A One-Dimensional Coordination Polymer Constructed from Cadmium(II) Cations and Sparfloxacinate Anions
1. Introduction
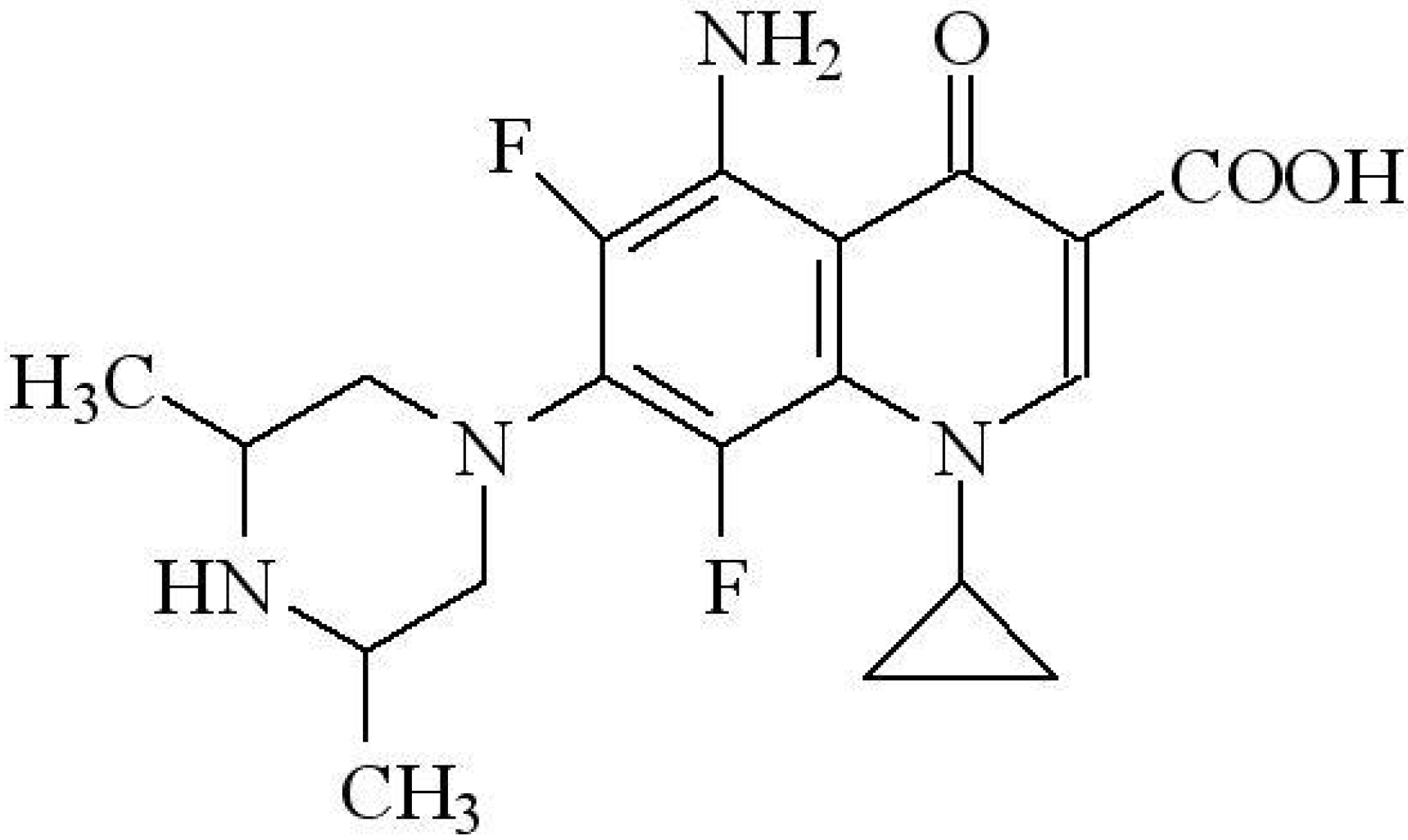
2. Results and Discussion
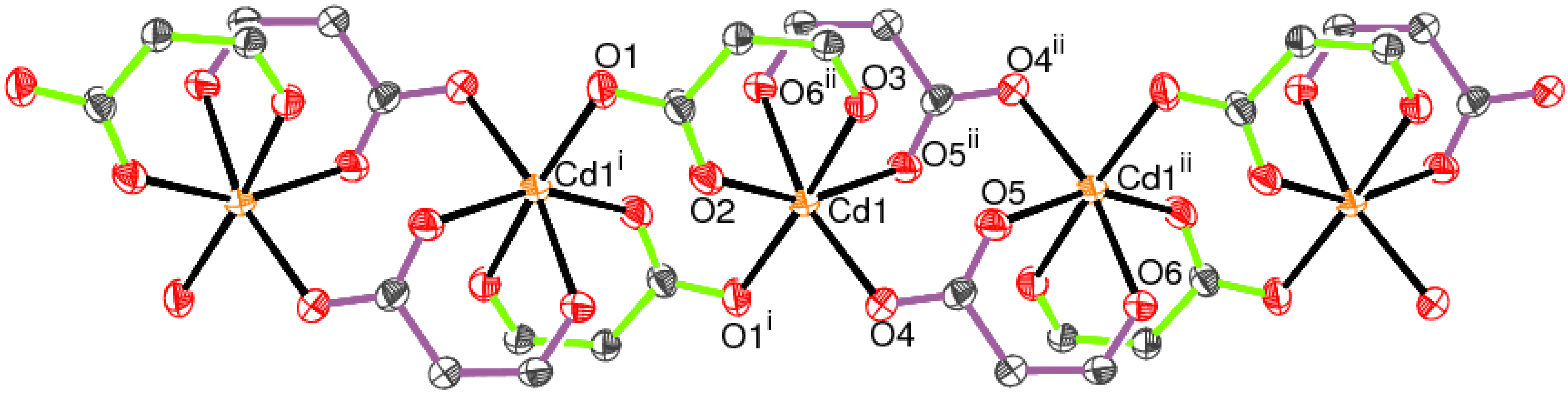
2.1. Crystal Structure of [Cd(spar)2]n·nH2O (1)
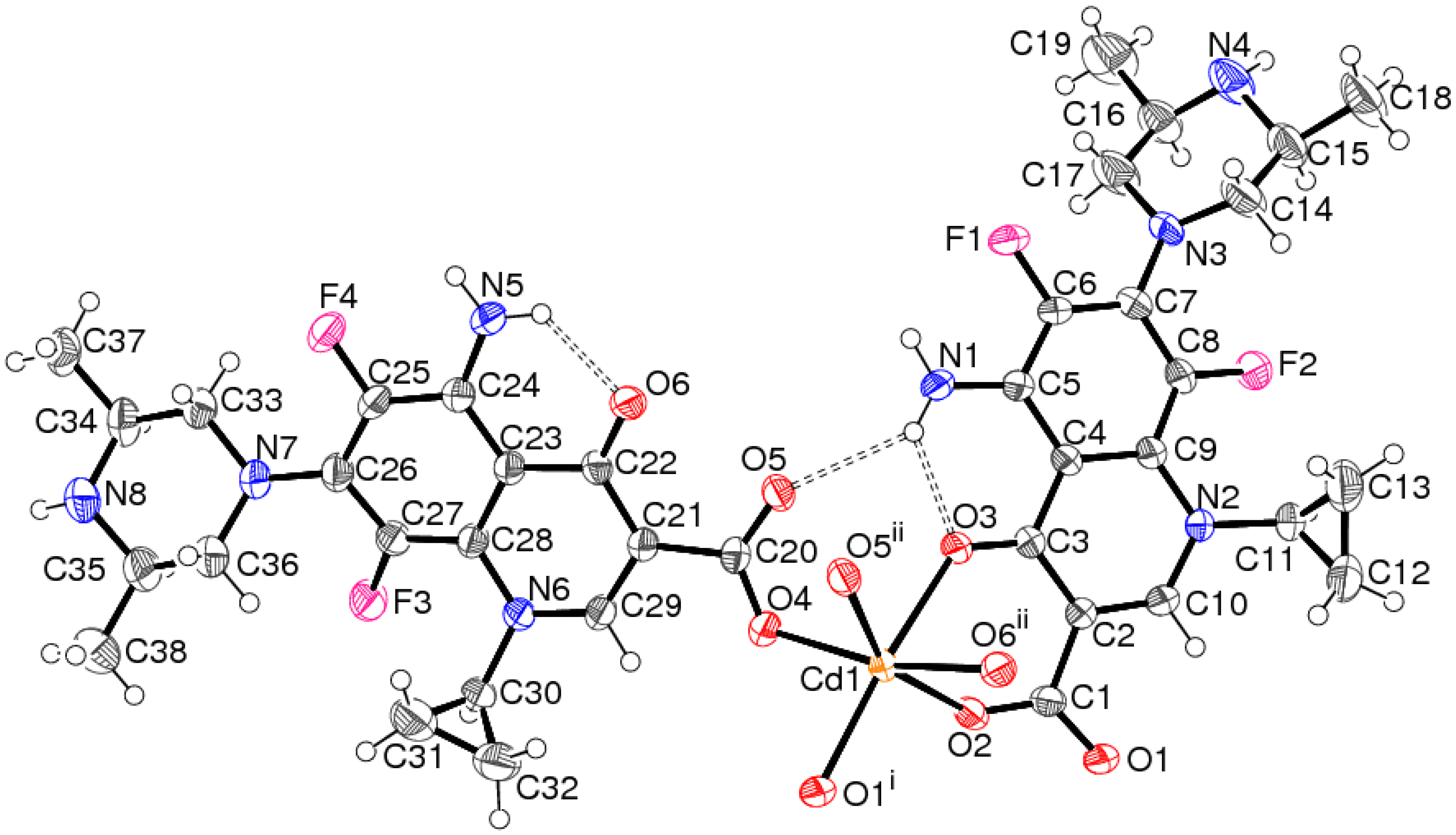
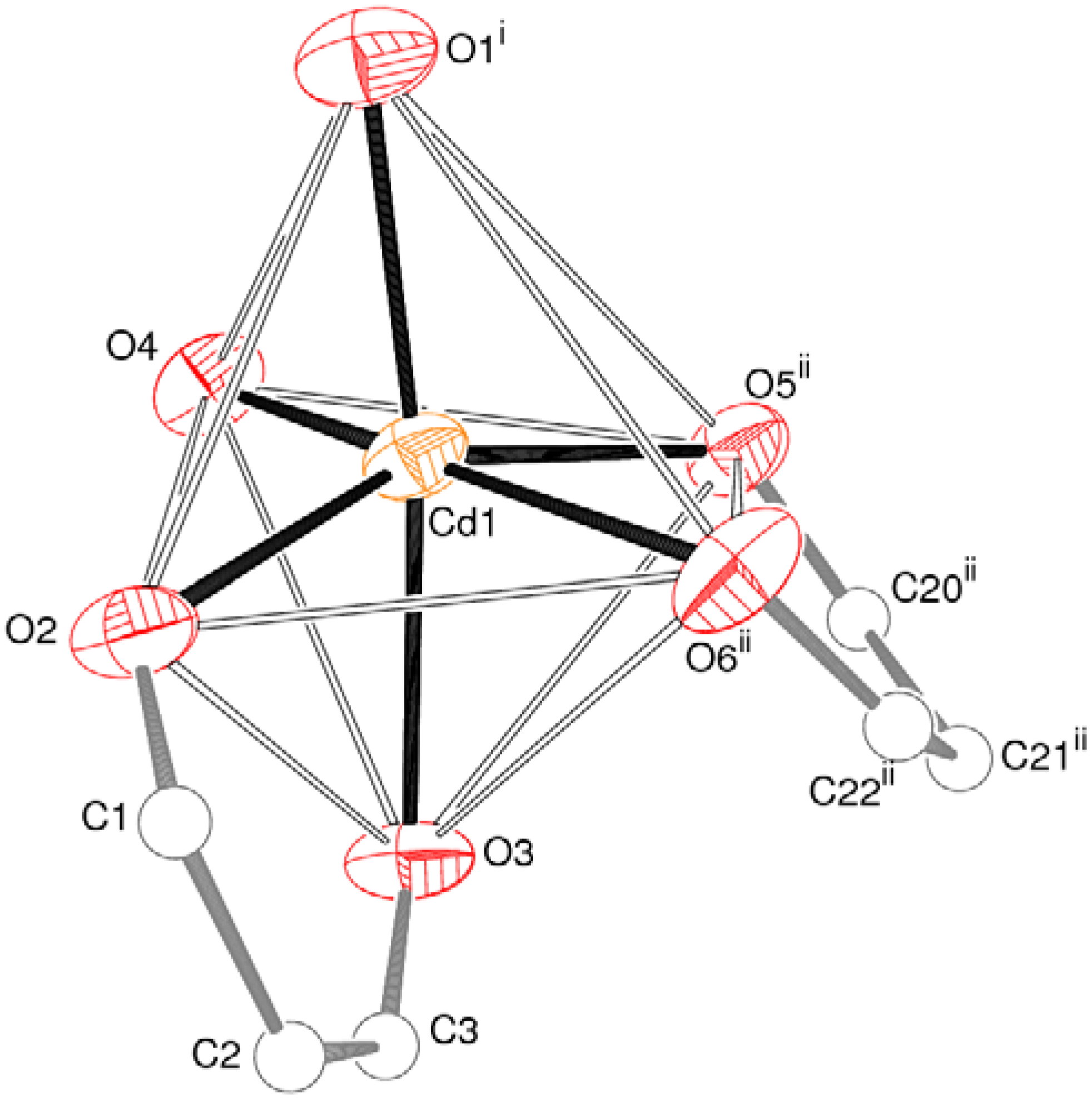
| Cd1–O4 | 2.264(2) | Cd1–O1 i | 2.269(2) |
| Cd1–O5 ii | 2.280(2) | Cd1–O2 | 2.292(2) |
| Cd1–O6 ii | 2.304(2) | Cd1–O3 | 2.3466(19) |
| O4–Cd1–O1 i | 87.58(8) | O4–Cd1–O5 ii | 90.75(8) |
| O1 i–Cd1–O5 ii | 102.81(8) | O4–Cd1–O2 | 104.12(8) |
| O1 i–Cd1–O2 | 98.16(7) | O5 ii–Cd1–O2 | 154.76(7) |
| O4–Cd1–O6 ii | 164.30(7) | O1 i–Cd1–O6 ii | 100.15(8) |
| O5 ii–Cd1–O6 ii | 74.28(8) | O2–Cd1–O6 ii | 88.40(8) |
| O4–Cd1–O3 | 92.27(7) | O1 i–Cd1–O3 | 170.82(8) |
| O5 ii–Cd1–O3 | 86.37(7) | O2–Cd1–O3 | 72.97(7) |
| O6 ii–Cd1–O3 | 82.26(8) |
| N1–H1A···O1 iii | 0.86 | 2.33 | 3.021(3) | 138 |
| N1–H1B···O3 | 0.86 | 1.96 | 2.598(3) | 130 |
| N1–H1B···O5 | 0.86 | 2.35 | 3.061(3) | 141 |
| N5–H5A···O4 iii | 0.86 | 2.59 | 3.221(3) | 131 |
| N5–H5B···O6 | 0.86 | 1.98 | 2.609(3) | 129 |
2.2. Spectroscopy
3. Experimental Section
3.1. Synthesis and Characterization
3.2. Single-Crystal Data Collection and Analysis
