(C5H12N)Cu2Br3: A Piperidinium Copper(I) Bromide with [Cu2Br3]− Ladders
Abstract
:1. Introduction
2. Results and Discussion
| Compound | (C5H12N)Cu2Br3 |
|---|---|
| Formula weight | 452.97 g/mol |
| Space group, Z | P-1 (no. 2), 2 |
| Lattice parameters | a = 6.2948(10) Å |
| b = 8.2624(14) Å | |
| c = 10.7612(17) Å | |
| α = 75.964(19)° | |
| β = 89.232(19)° | |
| γ = 84.072(19)° | |
| V | 540.04(15) Å3 |
| ρxray | 2.786 g/cm3 |
| Crystal size | 0.45 × 0.15 × 0.1 mm3 |
| MoKα radiation, λ | 0.71073 Å |
| Absorption coefficient | 14.978 mm−1 |
| Angle range | 1.95° < θ < 25.96° |
| Index ranges | −7 ≤ h ≤ 7 |
| −10 ≤ k ≤ 10 | |
| −13 ≤ l ≤ 13 | |
| Reflections measured | 4244 |
| Independent reflections, Rint | 1967, 0.0687 |
| No. of parameters | 101 |
| Transmission, max., min. | 0.566, 0.102 |
| R1[F2 > 2σ(F2)] | 0.0534 |
| wR2 | 0.1298 |
| e-density, min., max. | −1.679, 1.552 e/Å3 |
| Extinction coefficient | 0.0042(13) |
| Deposition no. | CCDC 873300 |
| Atom | x/a | y/b | z/c | Ueq a |
|---|---|---|---|---|
| C1 | 0.0365(14) | 0.7447(10) | 0.3342(8) | 28(2) |
| C2 | 0.1470(17) | 0.7054(11) | 0.4614(8) | 36(2) |
| C3 | 0.2956(17) | 0.8373(11) | 0.4685(9) | 36(2) |
| C4 | 0.4548(17) | 0.8549(11) | 0.3595(10) | 42(2) |
| C5 | 0.3429(16) | 0.8929(10) | 0.2324(9) | 30(2) |
| N1 | 0.1945(13) | 0.7657(8) | 0.2271(6) | 27(2) |
| Cu1 | 0.4716(2) | 0.6475(1) | 0.8950(1) | 41(1) |
| Cu2 | 0.9939(2) | 0.6409(1) | 0.8887(1) | 42(1) |
| Br1 | 0.2684(1) | 0.3883(1) | 0.9108(1) | 24(1) |
| Br2 | 0.1961(1) | 0.8693(1) | 0.9030(1) | 24(1) |
