Determination of the Absolute Configuration of Aegelinol by Crystallization of Its Inclusion Complex with β-Cyclodextrin
Abstract
:1. Introduction
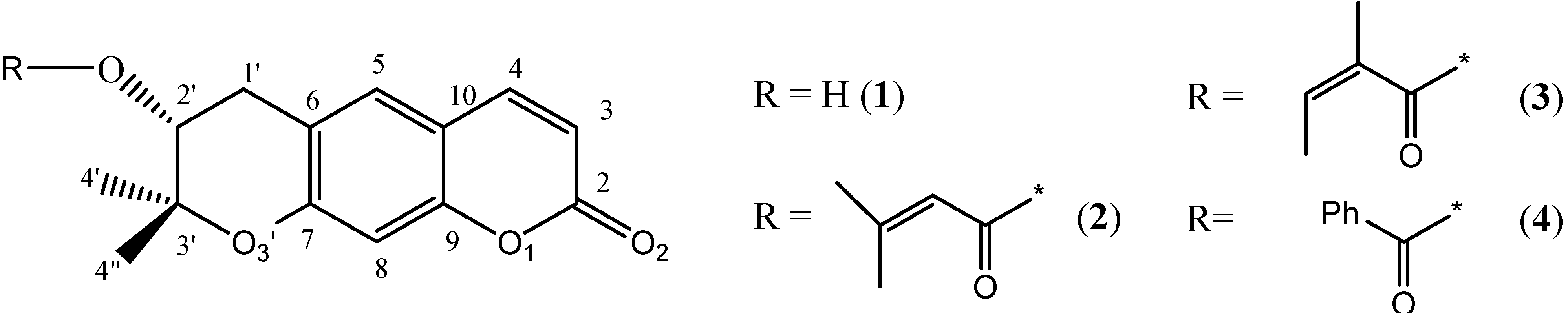
2. Results and Discussion
| Crystallographic data | Aegelinol | Β-CD-aegelinol |
|---|---|---|
| Chemical formula | C14H14O4 | 2C42H70O35. 2C14H14O4.23.5H2O |
| Temperature (K) | 150.05(10) | 150(2) |
| Wavelength (Å) | Mo Kα (0.71073) | Cu Kα (1.54179) |
| Crystal system | Orthorhombic | Triclinic |
| Space group | P212121 | P1 |
| a (Å) | 6.8921(3) | 15.404(1) |
| b (Å) | 11.4302(9) | 15.281(1) |
| c (Å) | 44.964(3) | 17.890(1) |
| α (°) | 90.00 | 99.662(1) |
| β (°) | 90.00 | 113.423(1) |
| γ (°) | 90.00 | 102.481(1) |
| Volume (Å3) | 3542.2(4) | 3618.4(5) |
| Z | 12 | 1 |
| Calculated density (g.cm−3) | 1.385 | 1.466 |
| F(0 0 0) | 1560 | 1704 |
| Absorption coefficient (mm−1) | 0.102 | 1.148 |
| Crystal description | Long Rod | Plate |
| Crystal size (mm) | 0.2 × 0.2 × 0.4 | 0.13 × 0.32 × 0.37 |
| Theta range for data collection (°) | 3.25- 25.02 | 2.81–69.59 |
| Limiting indices | −8 ≤ h ≤ 6, −11 ≤ k ≤ 13, −46 ≤ l ≤ 53 | −18 ≤ h ≤ 14, −18 ≤ k ≤ 18, −21 ≤ l ≤ 21 |
| Reflections collected/unique | 10327/5883 | 36967/15938 |
| R (int) | 0.0246 | 0.0355 |
| Data/restraints/parameters | 5883/0/491 | 15938/88/2082 |
| Goodness-of-fit (S) on F2 | 1.097 | 1.060 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Final R indices [I > 2sigma(I)] | 4.44% | 6.71% |