 (No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, F(000) = 936, T = 296(2) K, ρcalc = 1.558 g·cm−3, μ = 0.640 mm−1, 27884 reflections recorded (3.4° ≤ 2θ ≤ 50.0°; −10 ≤ h ≤ 10, −15 ≤ k ≤ 15, −20 ≤ l ≤ 20), RInt = 0.039, 6848 merged reflections, 6225 with I > 2σ(I), 541 variable parameters, R(F) = 0.036, wR(F2) = 0.082, min./max. ∆ρ = −0.58/0.46 e Å−3. Cambridge Structural Database deposition number: CCDC-888200.
(No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, F(000) = 936, T = 296(2) K, ρcalc = 1.558 g·cm−3, μ = 0.640 mm−1, 27884 reflections recorded (3.4° ≤ 2θ ≤ 50.0°; −10 ≤ h ≤ 10, −15 ≤ k ≤ 15, −20 ≤ l ≤ 20), RInt = 0.039, 6848 merged reflections, 6225 with I > 2σ(I), 541 variable parameters, R(F) = 0.036, wR(F2) = 0.082, min./max. ∆ρ = −0.58/0.46 e Å−3. Cambridge Structural Database deposition number: CCDC-888200.4. Conclusions
References
- Andersson, M.I.; MacGowan, A.P. Development of the quniolones. J. Antimicrob. Chemother. 2003, 51, 1–11. [Google Scholar] [CrossRef]
- Miyamoto, T.; Matsumoto, J.-I.; Chiba, K.; Egawa, H.; Shibamori, K.; Minamida, A.; Nishimura, Y.; Okada, H.; Kataoka, M.; Fujita, M.; Hirose, T.; Nakano, J. Pyridonecarboxylic acids as antibacterial agents. 14: Synthesis and structure-activity relationship of 5-substituted 6,8-difluoroquinolones, including sparfloxacin, a new quinoline antibacterial agent with improved potency. J. Med. Chem. 1990, 33, 1645–1656. [Google Scholar] [CrossRef]
