. Rev. 2004, 104, 5503–5533. [Google Scholar] [CrossRef]
- Thompson, J.A.; Murata, K.; Miller, D.C.; Stanton, J.L.; Broderick, W.E.; Hoffman, B.M.; Ibers, J.A. Synthesis of high-purity phthalocyanines (pc): High intrinsic conductivities in the molecular conductors H2(pc)I and Ni(pc)I. Inorg. Chem. 1993, 32, 3546–3553. [Google Scholar]
- Hasegawa, H.; Naito, T.; Inabe, T.; Akutagawa, T.; Nakamura, T. A highly conducting partially oxidized salt of axially substituted phthalocyanine. Structure and physical properties of TPP[Co(Pc)(CN)2]2 {TPP = tetraphenylphosphonium, [Co(Pc)(CN)2] = dicyano(phthalocyaninato)cobalt(III)}. J. Mater. Chem. 1998, 8, 1567–1570. [Google Scholar] [CrossRef]
- Matsuda, M.; Naito, T.; Inabe, T.; Hanasaki, N.; Tajima, H. Structure and electrical and magnetic properties of (PTMA)x[M(Pc)(CN)2]. y(solvent) (PTMA = phenyltrimethylammonium and [M(Pc)(CN)2] = dicynano(phthalocyaninato)MIII with M = Co and Fe. Partial oxidation by partial solvent occupation of the cationic site. J. Mater. Chem. 2001, 11, 2493–2497. [Google Scholar] [CrossRef]
- Takano, S.; Naito, T.; Inabe, T. Ladder-type phthalocyanine conductor, [PXX][Co(Pc)(CN)2] (PXX = peri-xanthenoxanthene, Co(Pc)(CN)2 = dicyano(phthalocyaninato)cobalt(III). Chem. Lett. 1998, 1249–1250. [Google Scholar]
- Asari, T.; Naito, T.; Inabe, T.; Matsuda, M.; Tajima, H. Novel phthalocyanine conductor containing two-dimensional Pc stacks, [PXX]2[Co(Pc)(CN)2] (PXX = peri-xanthenoxanthene, Co(Pc)(CN)2 = dicyano(phthalocyaninato)cobalt(III). Chem. Lett. 2004, 33, 128–129. [Google Scholar] [CrossRef]
- Asari, T.; Ishikawa, M.; Naito, T.; Matsuda, M.; Tajima, H.; Inabe, T. Nearly isotropic two-dimensional sheets in a partially oxidized Co(Pc)(CN)2 salt (Pc = phthalocyaninato). Chem. Lett. 2005, 34, 936–937. [Google Scholar] [CrossRef]
- Hanasaki, N.; Tajima, H.; Matsuda, M.; Naito, T.; Inabe, T. Giant negative magnetoresistance in a quasi-one-dimensional conductor TPP[FeIII(Pc)(CN)2]2: Interplay between local moments and one-dimensional conduction electrons. Phys. Rev. B 2000, 62, 5839–5842. [Google Scholar]
- Hanasaki, N.; Matsuda, M.; Tajima, H.; Ohmichi, E.; Osada, T.; Naito, T.; Inabe, T. Giant negative magnetoresistance reflecting molecular symmetry in dicyano(phthalocyaninato)iron compounds. J. Phys. Soc. Jpn. 2006, 75, 033702:2–033702:4. [Google Scholar]
- Hanasaki, N.; Masuda, K.; Kodama, K.; Matsuda, M.; Tajima, H.; Yamazaki, J.; Takigawa, M.; Yamaura, J.; Ohmichi, E.; Osada, T.; et al. Charge disproportionation in highly one-dimensional molecular conductor TPP[Co(Pc)(CN)2]2. J. Phys. Soc. Jpn. 2006, 75, 104713:1–104713:5. [Google Scholar]
- Matsuda, M.; Asari, T.; Naito, T.; Inabe, T.; Hanasaki, N.; Tajima, H. Structure and physical properties of low-dimensional molecular conductors, [PXX][CoIII(Pc)(CN)2] and [PXX][FeIII(Pc)(CN)2] (PXX = peri-xanthenoxanthene, Pc = phthalocyaninato). Bull. Chem. Soc. Jpn. 2003, 76, 1935–1940. [Google Scholar] [CrossRef]
- Ishikawa, M.; Asari, T.; Matsuda, M.; Tajima, H.; Hanasaki, N.; Naito, T.; Inabe, T. Giant magnetoresistance response by the π-d interaction in an axially ligatedphthalocyanine conductor with two-dimensional π-π stacking structure. J. Mater. Chem. 2010, 20, 4432–4438. [Google Scholar]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Chaikin, P.M.; Kwak, J.F.; Jones, T.E.; Garito, A.F.; Heeger, A.J. Thermoelectric power of tetrathiofulvalinium tetracyanoquinodimethane. Phys. Rev. Lett. 1973, 31, 601–604. [Google Scholar]
- Metz, J.; Hanack, M. Synthesis, characterization, and conductivity of (m-Cyano)(phthalocyaninato)cobalt(III). J. Am. Chem. Soc. 1983, 105, 828–830. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, M.; Giacovazzo, C.; Guagliardi, A.; Polidori, G. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar]
- Watkin, D.J.; Prout, C.K.; Carruthers, J.R.; Betteridge, P.W. CrystalStructure—Crystal Structure Analysis Software, version 3.5.1; Rigaku and Rigaku: The Woodlands, TX, USA, 1996. [Google Scholar]
- Chaikin, P.M.; Kwak, J.F. Apparatus for thermopower measurements on organic conductors. Rev. Sci. Instrum. 1975, 46, 218–220. [Google Scholar] [CrossRef]
- Ren, J.; Liang, W.; Whangbo, M.-H. Crystal and Electronic Structure Analysis Using CAESER; PrimeColor Software Inc.: Cary, NC, USA, 1998. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, Y.; Ishikawa, M.; Watanabe, N.; Takahashi, Y.; Naito, T.; Inabe, T. Metallic One-Dimensional Conductors Composed of Axially Ligated (Phthalocyanato)CoIII with Supramolecular Cations of A(EtOH)4 (A = Na and K). Crystals 2012, 2, 946-957. https://doi.org/10.3390/cryst2030946
Tanaka Y, Ishikawa M, Watanabe N, Takahashi Y, Naito T, Inabe T. Metallic One-Dimensional Conductors Composed of Axially Ligated (Phthalocyanato)CoIII with Supramolecular Cations of A(EtOH)4 (A = Na and K). Crystals. 2012; 2(3):946-957. https://doi.org/10.3390/cryst2030946
Chicago/Turabian StyleTanaka, Yasuhiro, Manabu Ishikawa, Naoko Watanabe, Yukihiro Takahashi, Toshio Naito, and Tamotsu Inabe. 2012. "Metallic One-Dimensional Conductors Composed of Axially Ligated (Phthalocyanato)CoIII with Supramolecular Cations of A(EtOH)4 (A = Na and K)" Crystals 2, no. 3: 946-957. https://doi.org/10.3390/cryst2030946