| Final R indices (all data) | 5.22% | 7.1% |
| Largest difference peak and hole (e/Å3) | 0.218 and −0.229 | 1.249 and −0.419 |
| Flack × parameter | 0.4(9) | 0.5(2) |

| Geometrical aspects | 1 A | 1 B | 1 C | 1-BCD A | 1-BCD B |
|---|---|---|---|---|---|
| C6-C1` | 1.503(3) | 1.501(3) | 1.501(3) | 1.49(2) | 1.52(2) |
| C1`-C2` | 1.518(4) | 1.523(4) | 1.520(4) | 1.45(2) | 1.49(3) |
| C2`-O2` | 1.431(3) | 1.422(3) | 1.429(3) | 1.43(2) | 1.43(2) |
| C2`- C3` | 1.522(4) | 1.527(4) | 1.521(4) | 1.52(2) | 1.45(3) |
| C3`-O3` | 1.463(3) | 1.470(3) | 1.467(3) | 1.46(2) | 1.50(2) |
| C7-O3` | 1.363(3) | 1.361(3) | 1.34(2) | 1.35(1) | 1.36(2) |
| C7-C6-C1` | 120.0(2) | 120.0(2) | 120.1(2) | 121(1) | 120(1) |
| C6-C1`-C2` | 110.4(2) | 111.6(2) | 110.2(2) | 110(1) | 113(2) |
| O2`-C2`-C1` | 111.4(2) | 111.8(2) | 111.3(2) | 110(1) | 114(2) |
| C1`-C2`-C3` | 110.3(2) | 110.7(2) | 110.6(2) | 114(1) | 112(2) |
| O3`-C3`-C2` | 109.7 (2) | 109.7(2) | 110.6(2) | 106(1) | 114(1) |
| O3`-C7-C6 | 122.9(2) | 123.0(2) | 123.2(2) | 122(1) | 125(1) |
| C7-O3`-C3` | 118.6 (2) | 118.4 (2) | 119.0(2) | 118(1) | 114(1) |
| C5-C6-C1`-C2` | −156.9(2) | −159.3(2) | −154.0(2) | −165(1) | 170(2) |
| C7-C6-C1`-C2` | 21.4(3) | 20.5(3) | 24.7(3) | 14(1) | −10(3) |
| C6-C1`-C2`-O2` | 70.3(3) | 79.0(3) | 71.9(3) | −172(1) | −78(3) |
| C6-C1`-C2`-C3` | −50.4(3) | −47.1(3) | −50.8(3) | −46(1) | 37(3) |