- Qadri, S.M.; Ueno, Y.; Burns, J.J.; Almodovar, E.; Rabea, N. In vitro activity of sparfloxacin (CI-978), a new broad-spectrum fluoroquinolone. Chemotherapy 1992, 38, 99–106. [Google Scholar] [CrossRef]
- Tacconelli, E.; De Angelis, G.; Cataldo, M.A.; Pozzi, E.; Cauda, R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 61, 26–38. [Google Scholar] [CrossRef]
- An, Z.; Gao, J.; Harrison, W.T.A. Two binuclear complexes containing the enrofloxacinate anion: Cd2(C19H21N3O3F)4(H2O)2·4H2O and Pb2(C19H21N3O3F)4·4H2O. J. Coord. Chem. 2010, 63, 3871–3879. [Google Scholar] [CrossRef]
- Xiao, D.-R.; Wang, E.-B.; An, H.-Y.; Su, Z.-M.; Li, Y.-G.; Gao, L.; Sun, C.-Y.; Xu, L. Rationally designed, polymeric, extended metal-ciprofloxacin complexes. Chem. Eur. J. 2005, 11, 6673–6686. [Google Scholar] [CrossRef]
- An, Z.; Liu, L.-R. Poly[bis[μ-1-cyclopropyl-6-fluoro-4-oxido-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato]nickel(II)]. Acta Cryst. 2008, E64, m176. [Google Scholar]
- Sivalakshmidevi, A.; Vyas, K.; Om Reddy, G. Sparfloxacin, an antibacterial drug. Acta Cryst. 2000, C56, e115–e116. [Google Scholar]
- Llinas, A.; Burley, J.C.; Prior, T.J.; Glen, R.C.; Goodman, J.M. Concomitant hydrate polymorphism in the precipitation of sparfloxacin from aqueous solution. Cryst. Growth Des. 2008, 8, 114–118. [Google Scholar] [CrossRef]