| Br3 | 0.7283(1) | 0.6547(1) | 0.7256(1) | 25(1) |
| Atoms | Atoms | ||
|---|---|---|---|
| Cu1–Cu1 (rung) | 2.889(2) | Cu2–Cu2 (rung) | 2.903(2) |
| Cu1–Cu2 (leg) | 3.015(2) | Cu1–Cu2 (leg) | 3.283(2) |
| Cu1–Br2 | 2.4065(14) | Cu2–Br3 | 2.4147(16) |
| Cu1–Br3 | 2.4134(15) | Cu2–Br2 | 2.4184(14) |
| Cu1–Br1 | 2.5755(15) | Cu2–Br1 | 2.5343(16) |
| Cu1–Br1’ | 2.6126(17) | Cu2–Br1’ | 2.6781(17) |
| Br1–Cu1–Br1’ | 112.34(5) | Br1–Cu2–Br1’ | 112.39(5) |
| Br1–Cu1–Br2 | 104.25(6) | Br1–Cu2–Br2 | 105.16(6) |
| Br1–Cu1–Br3 | 107.66(5) | Br1–Cu2–Br3 | 112.23(5) |
| Br1’–Cu1–Br2 | 108.18(5) | Br1’–Cu2–Br2 | 103.99(5) |
| Br1’–Cu1–Br3 | 99.62(6) | Br1’–Cu2–Br3 | 97.80(5) |
| Br2–Cu1–Br3 | 124.86(6) | Br2–Cu2–Br3 | 124.72(6) |
| C1–C2 | 1.492(12) | C4–C5 | 1.495(13) |
| C2–C3 | 1.524(14) | C5–N1 | 1.488(10) |
| C3–C4 | 1.522(14) | N1–C1 | 1.502(10) |
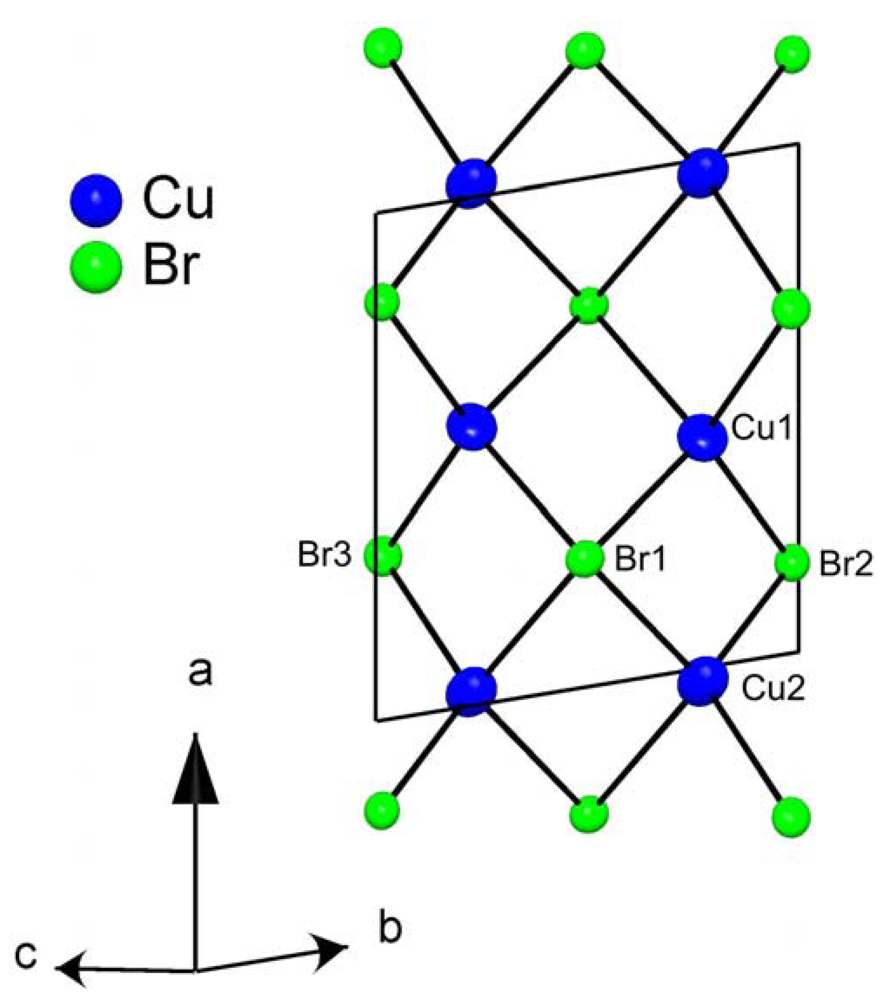
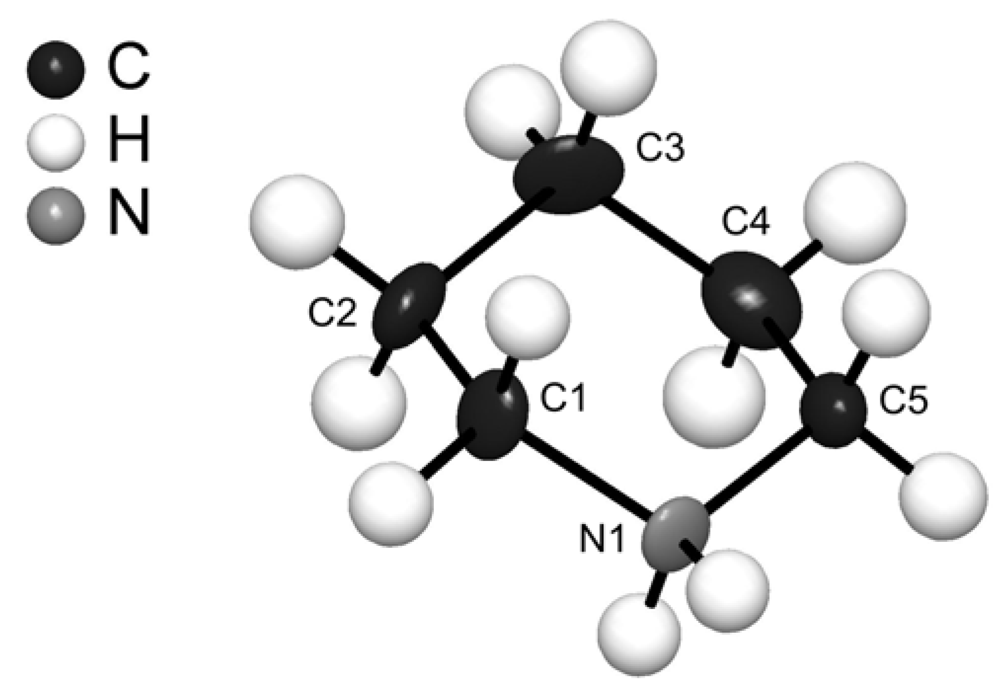
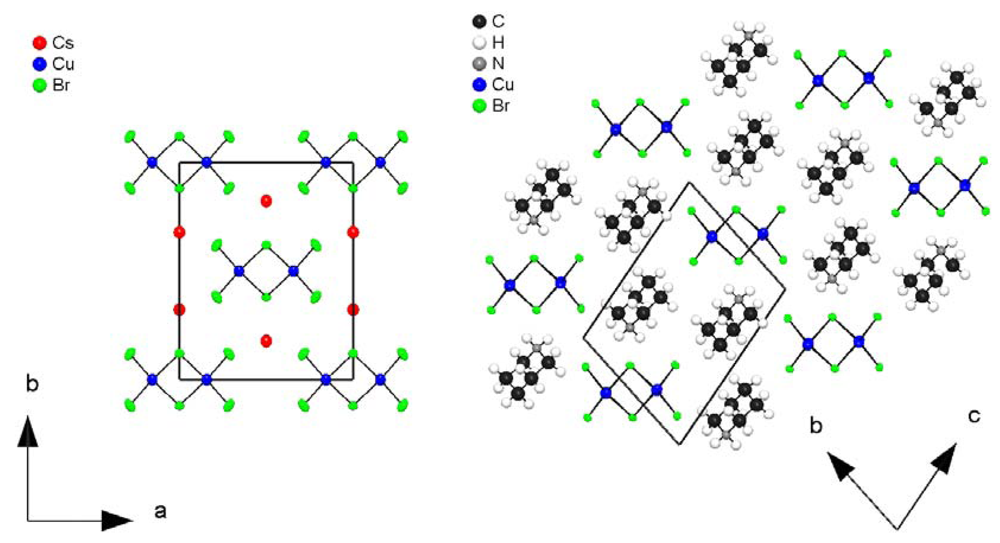
3. Experimental Section
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Meyer, G. Synproportionierung am metallischen Substrat: CsCu2Cl3 und CsCu2Br3. Z. Anorg. Allg. Chem. 1984, 515, 127–132. [Google Scholar] [CrossRef]
- Jouini, N.; Guen, L.; Tournoux, M. Structure cristalline de CsCu2I3. Rev. Chim. Mineral. 1980, 17, 486–491. [Google Scholar]
- Hull, S.; Berastegui, P. Crystal structures and ionic conductivities of ternary derivatives of the silver and copper monohalides—II: Ordered phases within the (AgX)x − (MX)1−x and (CuX)x − (MX)1−x (M = K, Rb and Cs; X = Cl, Br and I) systems. J. Solid State Chem. 2004, 177, 3156–3173. [Google Scholar] [CrossRef]
