Plasmonic Photonic-Crystal Slabs: Visualization of the Bloch Surface Wave Resonance for an Ultrasensitive, Robust and Reusable Optical Biosensor
Abstract
:1. Introduction
2. Model Calculation


3. Results
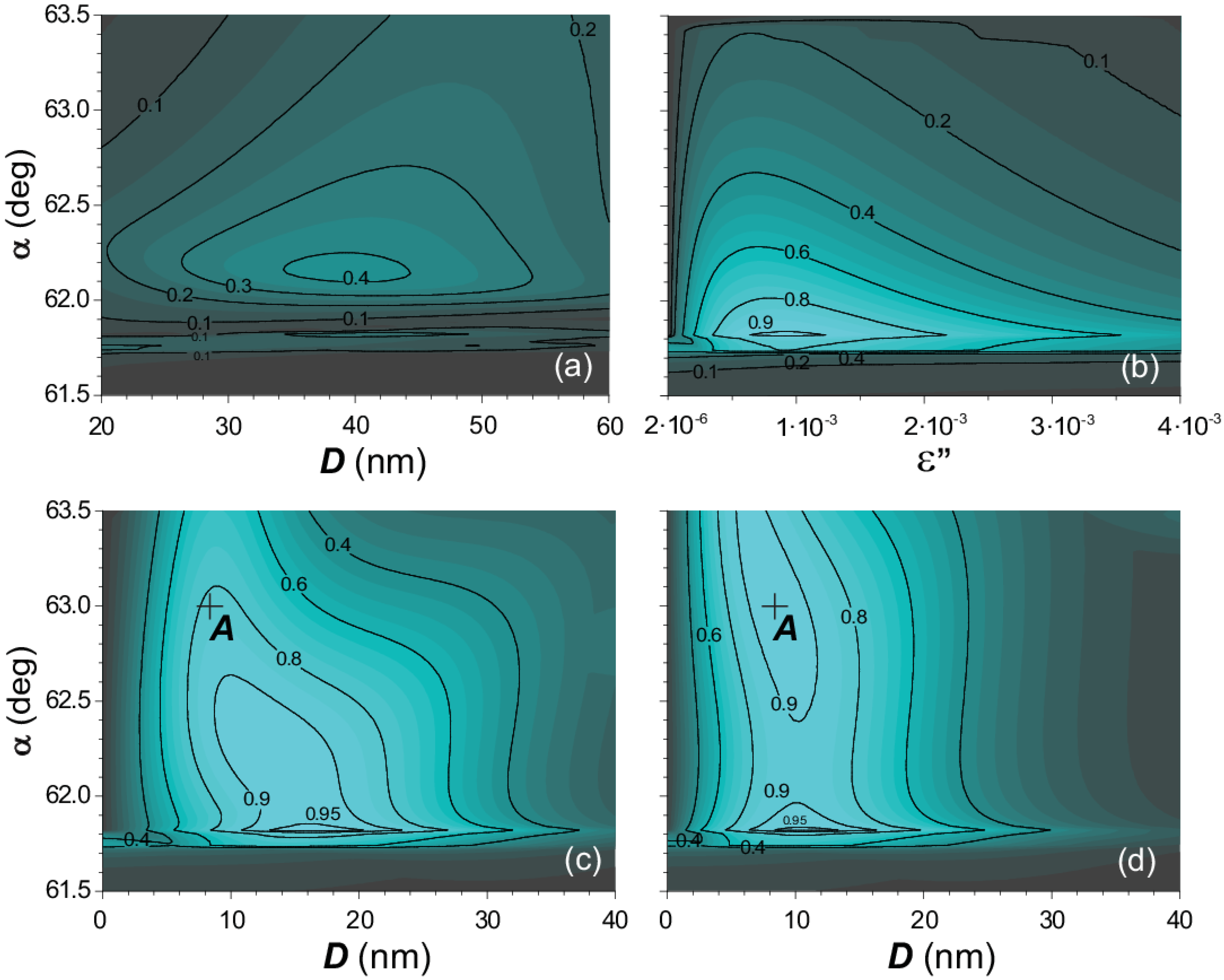



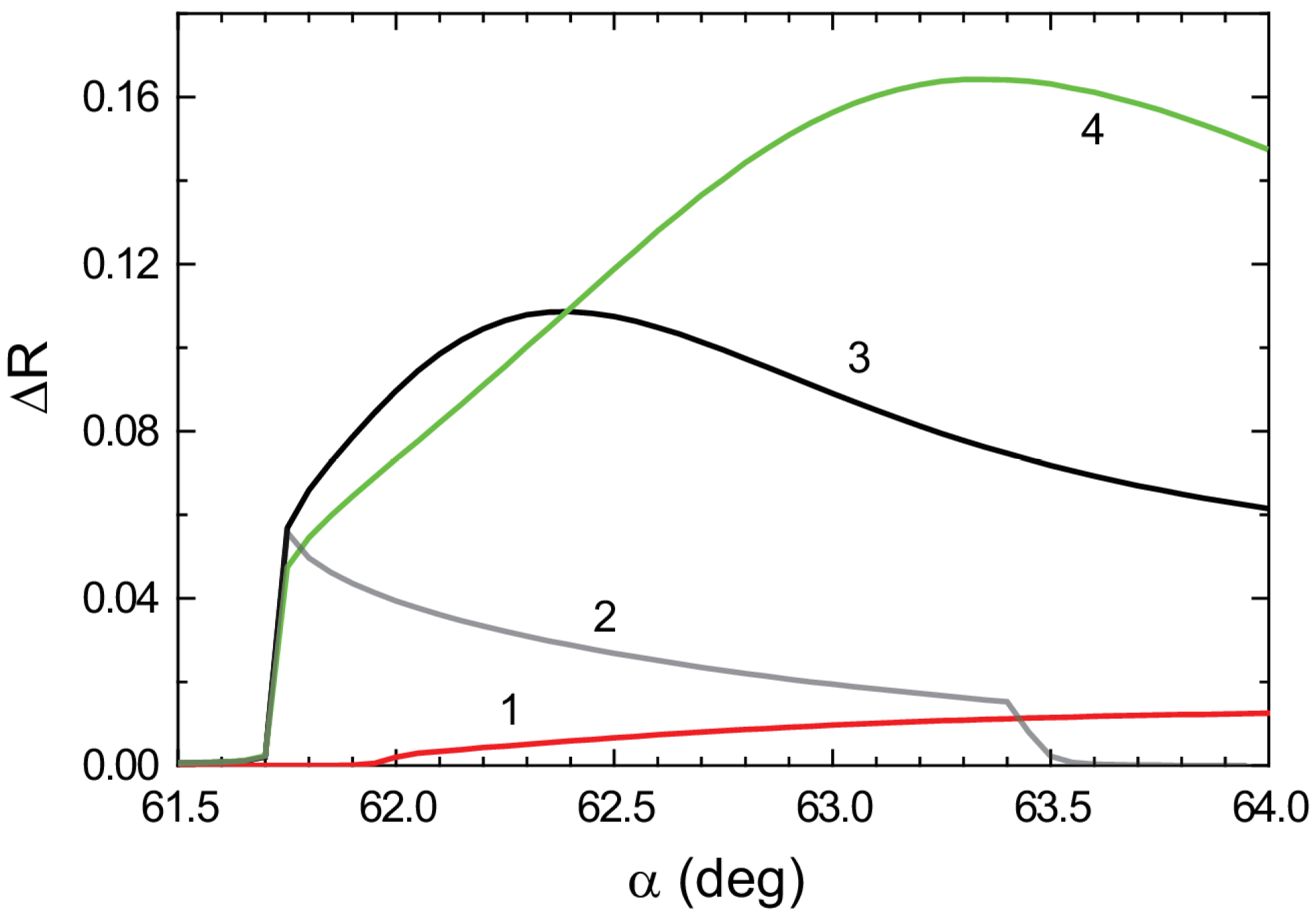
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wulfkuhle, J.D.; Liotta, L.A.; Petricoin, E.F. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer 2003, 3, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Nestor, P.J.; Scheltens, P.; Hodges, J.R. Advances in the early detection of Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 34–41. [Google Scholar] [CrossRef]
- Taitt, C.R.; Golden, J.P.; Shubin, Y.S.; Shriver-Lake, L.C.; Sapsford, K.E.; Rasooly, A.; Ligler, F.S. A Portable Array Biosensor for Detecting Multiple Analytes in Complex Samples. Microb. Ecol. 2004, 47, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.; Mullenix, M.C.; Swanson, S.J.; Koren, E. An acid dissociation bridging ELISA for detection of antibodies directed against therapeutic proteins in the presence of antigen. J. Immunol. Methods 2005, 304, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Stolz, L.; Granzow, R. Surface plasmon resonance and its use in biomolecular interaction analysis (BIA). Curr. Opin. Struct. Biol. 1995, 5, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, J.A.; Dhandapani, S.; Pennucci, J.J.; Abbott, C.M.; Mytych, D.T.; Kaliyaperumal, A.; Swanson, S.J.; Mullenix, M.C. Comparing ELISA and Surface Plasmon Resonance for Assessing Clinical Immunogenicity of Panitumumab. J. Immunol. 2007, 178, 7467–7472. [Google Scholar] [PubMed]
- Homola, J.; Piliarik, M. Surface Plasmon Resonance Based Sensors. In Springer Series on Chemical Sensors and Biosensors; Springer-Verlag: Berlin, Germany, 2006. [Google Scholar]
- Nenninger, G.G.; Piliarik, M.; Homola, J. Data analysis for optical sensors based on spectroscopy of surface plasmons. Meas. Sci. Technol. 2002, 13, 2038–2046. [Google Scholar] [CrossRef]
- Piliarik, M.; Párová, L.; Homola, J. High-throughput SPR sensor for food safety. Biosens. Bioelectron. 2009, 24, 1399–1404. [Google Scholar] [PubMed]
