Hexaethylguanidinium Salts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Considerations
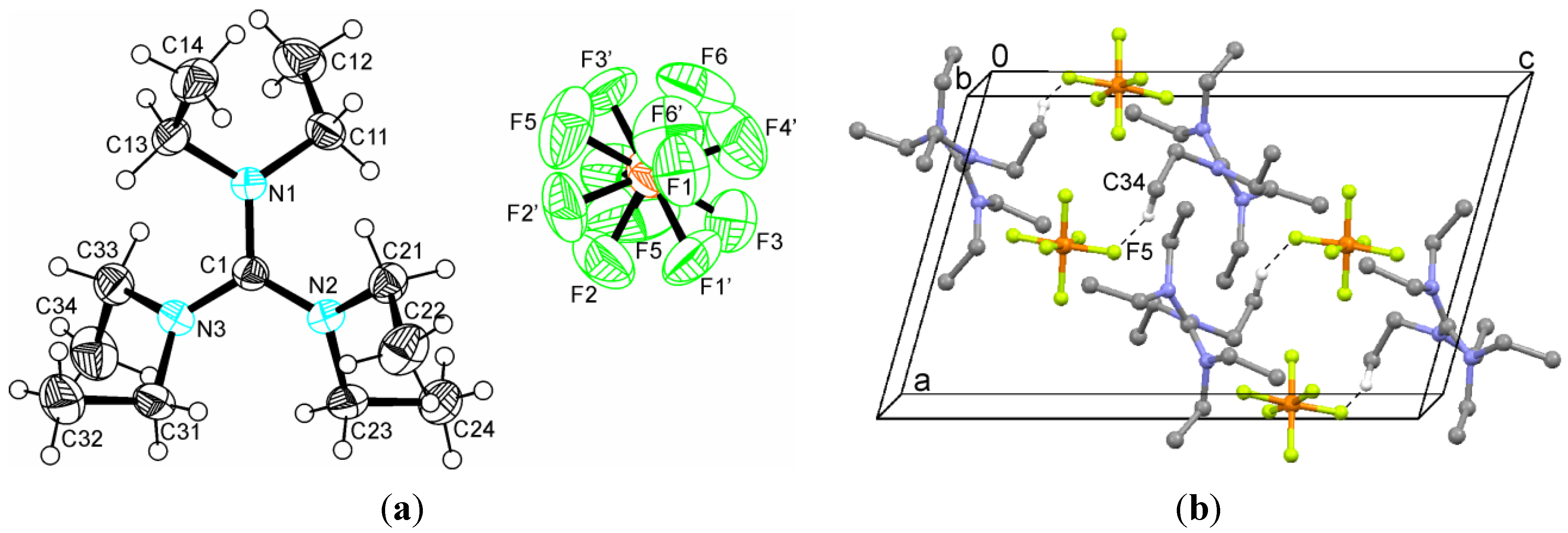
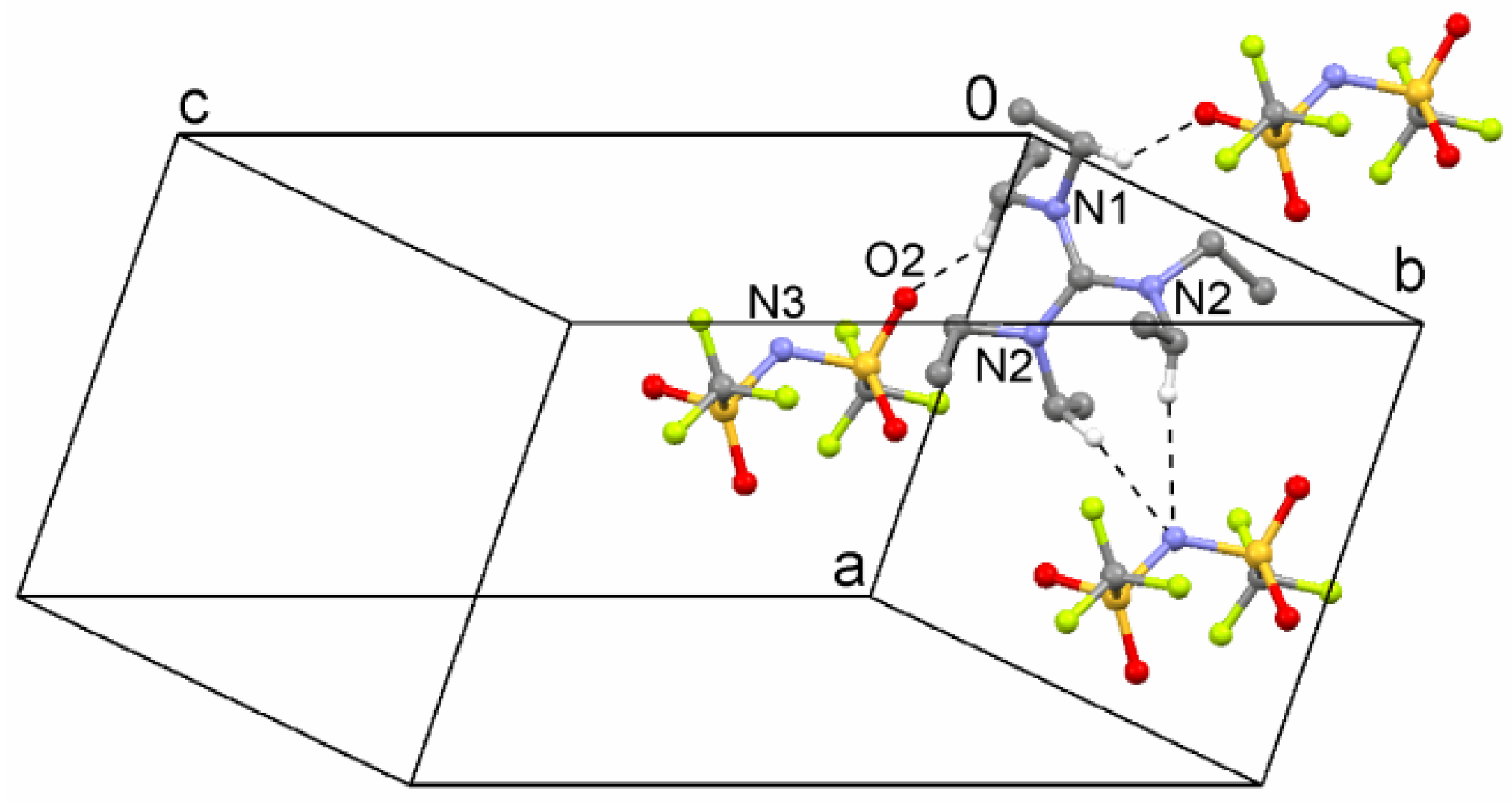
2.2. Crystal Structures

| Compound | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCDC No. | 976081 | 976082 | 976083 | 976084 | 976085 | |||||
| Chemical formula | C13H30N3·F6P | C13H30N3·Cl4Fe | (C13H30N3)2·Cl4Cu·(OH2)0.67 | C13H30N3·(Br4Co)0.42·Br0.16·OH2 | C13H30N3·C2F6NO4S2 | |||||
| Mr | 373.37 | 426.05 | 674.16 | 418.20 | 508.55 | |||||
| Crystal size/mm3 | 0.15 × 0.15 × 0.10 | 0.26 × 0.18 × 0.14 | 0.20 × 0.15 × 0.15 | 0.20 × 0.15 × 0.15 | 0.26 × 0.20 × 0.03 | |||||
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Trigonal | Tetragonal | |||||
| Space group | P21/n | P21/n | C2/c | R3c | P43212 | |||||
| a/Å | 9.6283(4) | 13.1905(4) | 16.2803(9) | 17.4600(2) | 9.05490(17) | |||||
| b/Å | 12.9449(4) | 10.3657(3) | 14.6412(7) | – | – | |||||
| c/Å | 15.5960(6) | 15.9222(5) | 17.1885(11) | 34.5190(3) | 29.3944(13) | |||||
| β/° | 105.511(4) | 102.698(3) | 117.797(8) | – | – | |||||
| V/Å3 | 1873.05(12) | 2123.78(11) | 3624.4(4) | 9113.33(17) | 2410.08(12) | |||||
| Z | 4 | 4 | 4 | 18 | 4 | |||||
| Dx/g·cm−3 | 1.32 | 1.33 | 1.24 | 1.37 | 1.40 | |||||
| μ/mm−1 | 1.83 | 1.21 | 0.92 | 4.02 | 2.69 | |||||
| F(000)/e | 792 | 892 | 1477 | 3865 | 1064 | |||||
| Diffractometer | Gemini-R Ultra | Gemini-R Ultra | Gemini-R Ultra | Nonius KappaCCD | Gemini-R Ultra | |||||
| Radiation | CuKα | MoKα | MoKα | MoKα | CuKα | |||||
| Data collection method | ω scans | ω scans | ω scans | φ and ω scans | ω scans | |||||
| T/K | 223 | 173 | 173 | 233 | 173 | |||||
| θmax/° | 67.5 | 25.4 | 25.1 | 25.0 | 67.0 | |||||
| h, k, l range | −11 ≤ h ≤ 10 −14 ≤ k ≤ 15 −18 ≤ l ≤ 16 | −15 ≤ h ≤ 13 −12 ≤ k ≤ 11 −19 ≤ l ≤ 19 | −19 ≤ h ≤ 19 −17 ≤ k ≤ 17 −20 ≤ l ≤ 20 | −20 ≤ h ≤ 20 −20 ≤ k ≤ 20 −40 ≤ l ≤ 40 | −9 ≤ h ≤ 10 −10 ≤ k ≤ 10 −31 ≤ l ≤ 35 | |||||
| Absorption correction | multi-scan | multi-scan | multi-scan | none | analytical | |||||
| Measured reflections | 18889 | 17064 | 49168 | 18293 | 9170 | |||||
| Independent reflections (Rint) | 3338 (0.040) | 3889 (0.032) | 3195 (0.065) | 1771 (0.038) | 2139 (0.040) | |||||
| Observed reflections [I ≥ 2σ(I)] | 2616 | 3263 | 2502 | 1580 | 1884 | |||||