- Clark, J.R.; Brown, G.E. Crystal structure of rasvumite, KFe2S3. Am. Mineral. 1980, 65, 477–482. [Google Scholar]
- Kageyama, H.; Nishi, M.; Aso, N.; Onizuka, K.; Yosihama, T.; Nukui, K.; Kodama, K.; Kakurai, K.; Ueda, Y. Direct evidence for the localized single-triplet excitations and the dispersive multitriplet excitations in SrCu2(BO3)2. Phys. Rev. Lett. 2000, 84, 5876–5879. [Google Scholar]
- Rüegg, C.; Cavadini, N.; Furrer, A.; Güdel, H.U.; Krämer, K.W.; Mutka, H.; Wildes, A.; Habicht, K.; Vorderwisch, P. Bose-Einstein condensation of the triplet states in the magnetic insulator TlCuCl3. Nature 2003, 423, 62–65. [Google Scholar] [CrossRef]
- Rüegg, C.; Kiefer, K.; Thielemann, B.; McMorrow, D.F.; Zapf, V.; Normand, B.; Zvonarev, M.B.; Bouillot, P.; Kollath, C.; Giamarchi, T.; et al. Thermodynamics of the spin luttinger liquid in a model ladder material. Phys. Rev. Lett. 2008, 101. [Google Scholar]
- Thielemann, B.; Rüegg, C.; Rønnow, H.M.; Läuchli, A.M.; Caux, S.; Normand, B.; Biner, D.; Krämer, K.W.; Güdel, H.U.; Stahn, J.; et al. Direct observation of magnon fractionalization in the quantum spin ladder. Phys. Rev. Lett. 2009, 102. [Google Scholar]
- Patyal, B.R.; Scott, B.L.; Willett, R.D. Crystal-Structure, magnetic-susceptibility, and EPR studies of bis(piperidinium)tetrabromocuprate(II): A novel monomer system showing spin diffusion. Phys. Rev. B 1990, 41, 1657–1663. [Google Scholar]