- Wu, B.M.; Pao, M.C. Sensitivity-tunable optical sensors based on surface plasmon resonance and phase detection. Opt. Express 2004, 12, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, I.; Brecht, A.; Gauglitz, G. Compact surface plasmon resonance-transducers with spectral readout for biosensing applications. Sens. Actuators B Chem. 1999, 54, 98–105. [Google Scholar] [CrossRef]
- Bardin, F.; Bellemain, A.; Roger, G.; Canva, M. Surface plasmon resonance spectro-imaging sensor for biomolecular surface interaction characterization. Biosens. Bioelectron. 2009, 24, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Thirstrup, B.; Zong, W. Data analysis for surface plasmon resonance sensors using dynamic baseline algorithm. Sens. Actuators B Chem. 2005, 106, 796–802. [Google Scholar] [CrossRef]
- Chinowsky, T.M.; Quinn, J.G.; Bartholomew, D.U.; Kaiser, R.; Elkind, J.L. Performance of the Spreeta 2000 integrated surface plasmon resonance affinity sensor. Sens. Actuators B Chem. 2003, 91, 266–274. [Google Scholar] [CrossRef]
- Piliarik, M.; Vala, M.; Tichý, I.; Homola, J. Compact and low-cost biosensor based on novel approach to spectroscopy of surface plasmons. Biosens. Bioelectron. 2009, 24, 3430–3435. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Robinson, G. The commercial development of planar optical biosensors. Sens. Actuators 1995, 29, 31–36. [Google Scholar]
- Raether, H. Surface Plasmons; Springer: Berlin, Germany, 1988. [Google Scholar]
- Yeh, P.; Yariv, A.; Hong, C.-S. Electromagnetic propagation in periodic stratified media. I. General theory. J. Opt. Soc. Am. 1977, 67, 423–438. [Google Scholar] [CrossRef]
- Yeh, P.; Yariv, A.; Cho, A.Y. Optical surface waves in periodic layered media. Appl. Phys. Lett. 1978, 32, 104–105. [Google Scholar]
- Robertson, W.M.; May, M.S. Surface electromagnetic waves on one-dimensional photonic band gap arrays. Appl. Phys. Lett. 1999, 74, 1800–1802. [Google Scholar] [CrossRef]
- Shinn, M.; Robertson, W.M. Surface plasmon-like sensor based on surface electromagnetic waves in a photonic band-gap material. Sens. Actuators B Chem. 2005, 105, 360–364. [Google Scholar] [CrossRef]
- Michelotti, F.; Sciacca, B.; Dominici, L.; Quaglio, M.; Descrovi, E.; Giorgis, F.; Geobaldo, F. Fast optical vapour sensing by Bloch surface waves on porous silicon membranes. Phys. Chem. Chem. Phys. 2010, 12, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Rivolo, P.; Michelotti, F.; Frascella, F.; Digregorio, G.; Mandracci, P.; Dominici, L.; Giorgis, F.; Descrovi, E. Real time secondary antibody detection by means of silicon-based multilayers sustaining Bloch surface waves. Sens. Actuators B Chem. 2012, 161, 1046–1052. [Google Scholar] [CrossRef]