| O2`-C2`-C3`-O3` | −62.3(3) | −67.7(3) | −65.7(3) | −173(1) | 68(2) |
| C1`-C2`-C3`-O3` | 60.0(3) | 58.2(3) | 57.7(3) | 62(1) | −55(2) |
| O2`-C2`-C3`-C4` | 177.6(2) | 172.2(2) | 173.7(2) | −60(1), 69(2) | −176(1), −53(2) |
| C1`-C2`-C3`-C4` | −60.0(3) | −62.0(3) | −62.9(3) | 175(1), −56(2) | 62(2), −175(2) |
| C1`-C6-C7-O3` | −0.4(4) | −4.0(4) | −4.3(4) | 1(1) | 0(2) |
| C8-C7-O3`-C3` | −172.5(2) | −166.6(2) | −172.4(2) | −162.8(9) | 162(1) |
| C6-C7- O3`-C3` | 10.1(3) | 15.8(3) | 11.0(4) | 18(2) | −16(2) |
| C2`-C3`-O3`-C7 | −39.6(3) | −42.6(3) | −37.6(3) | −47(1) | 44(2) |
| C4`-C3`-O3`-C7 | 82.3(3) | 79.1(3) | 84.9(3) | −165(1), 75(1) | −77(2), 172(2) |
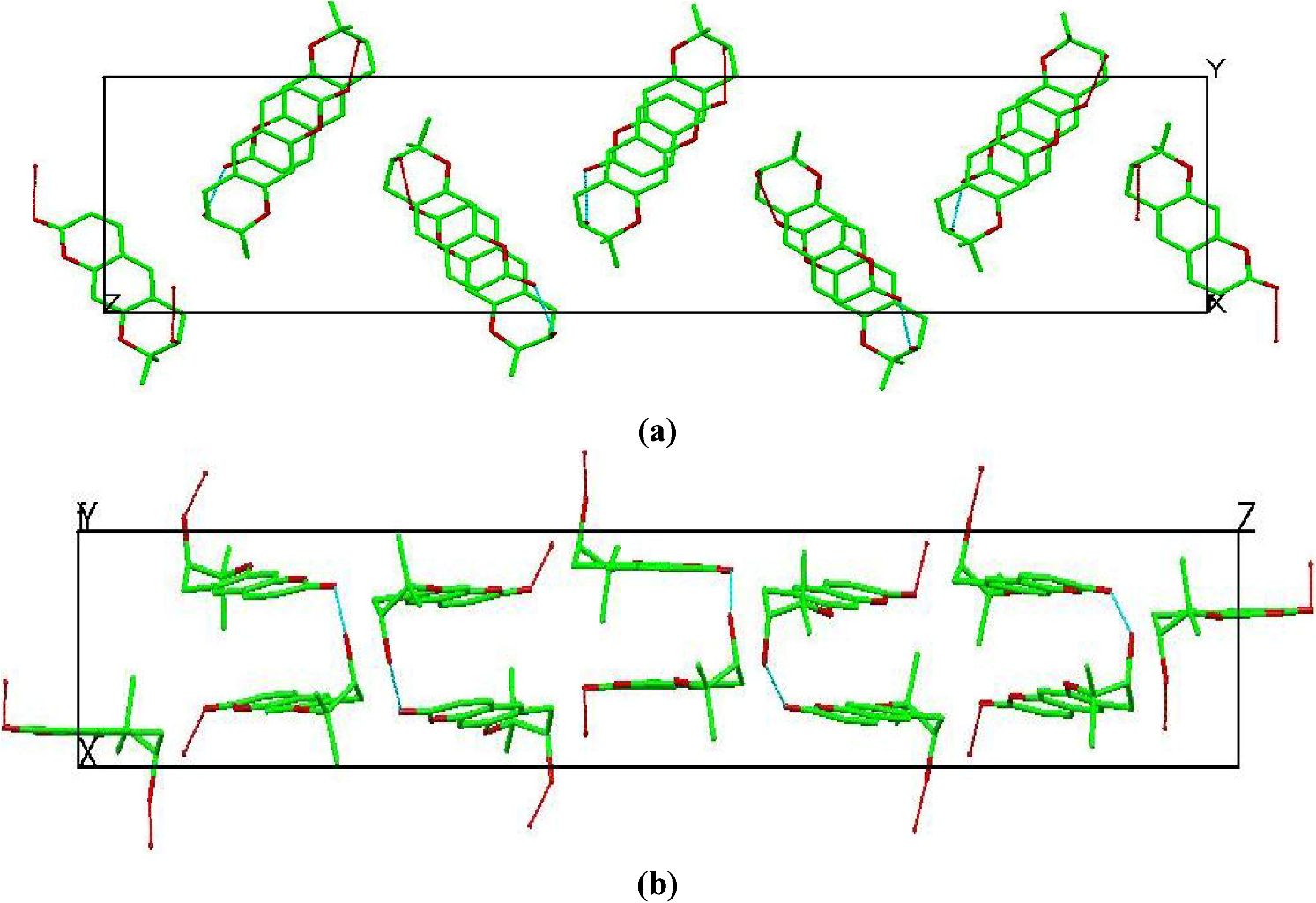
| Aegelinol (1) | |||||
|---|---|---|---|---|---|
| D | A | d (D-H) (Å) | d (H...A) (Å) | d (D...A) (Å) | <(DHA) (°) |
| O(2`A) | O(2A) i | 0.84 | 2.08 | 2.902(3) | 164.5 |
| O(2`B) | O(2C) ii | 0.84 | 2.01 | 2.816(2) | 159.4 |
| O(2`C) | O(2B) | 0.84 | 2.03 | 2.851(3) | 165.8 |
| BCD-1 complex ( 1-BCD) | |||||
| O2`A1 | O(13W) | 0.84 | 2.122 | 2.73(1) | 129.16 |
| O2`B1 | O(61A) | 0.84 | 2.524 | 3.278(3) | 150.01 |
| O2`A2 | O(17`W) | 0.84 | 2.04 | 2.49(2) | 145.6 |
| O2`B2 | O(63B) iii | 0.84 | 2.065 | 2.83 (2) | 151.37 |
| O(65B) | O2`A2 iv | 0.84 | 2.05 | 2.76(2) | 144.5 |
- (1) It implies that crystal packing does not severely limit conformational and translational freedom of guest molecules.