| Restraints/parameters | 54/300 | 0/196 | 2/213 | 0/98 | 9/151 | |||||
| R1/wR2 [I ≥ 2σ(I)] | 0.049/0.085 | 0.031/0.076 | 0.060/0.108 | 0.052/0.138 | 0.048/0.123 | |||||
| R1/wR2 (all data) | 0.064/0.090 | 0.042/0.083 | 0.075/0.114 | 0.057/0.141 | 0.054/0.130 | |||||
| Goodness of fit | 1.02 | 1.02 | 0.93 | 1.07 | 1.05 | |||||
| Flack parameter x | – | – | – | – | 0.04(4) | |||||
| ∆ρmax/min/e Å−3 | 0.24/−0.21 | 0.42/−0.23 | 0.64/−0.92 | 1.19/−0.68 | 0.31/−0.19 | |||||
| Compound | Interaction | H···A | D···A | <D–H···A | Symmetry A |
|---|---|---|---|---|---|
| 1 | C34–H···F5 | 2.589 | 3.455(5) | 148.6 | x,y,z |
| C14–H···F5 | 2.612 | 3.439(4) | 143.2 | −1 + x,y,z | |
| C14–H···F2 | 2.631 | 3.578(4) | 165.2 | −1/2 + x,1/2 − y,1/2 + z | |
| 2 | C2–H···Cl1 | 2.9110 | 3.699(2) | 137.3 | x,y,z |
| C11–H···Cl3 | 2.9164 | 3.697(2) | 136.4 | −1/2 + x,3/2 − y,1/2 + z | |
| 3 | O1–H···Cl2 | 2.18(3) | 2.996(7) | 156(7) | x,y,z |
| C14–H···O1 | 2.526 | 3.235(9) | 130.7 | 1 − x,−y,1 − z | |
| C13–H···O1 | 2.683 | 3.182(8) | 112.4 | 1 − x,−y,1 − z | |
| C11–H···Cl1 | 2.928 | 3.858(3) | 161.0 | 1/2 − x,1/2 − y,1 − z | |
| 4 | O2–H···Br1 | – | 2.76(2) | – | x − y,−y,1/2 − z |
| C5–H···O2 | 2.50 | 3.41(4) | 156.6 | x − y,−y,1/2 − z | |
| O2–H···Br1 | – | 3.19(3) | – | y,x,1/2 − z | |
| O1–H···Br1 | – | 3.30(1) | – | x − y,−y,1/2 − z | |
| C2–H···Br1 | 3.001 | 3.913(6) | 154.9 | −1/3 + x − y,1/3 + x,1/3 − z | |
| C5–H···O1 | 2.70 | 3.66(2) | 168.0 | x,y,z | |
| 5 | C7–H···O2 | 2.515 | 3.356(5) | 142.6 | y,−1 + x,−z |
| C2–H···N3 | 2.62 | 3.582(7) | 170 | x,y,z | |
| C8–H···O1 | 2.707 | 3.657(5) | 163.5 | −1 + y,−1 + x,−z |


2.3. Cyclic Voltammetry of Hexaethylguanidinium Tetrachloroferrate(III) (2)

3. Experimental Section
3.1. Hexaethylguanidinium Hexafluorophosphate (1)
3.2. Hexaethylguanidinium Tetrachloroferrate(III) (2)
3.3. Bis(Hexaethylguanidinium) Tetrachlorocuprate(II) Hydrate (3)
3.4. Bis(Hexaethylguanidinium) Tetrabromocobaltate(II)/Bromide Hydrate (4)
3.5. Hexaethylguanidinium Bis(Trifluoromethanesulfonyl)Imide (5)
4. Conclusions
Author Contributions
Appendix
Conflicts of Interest
References
- Starks, C.M. Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. J. Am. Chem. Soc. 1971, 93, 195–199. [Google Scholar] [CrossRef]