- Sinibaldi, A.; Danz, N.; Descrovi, E.; Munzert, P.; Schulz, U.; Sonntag, F.; Dominici, L.; Michelotti, F. Direct comparison of the performance of Bloch surface wave and surface plasmon polariton sensors. Sens. Actuators B Chem. 2012, 174, 292–298. [Google Scholar] [CrossRef]
- Frascella, F.; Ricciardi, S.; Rivolo, P.; Moi, V.; Giorgis, F.; Descrovi, E.; Michelotti, F.; Munzert, P.; Danz, N.; Napione, L.; et al. A Fluorescent One-Dimensional Photonic Crystal for Label-Free Biosensing Based on Bloch Surface Waves. Sensors 2013, 13, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ye, J.Y.; Divin, C.; Huang, B.; Thomas, T.P.; Baker, J.R., Jr.; Norris, T.B. Real-Time Biomolecular Binding Detection Using a Sensitive Photonic Crystal Biosensor. Anal. Chem. 2010, 82, 5211–5218. [Google Scholar]
- Konopsky, V.N.; Alieva, E.V. A biosensor based on photonic crystal surface waves with an independent registration of the liquid refractive index. Biosens. Bioelectron. 2010, 25, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Konopsky, V.N.; Karakouz, T.; Alieva, E.V.; Vicario, C.; Sekatskii, S.K.; Dietler, G. Photonic crystal biosensor based on optical surface waves. Sensors 2013, 13, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Baryshev, A.V.; Merzlikin, A.M.; Inoue, M. Efficiency of optical sensing by a plasmonic photonic-crystal slab. J. Phys. D Appl. Phys. 2013, 46, 125107:1–125107:5. [Google Scholar] [CrossRef]
- Baryshev, A.V.; Merzlikin, A.M.; Inoue, M. Plasmonic Photonic-Crystal Slab as An Ultrasensitive and Robust Optical Biosensor. In Proceedings of the SPIE 8632, Photonic and Phononic Properties of Engineered Nanostructures III, San Francisco, CA, USA, 21 February 2013.
- Palik, B.D. Handbook of Optical Constants of Solids; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Landolt-Bornstein Database. Available online: http://www.springermaterials.com (accessed on 14 November 2013).
- Goto, T.; Dorofeenko, A.V.; Merzlikin, A.M.; Baryshev, A.V.; Vinogradov, A.P.; Inoue, M.; Lisyansky, A.A.; Granovsky, A.B. Optical tamm states in one-dimensional magnetophotonic structure. Phys. Rev. Lett. 2008, 101. [Google Scholar] [CrossRef] [PubMed]