- (2) It provides an environment for the guest molecules that resembles a macromolecular binding pocket by providing a hydrophobic pocket rimmed with a scaffolding of hydrophilic binding sites that can interact directly with guest molecules or via bridging water molecules.

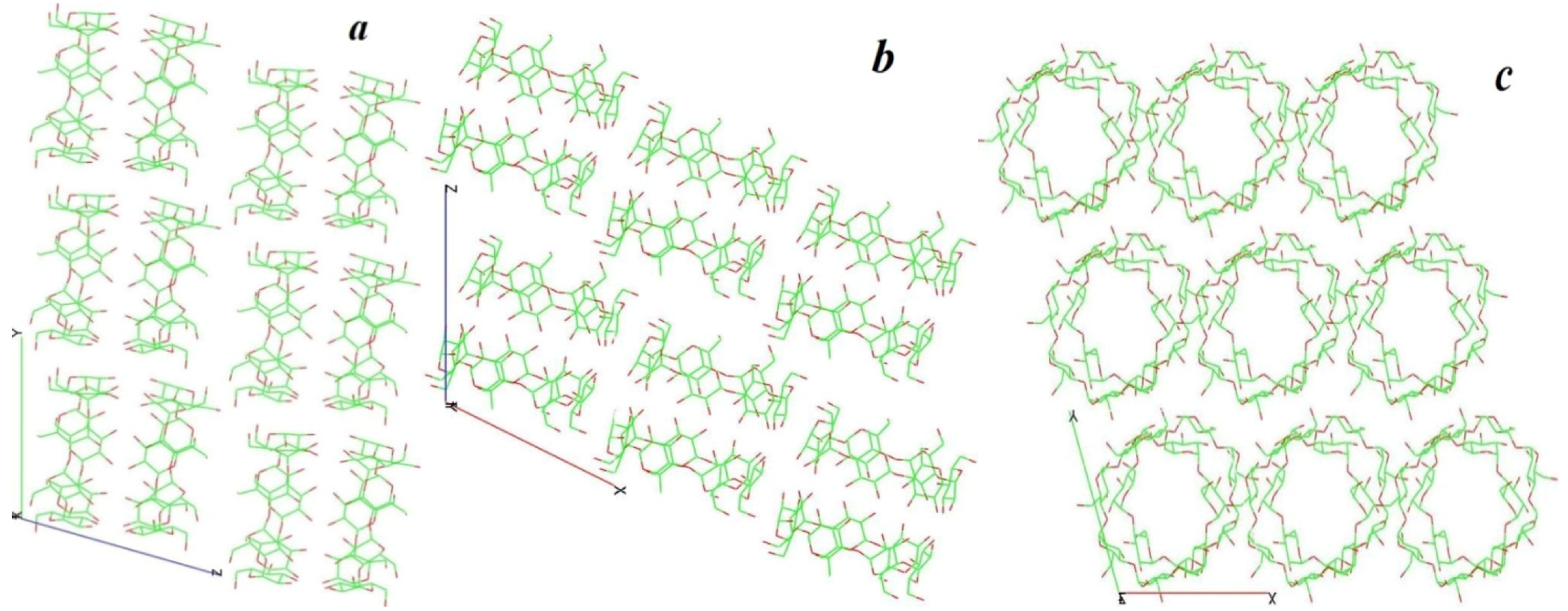
3. Experimental Section
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Alkhatib, R.; Hennebelle, T.; Roumy, V.; Sahpaz, S.; Suzgeç, S.; Akalın, E.; Meriçli, A.H.; Bailleul, F. Coumarins, caffeoyl derivatives and a monoterpenoid glycoside from Ferulago Asparagifolia. Biochem. Syst. Ecol. 2009, 37, 230–233. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sen, R.; Ganguly, D. Aegelinol, a minor lactonic constituent of Aegle Marmelos. Phytochemistry 1978, 17, 328–329. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant activities of coumarins from the roots of ferulago campestris (apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Abyshev, A.Z.; Gindin, V.A.; Semenov, E.V.; Agaev, E.M.; Abdulla-Zade, A.A.; Guseinov, A.B. Structure and biological properties of 2H-1-benzopyran-2-one (coumarin) derivatives. Pharm. Chem. J. 2006, 40, 607–610. [Google Scholar]
- Lemmich, J.; Nielsen, B.E. Stereochemistry of natural coumarins containing the 3-hydroxy-2,2-dimethylchroman system. Tetrahedron Lett. 1969, 1, 3–4. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. Coumarins derivatives from Eryngium Campestre. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Flack, H.D. On Enantiomorph-Polarity Estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar]
- Bernardinelli, G.; Flack, H.D. Least-squares absolute-structure refinement. Practical experience and ancillary calculations. Acta Cryst. 1985, A41, 500–511. [Google Scholar]
- Flack, H.D.; Bernardinelli, G. Reporting and evaluating absolute-structure and absolute-configuration determinations. J. Appl. Cryst. 2000, 33, 1143–1148. [Google Scholar]
- Harata, K.; Uekama, K.; Otagiri, M.; Hirayama, F. Crystal structures of cyclodextrin complexes with chiral molecules. J. Incl. Phenom. 1984, 2, 583–594. [Google Scholar] [CrossRef]
- Harata, K.; Uekama, K.; Otagiri, M.; Hirayama, F. The structure of the cyclodextrin complex. XIX. Crystal structures of hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin complexes with (S)- and (R)-mandelic acid. chiral recognition through the induced-fit conformational change of the macrocyclic ring. Bull. Chem. Soc. Jpn. 1987, 60, 497–502. [Google Scholar] [CrossRef]
- Harata, K.; Uekama, K.; Imai, T.; Hirayama, F.; Otagiri, M. Crystal structures of heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin complexes with (R)- and (S)-Flurbiprofen. J. Incl. Phenom. Molcycl. Chem. 1988, 6, 443–460. [Google Scholar]