- Jones, R.A. Applications of phase-transfer catalysis in organic synthesis. Aldrichim. Acta 1976, 9, 35–45. [Google Scholar]
- Halpern, M. What is Aliquat® 336 and Adogen® 464 HF? Available online: http://phasetransfer.com/WhatisAliquat336andAdogen464.pdf (accessed on 10 July 2014).
- BASF Homepage. Available online: http://www.phasetransfer.com/suppliers/cognis.htm (accessed on 10 July 2014).
- CAS Substance search. Available online: https://scifinder.cas.org/scifinder/login (accessed on 10 July 2014).
- Aliquat HTA-1. Available online: http://www.mining-solutions.basf.com/ev/internet/mining-solutions/en/function/conversions:/publish/content/mining-solutions/download-center/technical-data-sheets/pdf/xAliquat_HTA-1_TI_EVH_0145.pdf (accessed on 10 July 2014).
- Aliquat HTA-1. Available online: http://e-applications.basf-ag.de/data/basf-pcan/pds2/pds2-web.nsf/6A7E9E7EEC8E167BC125757700445100/$File/Aliquat_r_HTA-1_E.pdf (accessed on 10 July 2014).
- Hagen, H.; Kantlehner, W.; Speh, P. Verfahren zur Herstellung von N,N',N''-hexasubstituierten Guanidiniumhalogeniden. Germany Patent DE2826011A1, 3 January 1980. (In German)[Google Scholar]
- Kantlehner, W.; Haug, E.; Mergen, W.W.; Speh, P.; Maier, T.; Kapassakalidis, J.J.; Bräuner, H.-J.; Hagen, H. Herstellung von 1,1,2,3,3-pentasubstituierten und 1,1,2,2,3,3-hexasubstituierten Guanidiniumsalzen sowie von 1,1,2,3,3-Pentaalkylguanidinen. Liebigs Ann. Chem. 1984, 1984, 108–126. (In German) [Google Scholar]
- Kantlehner, W.; Haug, E.; Mergen, W.W.; Speh, P.; Maier, T.; Kapassakalidis, J.J.; Bräuner, H.-J.; Hagen, H. Ein Herstellungsverfahren für Hexaalkylguanidinium-chloride. Synthesis 1983, 15, 904–905. (In German) [Google Scholar]
- Caringi, J.J.; Faler, G.R.; Phelps, P.D.; Guggenheim, T.L.; Flowers, L.I.; Brunelle, D.J.; Odle, R.R. Preparation of Aqueous Solutions of Hexasubstituted Guanidinium Chlorides from Secondary Amines and Phosgene and Their Use as Phase-Transfer Catalysts. Europa Patent EP0784051A1, 16 July 1997. [Google Scholar]
- Brunelle, D.J.; Haitko, D.A.; Barren, J.P.; Singh, S. Process for Reaction of a Highly Polar Compound with a Nonpolar Compound in the Presence of Hexaalkylguanidinium Salts as Phase-Transfer Catalysts. Canada Patent CA2034435A1, 6 August 1991. [Google Scholar]