- Kaliteevski, M.; Iorsh, I.; Brand, S.; Abram, R.A.; Chamberlain, J.M.; Kavokin, A.V.; Shelykh, I.A. Tamm plasmon-polaritons: Possible electromagnetic states at the interface of a metal and a dielectric Bragg mirror. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Villa, F.; Gaspar-Armenta, J.A. Electromagnetic surface waves: Photonic crystal-photonic crystal interface. Opt. Commun. 2003, 223, 109–115. [Google Scholar]
- Kavokin, A.; Shelykh, I.; Malpuech, G. Lossless interface modes at the boundary between two periodic dielectric structures. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Namdar, A.; Shadrivov, I.V.; Kivshar, Y.S. Backward Tamm states in left-handed metamaterials. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Baryshev, A.V.; Kawasaki, K.; Lim, P.B.; Inoue, M. Interplay of surface resonances in one-dimensional plasmonic magnetophotonic crystal slabs. Phys. Rev. B 2012, 85. [Google Scholar] [CrossRef]
- Afinogenov, A.I.; Bessonov, V.O.; Nikulin, A.A.; Fedyanin, A.A. Observation of hybrid state of Tamm and surface plasmon-polaritons in one-dimensional photonic crystals. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Threm, B.; Nazirizadeh, Y.; Gerken, M. Photonic crystal biosensors towards on-chip integration. J. Biophotonics 2012, 5, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Baryshev, A.V.; Merzlikin, A.M. Approach to visualization of and optical sensing by Bloch waves in noble or base metal-based plasmonic photonic crystal slabs. Appl. Opt. 2014, 14, 3142–3146. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baryshev, A.V.; Merzlikin, A.M. Plasmonic Photonic-Crystal Slabs: Visualization of the Bloch Surface Wave Resonance for an Ultrasensitive, Robust and Reusable Optical Biosensor. Crystals 2014, 4, 498-508. https://doi.org/10.3390/cryst4040498
Baryshev AV, Merzlikin AM. Plasmonic Photonic-Crystal Slabs: Visualization of the Bloch Surface Wave Resonance for an Ultrasensitive, Robust and Reusable Optical Biosensor. Crystals. 2014; 4(4):498-508. https://doi.org/10.3390/cryst4040498
Chicago/Turabian StyleBaryshev, Alexander V., and Alexander M. Merzlikin. 2014. "Plasmonic Photonic-Crystal Slabs: Visualization of the Bloch Surface Wave Resonance for an Ultrasensitive, Robust and Reusable Optical Biosensor" Crystals 4, no. 4: 498-508. https://doi.org/10.3390/cryst4040498