- Brown, G.R.; Caira, M.R.; Nassimbeni, L.R.; van Oudtshoorn, B. Inclusion of ibuprofen by heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin: An X-ray diffraction and thermal analysis study. J. Incl. Phenom. Mol. Recogn. Chem. 1996, 26, 281–294. [Google Scholar] [CrossRef]
- Makedonopoulou, S.; Yannakopoulou, K.; Mentzafos, D.; Lamzin, V.; Popov, A.; Mavridis, I.M. Non-covalent interactions in the crystallization of the enantiomers of 1,7-dioxaspiro[5.5]undecane (olive fly sex pheromone) by enantiospecific cyclodextrin hosts, hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57, 399–409. [Google Scholar] [CrossRef]
- Yannakopoulou, K.; Mentzafos, D.; IMavridis, M.; Dandika, K. Chiral Recognition of (R)-(−)-1,7-Dioxaspiro-[5.5]undecane by Hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin. Angew. Chem. 1996, 35, 2480–2482. [Google Scholar] [CrossRef]
- Mentzafos, D.; Mavridis, I.M.; Yannakopoulou, K. Structure of the 1:1 Complex of Hexakis(2,3,6-tri-O-methyl) α-Cyclodextrin with (R)-(-)-1,7-Dioxaspiro[5.5]undecane. J. Incl. Phenom. Macrocycl. Chem. 1999, 33, 321–330. [Google Scholar] [CrossRef]
- Alexander, J.M.; Clark, J.L.; Brett, T.J.; Stezowski, J.J. Chiral discrimination in cyclodextrin complexes of amino acid derivatives: β-cyclodextrin N-acetyl-L-phenylalanine and N-acetyl-D-phenylalanine complexes. Proc. Nat. Acad. Sci. USA 2002, 99, 5115–5120. [Google Scholar] [CrossRef]
- Caira, M.R.; de Vries, E.; Nassimbeni, L.R.; Jacewicz, V.W. Inclusion of the antidepressant paroxetine in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2003, 46, 37–42. [Google Scholar] [CrossRef]
- Harata, K. The structure of the cyclodextrin complex. XII. Crystal structure of α-cyclodextrin-1-phenylethanol (1:1) tetrahydrate. Bull. Chem. Soc. Jpn. 1982, 55, 1367–1371. [Google Scholar] [CrossRef]
- Ferron, L.; Guillen, F.; Coste, S.; Coquerel, G.; Plaquevent, J.-C. Is the optical rotation sign/absolute configuration relationship still a problem? Examples taken from unnatural quaternary aminoacids. Chirality 2006, 18, 662–666. [Google Scholar] [CrossRef]
- Clark, J.L.; Stezowski, J.J. Molecular recognition in cyclodextrin complexes of amino acid derivatives. 1. Crystallographic studies of â-cyclodextrin complexes with N-Acetyl-L-phenylalanine methyl ester and N-Acetyl-L-phenylalanine amide pseudopeptides. J. Am. Chem. Soc. 2001, 123, 9880–9888. [Google Scholar] [CrossRef]
- Clark, J.L.; Booth, B.R.; Stezowski, J.J. Molecular recognition in cyclodextrin complexes of amino acid derivatives. 2.1. A new perturbation: The room-temperature crystallographic structure determination for the N-Acetyl-p-methoxy-L-phenylalanine methyl ester/â-cyclodextrin complex. J. Am. Chem. Soc. 2001, 123, 9889–9895. [Google Scholar]