- Fernandez, V.; Moran, M.; Gutierrez-Rios, M.T.; Foces-Foces, C.; Cano, F.H. EPR and X-ray structural study of some tetrahalocuprates CuX42− (X = Cl, Br) of protonated ammines with thermochromic properties. Inorg. Chim. Acta 1987, 128, 239–243. [Google Scholar] [CrossRef]
- Battaglia, L.P.; Corradi, A.B.; Geiser, U.; Willett, R.D.; Motori, A.; Sandrolini, F.; Antolini, L.; Manfredini, T.; Menabue, L.; Pellacani, G.C. The crystal structures, magnetic and electrical properties of two polymeric chlorocuprate(II) compounds. J. Chem. Soc. Dalton Trans. 1988, 265–271. [Google Scholar]
- A .cif file with the crystallographic data of (C5H12N)Cu2Br3 can be obtained from the Cambridge structure database at http://www.ccdc.cam.ac.uk/ under reference number CCDC 873300.
- Stoe & Cie, X-Area V1.17 & X-RED32 V1.04 Software; Stoe & Cie GmbH: Darmstadt, Germany, 2002.
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Cryst. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Gottingen, Germany, 1997. [Google Scholar]
- Spek, A.L. Single-Crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Komm, T.; Biner, D.; Neels, A.; Krämer, K.W. (C5H12N)Cu2Br3: A Piperidinium Copper(I) Bromide with [Cu2Br3]− Ladders. Crystals 2012, 2, 1434-1440. https://doi.org/10.3390/cryst2041434
Komm T, Biner D, Neels A, Krämer KW. (C5H12N)Cu2Br3: A Piperidinium Copper(I) Bromide with [Cu2Br3]− Ladders. Crystals. 2012; 2(4):1434-1440. https://doi.org/10.3390/cryst2041434
Chicago/Turabian StyleKomm, Theresa, Daniel Biner, Antonia Neels, and Karl W. Krämer. 2012. "(C5H12N)Cu2Br3: A Piperidinium Copper(I) Bromide with [Cu2Br3]− Ladders" Crystals 2, no. 4: 1434-1440. https://doi.org/10.3390/cryst2041434