- Brunelle, D.J.; Haitko, D.A.; Barren, J.P.; Singh, S. Method for Conducting Organic Reactions Using Guanidinium Salt as Phase Transfer Catalyst. Canada Patent CA2044470A1, 14 September 1992. [Google Scholar]
- Schlama, T.; Alcaraz, L.; Mioskowski, C. A convenient epoxidation of α,β-unsaturated ketones with sodium hypochlorite under phase transfer conditions catalyzed by hexaethylguanidinium chloride (HEGCl). Synlett 1996, 1996, 571–572. [Google Scholar]
- King, J.R.; Phelps, P.D. Hexasubstituted Guanidinium Salts and Ultracapacitors Employing Them as Electrolytes. U.S. Patent 5,726,856,10, 10 March 1998. [Google Scholar]
- Bogdanov, M.G.; Svinyarov, I.; Kunkel, H.; Steinle, C.; Arkhipova, M.; Kantlehner, W.; Maas, G. Empirical polarity parameters for hexaalkylguanidinium-based room-temperature ionic liquids. Z. Naturforsch. 2010, 65b, 791–797. [Google Scholar]
- Large, T.; Müller, T.; Kunkel, H.; Buck, S.; Maas, G. Ruthenium- and rhodium-catalyzed carbenoid reactions of diazoesters in hexaalkylguanidinium-based ionic liquids. Z. Naturforsch. 2012, 67b, 347–353. [Google Scholar]
- Branco, L.C.; Serbanovic, A.; Nunes da Ponte, M.; Afonso, C.A.M. Clean osmium-catalyzed asymmetric dihydroxylation of olefins in ionic liquids and supercritical CO2 product recovery. Chem. Commun. 2005, 107–109. [Google Scholar]
- Wu, Y.-M.; Song, G.-L.; Wang, K.-L.; Heng, J.; Zhu, H.-J. Hexaethylguanidinium chloride. Acta Cryst. 2006, E62, o3519–o3520. [Google Scholar] [CrossRef]
- Weller, F.; Petz, W. Concerning the reaction of Cp2TiCl2 with [C(NMe2)3][(CO)4FeC(O)NMe2]—Crystal structure of [C(NMe2)3]2[FeCl4]. Z. Anorg. Allg. Chem. 1994, 620, 343–345. (In German) [Google Scholar]
- Frey, W.; Vettel, M.; Edelmann, K.; Kantlehner, W. Crystal structure of N,N,N',N',N'',N''-hexamethylguanidinium tetraphenylborate, C31H38BN3. Z. Kristallogr. New Cryst. Struct. 1998, 213, 77–78. [Google Scholar]
- Tanaka, M.; Siehl, H.-U.; Viefhaus, T.; Frey, W.; Kantlehner, W. An ONIOM study of a guanidinium salt ionic liquid. Experimental and computational characterization of N,N,N',N',N''-pentabutyl-N''-benzylguanidinium bromide. Z. Naturforsch. 2009, 64b, 765–772. [Google Scholar]