- Clark, J.L.; Peinado, J.; Stezowski, J.J.; Vold, R.L.; Huang, Y.Y.; Hoatson, G.L. Molecular recognition in cyclodextrin complexes of amino acid derivatives: The effects of kinetic energy on the molecular recognition of a pseudopeptide in a nonconstraining host environment as revealed by a temperature-dependent crystallographic study. J. Phys. Chem. B 2006, 110, 26375–26387. [Google Scholar]
- Grandeury, A.; Petit, S.; Gouhier, G.; Agasse, V.; Coquerel, G. Enantioseparation of 1-(p-bromophenyl)ethanol by crystallization of host–guest complexes with permethylated β-cyclodextrin: Crystal structures and mechanisms of chiral recognition. Tetrahedron Asymmetry 2003, 14, 2143–2152. [Google Scholar]
- Amharar, Y.; Grandeury, A.; Sanselme, M.; Petit, S.; Coquerel, G. Crystallization and structural investigation of supramolecular compounds with modified cyclodextrins. Application to chiral discrimination. Ann. Pharm. Fr. 2010, 68, 212–217. [Google Scholar] [CrossRef]
- Grandeury, A.; Renou, L.; Dufour, F.; Petit, S.; Gouhier, G.; Coquerel, G. Chiral resolution by crystallization of host-guest supramolecular complexes: A paradoxal situation with an efficient discrimination despite structural similarities. J. Therm. Anal. Calorim. 2004, 77, 377–390. [Google Scholar] [CrossRef]
- Caira, M.R.; Griffith, V.J.; Nassimbeni, L.R.; van Oudtshoorn, B. X-ray structure and thermal analysis of a 1:1 complex between (S)-naproxen and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin. J. Incl. Phenom. Mol. Recogn. Chem. 1995, 20, 277–290. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Chen, L.Y. Crystal structures of inclusion complexes of β-Cyclodextrin with (S)-(+)- and (R)-(-)-fenoprofen. J. Am. Chem. Soc. 1988, 110, 4379–4391. [Google Scholar] [CrossRef]
- Harata, K. Role of hydrogen bond and spacial fitting in the chiral recognition by cyclodextrins. Crystal structures of hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin inclusion complexes with (R)- and (S)-1-phenylethanol. J. Chem. Soc. Perkin Trans. 2 1990, 5, 799–804. [Google Scholar] [CrossRef]
- Malpezzi, L.; Fronza, G.; Fuganti, C.; Mele, A.; Bruckner, S. Crystal architecture and conformational properties of the inclusion complex, neohesperidin dihydrochalcone-cyclomaltoheptaose (β-cyclodextrin), by X-ray diffraction. Carbohydr. Res. 2004, 339, 2117–2125. [Google Scholar] [CrossRef]
- Steiner, T.; Saenger, W. Relief of steric strain by intramolecular C-H···O interactions: Structural evidence for the 1,4-disubstituted cyclohexanes. J. Chem. Soc. Perkin Trans. 2 1998, 2, 371–378. [Google Scholar] [CrossRef]