- Li, T.; Yang, L.; Wang, Y.C.; Lian, Q. Bis[(2R,6S)-4-(5-amino-3-carboxy-1-cyclopropyl-6,8-difluoro-4-oxo-1,4-dihydroquinolin-7-yl)-2,6-dimethylpiperazin-1-ium] sulfate pentahydrate. Acta Cryst. 2011, E67, o3366. [Google Scholar]
- Skyrianou, K.C.; Raptopoulou, C.P.; Psycharis, V.; Kessissoglou, D.P.; Psomas, G. Structure, cyclic voltammetry and DNA-binding properties of the bis(pyridine)bis(sparfloxacinato)nickel(II) complex. Polyhedron 2009, 28, 3265–3271. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Raptopoulou, C.P.; Karaliota, K.; Katsaros, N.; Psomas, G. Crystal structure, spectroscopic, and biological study of the copper(II) complex with third-generation quinolone antibiotic sparfloxacin. Bioorg. Med. Chem. Lett. 2006, 16, 3864–3867. [Google Scholar] [CrossRef]
- Shingnapurkar, D.; Butcher, R.; Afrasiabi, Z.; Sinn, E.; Ahmed, F.; Sarkar, F.; Padhye, S. Neutral dimeric copper-sparfloxacin conjugate having butterfly motif with antiproliferative effects against hormone independent BT20 breast cancer cell line. Inorg. Chem. Commun. 2007, 10, 459–462. [Google Scholar] [CrossRef]
- Tarushi, A.; Polatoglou, E.; Kljun, J.; Turel, I.; Psomas, G.; Kessissoglou, D.P. Interaction of Zn(II) with quinolone drugs: Structure and biological evaluation. Dalton Trans. 2011, 40, 9461–9473. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation–quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-Valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst. 1985, B41, 244–247. [Google Scholar]