- Frey, W.; Orbegozo, T.; Spitzner, D.; Jäger, V. Crystal structure of (R)-N,N',N',N'',N''-pentamethyl-N-(1-phenylethyl)guanidinium iodide, [C14H24N3]I. Z. Kristallogr. New Cryst. Struct. 2009, 224, 251–252. [Google Scholar]
- Oelkers, B.; Sundermeyer, J. Pentaalkylmethylguanidinium methylcarbonates—Versatile precursors for the preparation of halide-free and metal-free guanidinium-based ILs. Green Chem. 2011, 13, 608–618. [Google Scholar]
- Castiglia, A.; Sehrawi, H.M.E.; Orbegozo, T.; Spitzner, D.; Claasen, B.; Frey, W.; Kantlehner, W.; Jäger, V. Synthesis and characterization of chiral guanidines and guanidinium salts derived from 1-phenylethylamine. Z. Naturforsch. 2012, 67b, 337–346. [Google Scholar]
- Tiritiris, I.; Kantlehner, W. 1-Benzyl-2-dimethylamino-3-methyl-3,4,5,6-tetrahydropyrimidin-1-ium bromide. Acta Crystallogr. 2012, E68, o2308. [Google Scholar] [CrossRef]
- Tiritiris, I.; Kantlehner, W. 2-Dimethylamino-1-(2-ethoxy-2-oxo-ethyl)-3-methyl-3,4,5,6-tetrahydropyrimidin-1-ium tetraphenylborate. Acta Crystallogr. 2012, E68, o2002. [Google Scholar] [CrossRef]
- Tiritiris, I.; Kantlehner, W. N,N,N',N',N''-Pentamethyl-N''-[3-(1,3,3-trimethylureido)-propyl]guanidinium tetraphenylborate. Acta Crystallogr. 2012, E68, o2223. [Google Scholar] [CrossRef]
- Frey, W.; Castiglia, A.; Jäger, V. Crystal structure of N,N',N''-trimethyl-N,N',N''-tris-(1-phenylethyl)guanidinium hexafluorophosphate, C28H36F6N3P. Z. Kristallogr. New Cryst. Struct. 2012, 227, 317–318. [Google Scholar]
- Froschauer, C.; Salchner, R.; Laus, G.; Weber, H.K.; Tessadri, R.; Griesser, U.; Wurst, K.; Kahlenberg, V.; Schottenberger, H. 1,3-di(alkoxy)imidazolium-based Ionic Liquids: Improved synthesis and crystal structures. Aust. J. Chem. 2013, 66, 391–395. [Google Scholar]
- Holbrey, J.D.; Reichert, W.M.; Rogers, R.D. Crystal structures of imidazolium bis(trifluoro-methanesulfonyl)imide “ionic liquid” salts: The first organic salt with a cis-TFSI anion conformation. Dalton Trans. 2004, 2267–2271. [Google Scholar]
- Xue, L.; DesMarteau, D.D.; Penningtn, W.T. Synthesis and structures of alkaline earth metal salts of bis[(trifluoromethyl)sulfonyl]imide. Solid State Sci. 2005, 7, 311–318. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Kabo, G.J.; Blokhin, A.V.; Shaplov, A.S.; Lozinskaya, E.I.; Golovanov, D.G.; Lyssenko, K.A.; Korlyukov, A.A.; Vygodskii, Y.S. IR and X-ray study of polymorphism in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides. J. Phys. Chem. B 2009, 113, 9538–9546. [Google Scholar] [CrossRef]