- Kokkinou, A.; Tsorteki, F.; Karpusas, M.; Papakyriakou, A.; Bethanis, K.; Mentzafos, D. Study of the inclusion of the (R)- and (S)-camphor enantiomers in α-cyclodextrin by X-ray crystallography and molecular dynamics. Carbohydr. Res. 2010, 345, 1034–1040. [Google Scholar] [CrossRef]
- Doriguetto, A.C.; Ellena, J.; Santos, M.H.; Moreira, M.E.C.; Nagem, T.J. Mammeigin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, o350–o352. [Google Scholar] [CrossRef]
- Gonzalez, J.C.; Lobo-Antunes, J.; Perez-Lourido, P.; Santana, L.; Uriarte, E. Synthesis of angular pyrrolocoumarins. Synthesis 2002, 4, 475–478. [Google Scholar]
- Saenger, W.; Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D. Inclusion Compounds; Academic Press: London, UK, 1984; Volume 2, pp. 231–259. [Google Scholar]
- Mentzafos, D.; Mavridis, M.; LeBas, G.; Tsoucaris, G. Structure of the 4-tert-butylbenzyl alcohol-[beta]-cyclodextrin complex. Common features in the geometry of [beta]-cyclodextrin dimeric complexes. Acta Crystallogr. Sect. B 1991, 47, 746–757. [Google Scholar] [CrossRef]
- Brett, T.J.; Alexander, J.M.; Stezowski, J.J. Chemical insight from crystallographic disorder-structural studies of supramolecular photochemical systems. Part 2. The β-cyclodextrin-4,7-dimethylcoumarin inclusion complex: A new β-cyclodextrin dimer packing type, unanticipated photoproduct formation, and an examination of guest influence on β-CD dimer packing. Chem. Soc. Perkin Trans. 2 2000, 6, 1095–1103. [Google Scholar]
- CrysAlisPro, version 1.171.33.55, Oxford Diffraction Ltd.: Oxfordshire, UK, 2010.
- Sheldrich, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Zhao, Y.L.; Benitez, D.; Yoon, I.; Stoddart, J.F. Inclusion behavior of β-cyclodextrin with bipyridine molecules: Factors governing host-guest inclusion geometries. Chem. Asian J. 2009, 4, 446–456. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elasaad, K.; Alkhatib, R.; Hennebelle, T.; Norberg, B.; Wouters, J. Determination of the Absolute Configuration of Aegelinol by Crystallization of Its Inclusion Complex with β-Cyclodextrin. Crystals 2012, 2, 1441-1454. https://doi.org/10.3390/cryst2041441
Elasaad K, Alkhatib R, Hennebelle T, Norberg B, Wouters J. Determination of the Absolute Configuration of Aegelinol by Crystallization of Its Inclusion Complex with β-Cyclodextrin. Crystals. 2012; 2(4):1441-1454. https://doi.org/10.3390/cryst2041441
Chicago/Turabian StyleElasaad, Kossay, Racha Alkhatib, Thierry Hennebelle, Bernadette Norberg, and Johan Wouters. 2012. "Determination of the Absolute Configuration of Aegelinol by Crystallization of Its Inclusion Complex with β-Cyclodextrin" Crystals 2, no. 4: 1441-1454. https://doi.org/10.3390/cryst2041441