- APEX2 and SAINT Diffractometer Control Software; Bruker AXS Inc.: Madison, WI, USA, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar]
- Lopez-Gresa, M.P.; Ortiz, R.; Perello, L.; Latorre, J.; Liu-Gonzalez, M.; Garcia-Granda, S.; Perez-Priede, M.; Canton, E. Interactions of metal ions with two quinolone antimicrobial agents (cinoxacin and ciprofloxacin)—Spectroscopic and X-ray structural characterization. Antibacterial studies. J. Inorg. Biochem. 2002, 92, 65–74. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
An, Z.; Gao, J.; Harrison, W.T.A. A One-Dimensional Coordination Polymer Constructed from Cadmium(II) Cations and Sparfloxacinate Anions. Crystals 2012, 2, 1366-1373. https://doi.org/10.3390/cryst2041366
An Z, Gao J, Harrison WTA. A One-Dimensional Coordination Polymer Constructed from Cadmium(II) Cations and Sparfloxacinate Anions. Crystals. 2012; 2(4):1366-1373. https://doi.org/10.3390/cryst2041366
Chicago/Turabian StyleAn, Zhe, Jing Gao, and William T. A. Harrison. 2012. "A One-Dimensional Coordination Polymer Constructed from Cadmium(II) Cations and Sparfloxacinate Anions" Crystals 2, no. 4: 1366-1373. https://doi.org/10.3390/cryst2041366