- Bentivoglio, G.; Schwärzler, A.; Wurst, K.; Kahlenberg, V.; Nauer, G.; Bonn, G.; Schottenberger, H.; Laus, G. Hydrogen bonding in the crystal structures of new imidazolium triflimide protic ionic liquids. J. Chem. Crystallogr. 2009, 39, 662–668. [Google Scholar]
- Laus, G.; Hummel, M.; Többens, D.M.; Gelbrich, T.; Kahlenberg, V.; Wurst, K.; Griesser, U.J.; Schottenberger, H. The 1:1 and 1:2 salts of 1,4-diazabicyclo[2.2.2]octane and bis(trifluoro-methylsulfonyl)amine: Thermal behaviour and polymorphism. CrystEngComm 2011, 13, 5439–5446. [Google Scholar] [CrossRef]
- Gnahm, M.; Berger, C.; Arkhipova, M.; Kunkel, H.; Pajkossy, T.; Maas, G.; Kolb, D.M. The interfaces of Au(111) and Au(100) in a hexaalkyl-substituted guanidinium ionic liquid: An electrochemical and in situ STM study. Phys. Chem. Chem. Phys. 2012, 14, 10647–10652. [Google Scholar]
- Hess, S.; Arkhipova, M.; Wohlfahrt-Mehrens, M.; Maas, G.; Wachtler, M. Synthesis and characterization of guanidinium-based ionic liquids as possible electrolytes in lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A753–A761. [Google Scholar]
- Bucher, N.; Hartung, S.; Arkhipova, M.; Yu, D.; Kratzer, P.; Maas, G.; Srinivasan, M.; Hoster, H.E. A novel ionic liquid for Li ion batteries—Uniting the advantages of guanidinium and piperidinium cations. RSC Adv. 2014, 4, 1996–2003. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salchner, R.; Kahlenberg, V.; Gelbrich, T.; Wurst, K.; Rauch, M.; Laus, G.; Schottenberger, H. Hexaethylguanidinium Salts. Crystals 2014, 4, 404-416. https://doi.org/10.3390/cryst4030404
Salchner R, Kahlenberg V, Gelbrich T, Wurst K, Rauch M, Laus G, Schottenberger H. Hexaethylguanidinium Salts. Crystals. 2014; 4(3):404-416. https://doi.org/10.3390/cryst4030404
Chicago/Turabian StyleSalchner, Robert, Volker Kahlenberg, Thomas Gelbrich, Klaus Wurst, Martin Rauch, Gerhard Laus, and Herwig Schottenberger. 2014. "Hexaethylguanidinium Salts" Crystals 4, no. 3: 404-416. https://doi.org/10.3390/cryst4030404






